Abstract
Although hippocampal neurogenesis has been described in many adult mammals, the functional impact of this process on physiology and behavior remains unclear. In the present study, we used two independent methods to ablate hippocampal neurogenesis and found that each procedure caused a limited behavioral deficit and a loss of synaptic plasticity within the dentate gyrus. Specifically, focal X irradiation of the hippocampus or genetic ablation of glial fibrillary acidic protein-positive neural progenitor cells impaired contextual fear conditioning but not cued conditioning. Hippocampal-dependent spatial learning tasks such as the Morris water maze and Y maze were unaffected. These findings show that adult-born neurons make a distinct contribution to some but not all hippocampal functions. In a parallel set of experiments, we show that long-term potentiation elicited in the dentate gyrus in the absence of GABA blockers requires the presence of new neurons, as it is eliminated by each of our ablation procedures. These data show that new hippocampal neurons can be preferentially recruited over mature granule cells in vitro and may provide a framework for how this small cell population can influence behavior.
Keywords: long-term potentiation, learning, memory
New neurons are born in the dentate gyrus (DG) of the hippocampus throughout the life of mammals (1) and derive from dividing progenitor cells located in the innermost part of the granule cell layer, a region called the subgranular zone. Young granule neurons integrate into the existing circuitry of the hippocampus, as evidenced by the development of functional synaptic inputs provided by the medial perforant path (MPP) and growth of axons to target cells in CA3 (2). Although a variety of environmental and pharmacological manipulations can affect neurogenesis (2, 3), it is unclear whether adult-born neurons provide a significant contribution to hippocampal function and, ultimately, how it might impact behavior.
Recent studies have shown that various strategies to disrupt neurogenesis produce a limited impairment in some hippocampal-dependent learning and memory tasks and in responses to antidepressant drugs (4–11). Unfortunately, the lack of spatial and cellular specificity provided by most ablation techniques has made it difficult to ascertain whether the consequent behavioral effects were caused by ablation of neurogenesis or other impairments. To circumvent these problems we have used two independent strategies of ablation. The first is a previously reported x-ray procedure that differs from similar methods in two ways: (i) the x-ray administration is restricted to a fraction of the brain containing the hippocampus and spares neurogenesis in the neighboring subventricular zone; and (ii) mice are allowed to recover for 3 months before testing to allow for the disappearance of markers of inflammation, such as reactive microglia (9). The second method of ablation is a genetic strategy that directly targets dividing progenitors throughout the brain and avoids potential radiation–induced side effects. Therefore, the two strategies are complementary: irradiation affords spatial but not cellular specificity, whereas genetic ablation is specific to progenitor cells, but impacts both neurogenic regions of the brain.
Using these strategies, we examined whether loss of neurogenesis in adult mice altered hippocampal-dependent behaviors or changed electrophysiological responses within the hippocampus. Our results demonstrate a specific impairment in contextual fear conditioning and a loss of long-term potentiation (LTP) in the DG after ablation of neurogenesis. These findings show that new neurons make an essential contribution to a subset of hippocampal functions.
Results
Ablation of Neurogenesis Impairs Contextual Fear Conditioning.
We have previously shown that a series of low-dose X irradiations targeted to the hippocampus of adult mice produces a lasting ablation of neurogenesis in the DG, while sparing cell proliferation in surrounding brain areas including the subventricular zone (4, 9). Although brain irradiation can induce a rapid inflammatory response, evidenced primarily by an increase in activated microglia (12, 13), we have shown that our procedure results in only a transient increase in inflammatory markers that subsides within 2–3 months (9). Therefore, animals were allowed to recuperate from irradiation for 3 months before functional analyses were performed.
Cued and contextual fear learning are forms of Pavlovian conditioning elicited by pairing a neutral conditioned stimulus (CS) with an aversive unconditioned stimulus (US) in a distinctive context. Acquisition of the CS–US association is known to require the amygdala (14, 15), whereas acquisition of a context–US association usually requires both the hippocampus and amygdala (16, 17). Sham and irradiated mice were trained in a cued conditioning protocol 3 months after x-ray treatment (Fig. 1A–C). Fig. 1D shows that irradiation did not alter freezing either before or during the CS in the cued conditioning test, suggesting that amygdala function is not grossly impaired by this procedure. However, irradiated mice froze significantly less than shams in the test of context-elicited fear (Fig. 1E).
Fig. 1.
Irradiation impairs contextual but not cued fear conditioning. (A-C) Schematic diagram of the fear conditioning procedure (n = 14 per group). (A) Day 1: training (context A). (B) Day 2: cued test (context B). (C) Day 3: context test (context A). (D) Conditioned freezing during the tone test. Percent time freezing during the 60 s before tone presentation (pretone) and during the 20-s tone presentation (Tone). Data were subjected to a 2 (treatment) × 2 (period: pretone vs. tone) ANOVA (n = 14 per group). Mice froze significantly more during the tone than during the pretone period [F(1,26) = 38.7, P < 0.001]. There was no effect of X irradiation [F(1,26) = 0.3] and no treatment × period interaction [F(1,26) < 1]. (E) Percent time freezing during the 4 min of the context test. Data were subjected to a 2 (treatment) × 4 (minute) ANOVA. Irradiated mice spent significantly less time freezing than sham mice [F(1,26) = 5.4, P = 0.03]. Error bars represent ± 1 SEM.
The fact that tone-elicited freezing was normal in irradiated mice suggests that the deficit in context-elicited freezing was not caused by an impairment in the motor control of freezing or a reduction in shock sensitivity. We also sought to confirm that the reduction in context-elicited freezing in these animals was not caused by a general reduction in anxiety-like behaviors. Therefore, irradiated and sham mice were subjected to two tests of anxiety-like behavior: the elevated-plus maze and the light–dark choice task. Each test examines the exploratory behavior of animals within an apparatus that contains anxiogenic areas. The number of entries and time spent in the anxiogenic areas are increased by treatment with classical anxiolytic drugs (18–20). As shown in Fig. 4 A–C, which is published as supporting information on the PNAS web site, there was no effect of irradiation on activity in either the open versus closed arms (elevated-plus maze) or the light versus dark compartments (light–dark choice task).
Spatial Memory Is Normal in Irradiated Mice.
The performance of sham and irradiated mice was compared by using several water maze tasks: the visible and hidden platform tasks and the delayed matching-to-place task (21, 22). As shown in Fig. 5 A–C, which is published as supporting information on the PNAS web site, irradiation resulted in no detectable impairments in either the visible platform task or the training phase of the hidden platform task, which assesses the ability to use extra-maze spatial cues to locate a submerged platform. Likewise, sham and irradiated mice displayed similar memory for the hidden platform location in probe trials conducted on the final day of training (Fig. 5D Left) and 2 weeks after the end of training (Fig. 5D Right).
The delayed matching-to-place task assessed the ability to rapidly acquire and reacquire new hidden platform locations. Mice received four trials per day with a new platform location each day. The performance of sham and irradiated mice was compared over a period of 4 weeks. Again no effect of irradiation was observed (Fig. 5E). Sham and irradiated mice were also tested in a place recognition task, the Y maze (23). Mice are allowed to freely explore two arms of a three-armed maze for 10 min and, after a 30-min delay, are returned to the maze with all arms open for a 5-min recognition test. During this test, intact rodents typically make more visits to the novel unexplored arm overall. Fig. 4D shows that novel arm preference was unaffected by irradiation.
Fear Conditioning in Glial Fibrillary Acidic Protein (GFAP)-Thymidine Kinase (TK) Transgenic (TG) Mice.
Because the impairment of contextual fear conditioning was the only significant effect of our irradiation procedure on learning and memory processes, we sought to confirm this result by using an independent method to ablate neurogenesis. TG mice that express herpes virus TK under the regulation of the mouse GFAP promoter were generated as described (24). TK is expressed by both proliferating and nonproliferating GFAP-positive cells in the brains of these mice; however, delivery of the antiviral prodrug ganciclovir (GCV) only kills proliferating TK-positive cells. Because neurons in the adult hippocampus derive from GFAP-positive progenitor cells, neurogenesis is ablated by chronic delivery of GCV (25, 26).
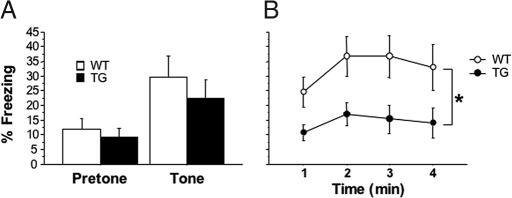
To determine whether the impairment in contextual conditioning observed after irradiation was caused by a loss of young neurons, WT and GFAP-TK TG mice were treated with GCV for 6 weeks to ablate neurogenesis and then subjected to the fear conditioning procedure. GCV-mediated ablation of neurogenesis did not alter cued fear conditioning, but produced a significant reduction of freezing during the contextual conditioning test (Fig. 2). Similar to our observations in irradiated mice, GCV-treated TG mice displayed normal levels of anxiety-related behaviors in several conflict tests (data not shown).
Fig. 2.
GCV treatment impairs contextual but not cued fear conditioning in GFAP-TK TG mice. (A) Conditioned freezing during the tone test. Percent of time freezing during the 60 s before tone presentation (pretone) and during the 20-s tone presentation (tone) is shown. Data were subjected to a 2 (treatment) × 2 (period: pretone vs. tone) ANOVA [n = 19 (WT) and 11 (TG)]. Mice froze significantly more during the tone than during the pretone period [F(1,28) = 14.1, P < 0.001]. There was no effect of genotype [F(1,28) = 0.5] or genotype × period interaction [F(1,28) < 1]. (B) Percent time freezing during the 4 min of the context test. Data were subjected to a 2 (treatment) × 4 (minute) ANOVA. Irradiated mice spent significantly less time freezing than did sham mice [F(1,28) = 4.7, P = 0.04].
Neurogenesis-Dependent Plasticity in the DG.
The physiological consequences of ablating neurogenesis were examined in two hippocampal subregions that exhibit LTP, the MPP/DG connection and the Schaffer collateral commissural projections to CA1 (SC/CA1). In the first experiment, slices derived from mice 3 months after x-ray treatment were used for extracellular recordings. The slope of excitatory postsynaptic potentials (EPSPs) evoked by stimulation of the MPP was examined by placing an extracellular recording electrode in the molecular layer of the upper blade of the DG. Because no difference was detected in either the input–output relationships (Fig. 6A, which is published as supporting information on the PNAS web site) or paired-pulse depression (Fig. 6B) in the DG of irradiated animals, we next investigated long-term plasticity in this synaptic connection.
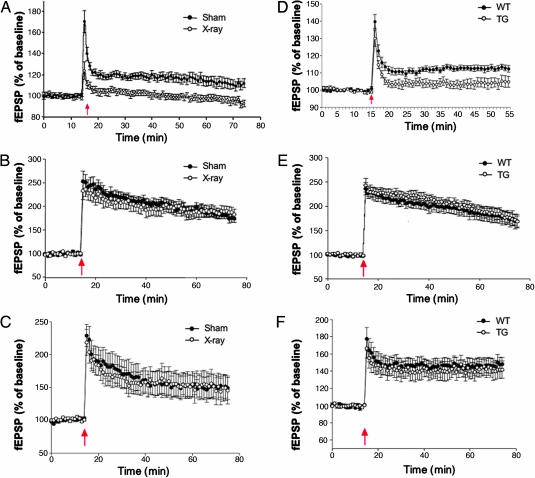
LTP of the MPP/DG connection has proven difficult to evoke in slice preparations because of the prominent GABAergic innervation of this hippocampal subfield (27); yet recent studies in rats have identified a small but stable form of LTP that can be elicited with a 100-Hz stimulation protocol and is disrupted by cranial gamma-irradiation (28, 29). To confirm these findings in our experimental system, we elicited LTP by using this 100-Hz protocol and compared responses evoked in the DG of sham and irradiated mice. Fig. 3A shows that a stable potentiation of ≈15% was observed in sham mice, but was completely absent in irradiated animals. In contrast, stimulation of MPP afferents in the presence of the GABA blocker picrotoxin produced a larger potentiation (≈200%) in the DG that was not affected by irradiation (Fig. 3B). These results support previous reports that young neurons in the DG exhibit a lack of GABAergic inhibition and are, therefore, more receptive to the induction of LTP (28–31).
Fig. 3.
Ablation of neurogenesis prevents a form of LTP in the DG. Slices for physiological experiments were derived either 3 months after x-ray treatment (A-C) or immediately after a 6-week GCV treatment was completed (D-F). (A) MPP/DG LTP in sham (n = 8) and irradiated (n = 8) mice 3 months after treatment. Arrow indicates the delivery of the 100-Hz stimulation. Repeated-measures ANOVA performed on the last 10 min of recording confirmed a significant difference between sham and x-irradiated animals [F(1,14) = 11.2, P < 0.001]. (B) LTP evoked in the DG after the addition of 50 μM picrotoxin. Stimulation parameters are the same as above. There is no difference in LTP between sham and irradiated mice [F(1,14) < 1]. (C) LTP in the Schaffer collateral to CA1 connection was not altered by irradiation [F(1,14) < 1]. (D) Six weeks of GCV treatment caused a loss of LTP in the DG in TG (n = 9) but not WT (n = 15) mice. Repeated measures ANOVA on the last 10 min confirmed a significant difference between WT and TG animals [F(1,24) = 7.2, P = 0.01]. (E) GCV treatment did not alter DG LTP elicited in the presence of 50 μM picrotoxin [F(1,24) < 1]. (F) LTP in Schaffer collateral/CA1 connections was also unaffected by GCV [F(1,24) < 1]. Error bars represent ± 1 SEM.
To determine whether the effects of irradiation on hippocampal physiology were specific to the DG, we also examined plasticity in the CA1 subfield, a region of the hippocampus that is devoid of neurogenesis in adulthood. We found that sham and irradiated slices displayed similar input–output relationships and paired-pulse facilitation (Fig. 6 C and D) and robust LTP in the Schaffer/CA1 synaptic connection induced by a similar 100-Hz stimulation protocol (Fig. 3C). Thus, irradiation does not impair synaptic plasticity in the CA1 region but completely abolishes LTP evoked in the DG. We next sought to confirm these findings by repeating the above experiments by using the genetic model of ablation.
WT and GFAP-TK TG mice were treated with GCV for 6 weeks before we collected hippocampal slices for extracellular field recordings. Similar to our findings using irradiation, this method of ablating neurogenesis abolished LTP evoked in the DG without picrotoxin (Fig. 3D). However, no difference in DG LTP was observed between WT and TG mice when picrotoxin was added to the bath solution (Fig. 3E). To determine whether the effects of GCV treatment were specific to plasticity associated with young neurons, additional comparisons between GCV-treated WT and TG mice were performed. As shown in Fig. 7, which is published as supporting information on the PNAS web site, we detected no differences between WT and TG mice in input–output relationships and paired-pulse depression in the MPP/DG connections or in input–output and paired-pulse facilitation in the Schaffer/CA1 connections. Finally, LTP in the Schaffer/CA1 connection was normal in GCV-treated TG mice (Fig. 3F). Thus, genetically targeted disruption of neurogenesis causes an impairment of LTP within the DG without altering synaptic plasticity in the CA1 subfield, in good agreement with our observations using irradiation. It is noteworthy that, after induction of LTP in the DG, posttetanic potentiation is significantly reduced in irradiated mice, but not in GCV-treated TG mice. It is possible that this difference is because the loss of neurogenesis is not as profound in TG mice when compared with irradiated mice. Alternatively, non-neurogenesis-dependent mechanisms may contribute to the posttetanic potentiation changes observed in irradiated mice.
Discussion
New Neurons Contribute to LTP in the DG.
Recent studies have identified a form of DG LTP that can be elicited in the absence of GABAA blockers and is disrupted by gamma irradiation (28, 29). In keeping with these results, we observed a small but stable LTP in response to stimulation of the MPP that did not require the use of GABA blockers. The fact that this LTP was abolished by both of our ablation paradigms indicates that it requires neurogenesis. In contrast, LTP evoked in the presence of a GABA blocker was larger in magnitude and, importantly, was not altered by ablation of neurogenesis.
Single-cell recordings have demonstrated that young neurons are more excitable than mature granule cells because of properties such as an elevated resting membrane potential, high input resistance, and low-threshold calcium channel expression (29, 31). In addition, young neurons (2–4 wk old) are not inhibited, and can in fact be depolarized, by GABAergic activity (30, 32). These properties may explain why young granule neurons are more receptive to the induction of LTP. We suspect that in the presence of GABA blockers both young and mature neurons are recruited, resulting in a larger LTP. In this case, LTP is not affected by ablation because the new neurons represent only a small fraction of the total number of granule cells responding to stimulation (33) or, alternatively, because the excitatory influence of GABA on new neurons is blocked.
Role for Hippocampal Neurogenesis in Contextual Fear Conditioning.
As in the case of the electrophysiological studies, we examined animal behavior after ablation of neurogenesis with two different strategies. Although we found no deficit in spatial learning or memory as assessed in several water maze paradigms or in novel place recognition in the Y maze, each ablation method produced a significant impairment of contextual fear conditioning. The effect of ablation was specific to contextual fear conditioning because conditioned responses to the tone alone were not altered. Moreover, the reduction in context-elicited freezing does not appear to arise from reduced basal levels of anxiety or fear, as we observed no differences in classic tests of anxiety-like behaviors such as the elevated-plus maze or light–dark choice task.
Our finding of impaired contextual fear conditioning conflicts with previous studies reporting a reduction in hippocampal-dependent trace conditioning after ablation of neurogenesis by systemic antimitotic treatment in rats (5), but no change in contextual conditioning (6). Our results also conflict with a report that whole-head irradiation in rats impairs long-term memory in the water maze (10). One explanation for the discrepancies may be the greater spatial specificity of our ablation method. Another possibility is that the behavioral significance of neurogenesis differs between mice and rats. However, a recent study reported that contextual fear conditioning was impaired in rats after whole-head gamma irradiation (11). Integrating these findings will require further information on whether extrahippocampal neurogenesis impacts these tasks and how subtle methodological variations in the tasks might impact their neurogenesis dependence.
It is intriguing that ablation of hippocampal neurogenesis reduced contextual fear conditioning but did not affect performance in spatial maze tasks that are also known to be sensitive to hippocampal lesions. One explanation may come from reports that spatial maze learning and contextual conditioning use different molecular signaling pathways within the hippocampus (34) and, importantly, require different extrahippocampal circuitry. Lesioning the amygdala, for example, does not impair spatial maze learning but disrupts fear conditioning, and activation of the amygdala during strong emotional stimuli like those experienced in fear conditioning can enhance hippocampal-dependent learning (35, 36). Therefore, one interpretation of our results is that new neurons may influence learning and memory primarily in tasks that involve significant emotional arousal or coactivation of the hippocampus and the amygdala.
An alternative hypothesis is suggested by experiments showing that exposure to novel environments increases GABAergic tone in the DG and facilitates the generation of LTP (37–39). Because young neurons are not inhibited by GABA, they may be preferentially recruited under such conditions. If so, the effects of blocking neurogenesis would be largest in tasks that take place in a novel environment. This would explain why loss of neurogenesis impairs context conditioning but not water maze performance because contextual conditioning occurs in a single exposure to a novel environment, whereas learning in the water maze requires repeated exposure to an environment over multiple days. It is also interesting to note that our previous studies have revealed a role for neurogenesis in the behavioral response to antidepressant drugs (4). The principal test used in these experiments (the novelty-suppressed feeding paradigm) relies on the fear elicited by placement of the animal in a novel environment.
In summary our studies reveal that, under certain conditions, the activity of young neurons may be preferentially recruited over that of mature ones and provide a potential framework for how a small number of new neurons in the DG may impact behavior. Specifically, we hypothesize that certain behaviors, such as contextual fear conditioning, will elicit a form of neurogenesis-dependent plasticity similar to that observed in vitro.
Materials and Methods
Animals.
A total of 129/SvEv age-matched adult male mice (ages 12–25 weeks) purchased from Taconic (Hudson, NY) were used in all experiments with X irradiation. GFAP-TK TG mice (line 7.1) were generated as described (24, 40). We transferred the GFAP-TK transgene onto a C57/BL6-BALB/c mixed background and used 12- to 20-week-old male littermates derived from heterozygote crossings. Mice were housed four or five per cage in a 12-h (06:00–18:00) light–dark colony room at 22°C with freely available food and water.
Drugs.
GCV (Roche, Indianapolis, IN) was dissolved in sterile saline at a concentration of 25 mg/ml and delivered through Alzet (Palo Alto, CA) osmotic minipumps implanted s.c. under anesthesia. An average dose of 10 mg/kg per day was delivered for ≈6 weeks.
Irradiation Procedure.
This procedure was performed as described (4).
Morris Water Maze.
The water maze procedure (n = 16 per group) consisted of three phases: visible platform training, hidden platform training, and long-term memory testing (Fig. 5 A and B). The pool was 1.7 m in diameter, and during training a circular platform (14.6-cm diameter) was submerged 0.5 cm below the surface of the water in the center of one quadrant of the pool. Data (swim speed, path length, and location) were collected by using a video tracking system (HVS Image, Hampton, U.K.).
During visible and hidden platform training there were four trials per day, with an intertrial interval of 15 min. The start location varied between trials. During the visible platform training (days 1 and 2), the platform location was indicated by a marker rising above the water. In hidden-platform training (days 3–12), the platform location was not marked and thus could only be discerned by using extra-maze cues. Long-term memory for the platform location was assessed in probe trials 2 and 4 weeks after the final hidden-platform training day. On probe trials the platform was removed from the pool, and the mouse was placed into the pool for 60 s. Percent time in each quadrant and number of crossings of the platform location were recorded.
Delayed Matching-to-Place Water Maze Task.
A naïve group of mice (n = 15 sham, 14 x-ray) received four trials per day for 16 days (4 days per week) using the pool and (hidden) platform described above. A new platform location was used each day, but within each day the platform location was constant. The start position was varied randomly between trials. In this way, mice were required to learn a new platform location each day.
Y Maze.
The Y maze consisted of three Plexiglas arms of equal size joined together in a Y configuration. Each arm was 40 cm long and 10 cm wide with 12-cm-high walls. The floor of each arm was of black plastic and the walls were of clear plastic. The apparatus was placed on the floor of the experimental room and illuminated with a 100-W bulb from 200 cm above. A trial consisted of two exposures to the maze. Mice (n = 16 per group) were first allowed to explore two of the three arms for a total of 10 min while the third arm was blocked. Thirty minutes later mice were placed back in the maze for 5 min with all arms open, and the number of entries and time spent in each arm were recorded.
Fear Conditioning.
Fear conditioning was conducted in chambers with internal dimensions of ≈20 cm wide × 16 cm deep × 20.5 cm high (Med Associates, St. Albans, VT). A house light (CM1820 bulb) mounted directly above the chamber provided illumination. Each chamber was located inside a larger, insulated plastic cabinet that provided protection from outside light and noise. The behavior of mice was recorded by analog (x-ray experiment) or digital (GFAP-TK experiment) video cameras mounted above the conditioning chamber.
To assess freezing, analog videos were scored by an observer using the Stopwatch+ program (Center for Behavioral Neuroscience, Atlanta, GA). Freezing was defined as the complete absence of motion, including motion of the vibrissae, for a minimum of 0.5 s. Each test session was scored continuously in its entirety. Digital video recordings were analyzed with FreezeFrame software from Actimetrics (Evanston, IL). This software assesses freezing by measuring changes in the intensity of each pixel between successive frames of the video file. We have determined that the scores obtained with this software are highly correlated with the scores assigned by human observers (r = 0.90).
The fear conditioning procedure was conducted over 3 days (Fig. 4A). On day 1, mice were placed in the conditioning chamber and received pairings between a tone (20 s, 80 dB, 2 KHz) and a coterminating shock (1 s, 0.7 mA). The irradiated and GFAP-TK mice were of different background strains, and pilot studies indicated that it was necessary to use different numbers of tone-shock pairings to produce similar levels of conditioning in the two strains. Therefore, mice in the irradiation experiment received three tone-shock pairings, and mice in the GFAP-TK experiment received five pairings. In both cases, the intertrial interval was variable with a mean of 125 s, and the first tone presentation commenced ≈120 s after the mouse was placed into the chamber. All other procedural details were identical between the two experiments. The chambers were cleaned with 70% isopropanol between each set of mice. Each chamber was scented by a paper towel dabbed with mint solution and placed underneath the chamber floor.
On day 2, the procedure and context were changed in several ways to test conditioned fear of the tone CS in the absence of contextual cues associated with shock. The floor and walls of the chamber were covered by white and black plastic inserts; the chamber was scented with limonene; the ventilation fan was not operated; the experimenter wore a different style of gloves; chambers were cleaned with a nonalcohol disinfectant between runs; and mice were kept is a different holding room before testing. Each mouse was placed into the chamber for 5.5 min. The tone was presented twice for 20 s at 120 and 290 s into the session. No shocks were administered. Freezing was scored for the 1 min before the first tone presentation (pretone freezing) and during the 20 s of the first tone presentation (tone-elicited freezing). On day 3, mice were tested for conditioned fear of the training context. The testing procedure and context were identical to those used on day 1, except the CS was not presented. Mice were placed into the chambers for 4 min. The entire session was scored for freezing.
Elevated-Plus Maze.
The elevated plus maze was performed as described (41).
Light–Dark Choice Task.
The light–dark test (n = 16 per group) was conducted in an open field chamber measuring 43.2 × 43.2 cm (Med Associates) with a white floor and clear walls. A dark plastic box that is opaque to visible light but transparent to infrared covered half of the chamber area, thus creating dark and light compartments of equal size. An opening at floor level in the center of one wall of the dark compartment allowed passage between the light and dark compartments. The light compartment was brightly illuminated with an 8-W fluorescent tube (400 lux). The test was performed in a quiet, darkened room, and the mice were kept in this room at least 1 h before the test. Between each trial, the whole apparatus was cleaned. At the beginning of the test, the mouse was placed in the dark compartment and allowed to freely explore both compartments for 5 min. Ambulation distance and time spent in the dark and the light compartments were recorded.
Electrophysiology.
After cervical dislocation of 3- to 4-month-old mice (male), transverse hippocampal slices (400 μm) were prepared by using a tissue chopper or a vibratome. The slices were incubated in an interface chamber at 32°C and perfused with oxygenated artificial cerebrospinal fluid containing 119 mM NaCl, 2.3 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4, and 11 mM glucose. Slices were allowed to equilibrate for at least 2 h before positioning the electrodes and beginning stimulation.
To record from the DG, the MPP was stimulated by using a WPI (Waltham, MA) stimulation isolation unit and a bipolar tungsten electrode. Evoked potentials were recorded in the molecular layer above the upper blade of the DG by using a glass capillary microelectrode filled with artificial cerebrospinal fluid (tip resistance 1–3 MΩ). Isolation of the MPP was confirmed by assessing paired-pulse inhibition of the MPP/DG synaptic connection (42). Input–output curves were obtained after 10 min of stable recordings. The stimulation intensity that produced a ⅓ maximal response was used for the test pulses and tetanus. After 15 min of stable baseline response to test stimulation (once every 20 s), the ability to elicit LTP was assessed. LTP was induced with a weak stimulation paradigm consisting of four trains of 1 s each, 100 Hz within the train, repeated every 15 s (28). Responses were recorded every 20 s for 40 or 60 min after LTP induction. In a parallel set of experiments, the artificial cerebrospinal fluid contained 50 μM picrotoxin (Sigma, St. Louis, MO) to block GABAA receptor-mediated activity.
To record field EPSPs (fEPSPs) in the CA1 region of the hippocampus, afferent fibers of the Schaffer collateral pathway were stimulated by using a Grass (Quincy, MA) S48 stimulation unit, and recordings were made in the CA1 stratum radiatum. Basal synaptic transmission was determined by plotting the stimulus voltages against the fEPSP slope. The stimulation intensity (0.05 ms in duration) was adjusted to give fEPSP slopes approximately one-third of the maximum. Paired-pulse facilitation was tested by using four different interstimulus intervals (50, 100, 200, and 300 ms) and defined by expressing the second fEPSP slope as a percentage of the first. For LTP experiments, a 15-min baseline was recorded by stimulating every 20 s at the intensity that produced one-third maximum response. LTP was induced by using a 100-Hz stimulation (one train, 1 s in duration), after which responses were elicited once every 20 s at the same stimulation intensity for 60 min.
Supplementary Material
Abbreviations
- GFAP
glial fibrillary acidic protein
- LTP
long-term potentiation
- DG
dentate gyrus
- MPP
medial perforant path
- CS
conditioned stimulus
- US
unconditioned stimulus
- TK
thymidine kinase
- GCV
ganciclovir
- EPSP
excitatory postsynaptic potential
- TG
transgenic.
Footnotes
The authors declare no conflict of interest.
References
- 1.Alvarez-Buylla A, Lim DA. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doetsch F, Hen R. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 5.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 6.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 8.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 9.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 10.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 12.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 13.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Neuropsychopharmacology. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daumas S, Halley H, Frances B, Lassalle JM. Learn Mem. 2005;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips RG, LeDoux JE. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 18.Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 19.Hogg S. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 20.Pellow S, Chopin P, File SE, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 21.Girard TA, Xing H, Ward GR, Nguyen H, Wainwright PE. Pharmacol Biochem Behav. 2001;68:515–523. doi: 10.1016/s0091-3057(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 22.Steele RJ, Morris RG. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Neurobiol Learn Mem. 2000;73:31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- 24.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 25.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 26.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigstrom H, Gustafsson B. Brain Res. 1983;275:153–158. doi: 10.1016/0006-8993(83)90428-6. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JS, Kee N, Wojtowicz JM. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Scott BW, Wojtowicz JM. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 30.Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Hieber C, Jonas P, Bischofberger J. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 32.Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 33.McDonald HY, Wojtowicz JM. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno K, Giese KP. J Pharmacol Sci. 2005;98:191–197. doi: 10.1254/jphs.crj05005x. [DOI] [PubMed] [Google Scholar]
- 35.Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- 36.Cahill L, McGaugh JL. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 37.Davis CD, Jones FL, Derrick BE. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser EI. J Neurosci. 1996;16:1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straube T, Korz V, Frey JU. Neurosci Lett. 2003;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 40.Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- 41.Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNaughton BL. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.