Abstract
Ischemia and chronic hypoxia (CH) trigger a variety of adverse effects arising from metabolic stress that injures cells. In response to reduced O2, hypoxia-inducible factor 1α (HIF-1α) activates erythropoietin (Epo) as well as many other target genes that counteract the effects of O2 deficiency. Epo produced by the kidney stimulates erythrocyte production, leading to decreased HIF-1α production by improved tissue O2 delivery. However, Epo is produced by many other tissues, and it is currently unclear to what extent, if any, locally produced Epo modulates HIF-1α expression. Derivatives of Epo that possess tissue-protective activities but do not stimulate erythropoiesis [e.g., carbamylated Epo (CEpo)] are useful tools with which to determine whether exogenous Epo modulates HIF-1α in the absence of changes in hemoglobin concentration. We compared the effects of CH (6.5% O2 for 10 days) with or without CEpo administered by daily s.c. injection (10 μg/kg of body weight). CEpo administration did not alter the survival rate, weight loss, or increased hemoglobin concentration associated with CH. Therefore, CEpo does not directly suppress HIF-mediated erythropoiesis. CEpo does, however, prevent CH-induced neuronal increases of HIF-1α and Epo receptor-associated immunoreactivity (a measure of stress) while reducing the apoptotic index. In contrast, the myocardium did not exhibit increased HIF-1α expression during CH, although CEpo did reduce the apoptotic index. These observations therefore demonstrate that CEpo administration reduces the metabolic stress caused by severe CH, resulting in improved cellular survival independent of erythrocyte production.
Keywords: apoptosis, brain, hypoxia-inducible factor 1α, heart, Epo receptor
Chronic hypoxia (CH), a condition whereby the O2 supply to tissues is inadequate with respect to need, occurs in several settings encompassing physiological (high altitude and airline flights) and pathological (congenital heart disease, anemia, carbon monoxide poisoning, and chronic lung diseases) situations. A potentially lethal challenge, CH activates an array of molecular responses that enable the organism to compensate for the lack of O2 at multiple levels. Among the most prominent adaptive responses, an increase in RBCs and Hb represents an attempt to maximize the blood O2-carrying capacity (1). Erythropoiesis is under the control of the glycoprotein erythropoietin (Epo) that is primarily produced by the kidney to stimulate survival and differentiation of erythroid precursor cells into RBCs (2). Hypoxia-inducible factor 1α (HIF-1α), the molecular link between hypoxia and the induction of the Epo gene (3), regulates the transcription not only of the Epo gene but also of many other genes (4), including the Epo receptor (EpoR) (4) and proapoptotic proteins. In addition to its erythropoietic function, Epo is also produced locally, and it functions to protect a variety of tissues from ischemic injury, including the central nervous (5–7) and cardiovascular systems (8, 9). Both endogenous (10) and exogenous Epo (11, 12) protect tissues by signaling pathways in common with ischemic preconditioning, a process whereby repeated exposures to sublethal ischemia induce protection against a major ischemic challenge (13).
Carbamylation of Epo lysine residues results in a derivative (CEpo) that lacks erythropoietic activity, yet it appears to have identical cytoprotective properties compared with Epo when evaluated in a variety of cell lines in vitro and organs in vivo (14). It is notable that both Epo (15) and CEpo (16) may have direct metabolic effects, e.g., by directly increasing the threshold of the mitochondrial permeability transition pore to reactive oxygen species. Because the pharmacodynamics of CEpo are very similar to recombinant human Epo (rhEpo) (14), CEpo is under consideration as a candidate drug in various models of brain and heart ischemia. Use of CEpo to protect from hypoxia-related injury is especially promising because the lack of erythropoietic activity will avoid polycythemia, an undesirable specific consequence of CH that leads to cardiac dysfunction (17), a situation only partly overcome by increased in vivo nitric oxide production (18).
In a model in which rats are exposed to normobaric CH for 15 days, we observed marked deterioration of myocardial protection (19) and sustained apoptosis in a number of organs (20). Although plasma Epo was not measured, an increase in blood Hb by >70% indicates a substantial Epo stimulation. Although it is well known that Epo is made locally in many tissues in the setting of hypoxia (for review, see ref. 21), the increased apoptosis we have observed suggests that endogenous Epo levels are not sufficient to provide protection during prolonged hypoxia. It is also well known that administration of exogenous Epo provides protection from a wide variety of injuries, including ischemia and hypoxia (21), but it is unknown whether these effects are through an increased resistance to metabolic stress or a primary reduction of metabolic stress. The objective of this work was to determine whether CEpo provides antiapoptotic effects during severe CH in vivo without an increase in RBC mass, as happens with treatment by rhEpo. Here, we demonstrate that CEpo administration reduced metabolic stress as measured by increased brain and heart cell survival and decreased HIF-1α and EpoR-associated immunoreactivity after exposure of mice to severe CH (6.5% O2, corresponding to an altitude of 8,500 m).
Results
A total of 45 mice (30.1 ± 0.3 g at the time of study initiation) were used, having baseline parameters as summarized in Table 1. Although the survival rate in the normoxic groups was 17 of 17 (100%), exposure to hypoxia was associated with mortality in both vehicle- (6 of 14 or 43%) and CEpo-treated (4 of 14 or 29%) groups. Deaths generally occurred within a few hours after the onset of hypoxia. Fisher's exact test did not support any significant association between the treatments (P not significant). Body weight decreased in both the CH groups, with no difference between vehicle- and CEpo-treated mice. Likewise, the blood Hb concentration increased in response to CH in both vehicle- and CEpo-treated mice. As expected, CEpo treatment did not increase the blood Hb concentration in normoxic animals (Table 1).
Table 1.
Main characteristics (mean ± SEM) of the tested animals
| Normoxia |
Hypoxia |
|||
|---|---|---|---|---|
| Vehicle | CEpo | Vehicle | CEpo | |
| Total, n | 7 | 10 | 14 | 14 |
| Survived, n | 7 | 10 | 8 | 10 |
| Body weight change, g | 1.2 ± 0.7 | 0.2 ± 0.5 | −12.2 ± 0.4* | −11.1 ± 0.8* |
| Hemoglobin, mM | 6.61 ± 0.70 | 5.86 ± 0.33 | 9.43 ± 0.48† | 8.61 ± 0.50† |
Mice were exposed for 10 days to either normoxia (21% O2) or hypoxia (6.5% O2), and they were treated with either vehicle (PBS) or CEpo (10 μg/kg), both injected s.c. once a day. When significant (P < 0.05), the one-way ANOVA was followed by the Bonferroni multiple-comparison test aimed to test normoxia vs. hypoxia or vehicle vs. CEpo differences. No differences were assessed between vehicle- and CEpo-treated animals under either hypoxia or normoxia.
*, P < 0.0001 by ANOVA;
†, P < 0.0002 by ANOVA.
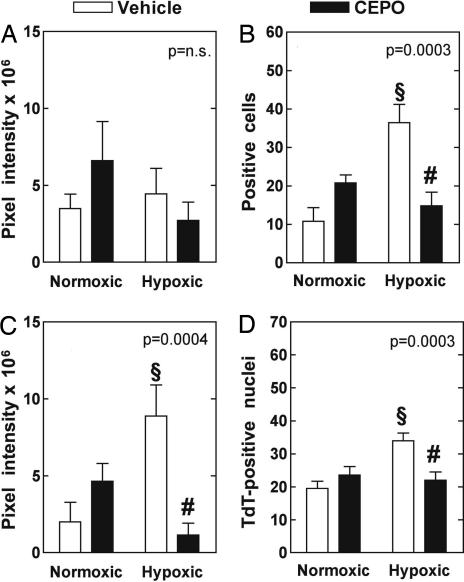
Brain tissue was obtained and frozen in liquid nitrogen within 1 min after the animals were euthanized. Fig. 1 summarizes the expression level of the proteins assessed and the results of the TUNEL measurements in the studied groups. Whereas TUNEL positivity and EpoR-associated immunoreactivity are expressed as the number of terminal deoxynucleotidyltransferase (TdT)-labeled nuclei and EpoR-associated immunoreactivity-positive cells per unit area, respectively, the levels of HIF-1α and Epo protein expression are expressed as total pixel intensity per unit area. To obtain a comparative evaluation that takes into account the possible occurrence of significant differences by chance alone, we first performed ANOVA of pooled data by one-way ANOVA separately for each parameter. Because the variations among the means were significantly greater than expected by chance alone for all parameters except Epo, the Bonferroni multiple comparison test could be used to detect differences between selected pairs of groups for TdT-labeled nuclei, EpoR-associated immunoreactivity-positive cells, and HIF-1α expression. Treatment with CEpo in normoxic animals did not induce appreciable differences in any parameter. CH increased the number of TdT-labeled nuclei, the number of EpoR-associated immunoreactivity-positive cells, and the expression level of HIF-1α. Treatment with CEpo blunted the CH-induced increase in all of these parameters to a value close to that measured in samples from normoxic animals.
Fig. 1.
CEpo treatment moderates the CH-induced neuronal stress response. Epo (A), EpoR (B), HIF-1α (C), and TUNEL (D) positivity are expressed as, respectively, protein level (pixel intensity from immunofluorescence images), no. of cells with EpoR-associated immunoreactivity, protein level (pixel intensity from immunofluorescence images) and no. of TdT-positive nuclei in brain tissue. All data (mean ± SEM) refer to 0.037-mm2 unit area. (Insets) P value of one-way ANOVA. §, P < 0.05 vs. respective value in normoxic animals; #, P < 0.05 vs. respective value in vehicle-treated animals (Bonferroni post hoc test).
Next, we determined whether the observed increase in the number of TdT-labeled nuclei and EpoR-associated immunoreactivity-positive cells could be attributed to neuronal cells. To that aim, we labeled the primary antibody against EpoR-associated immunoreactivity with rhodamine, which yields a red signal, and the primary antibody against neuronal nuclei (NeuN) with fluorescein, which yields a green signal that was digitally converted to blue to increase visibility. After merging the images, the combination of the two dyes yields a bright purple color. Fig. 2Left shows sample images taken from hypoxic brains from vehicle- and CEpo-treated mice. This procedure was performed in five sections per animal, and five fields were examined per section. Essentially all EpoR-associated immunoreactivity resides in neurons in either vehicle- (95 ± 2%) or CEpo-treated (96 ± 3%) animals (P not significant). In contrast, the proportion of neurons that express EpoR-associated staining is dramatically reduced in CEpo-treated animals (35 ± 1.5% versus 79 ± 4%; P < 0.0001, two-tailed). Together, these data argue that neurons respond to CH stress by increasing EpoR-associated expression and that CEpo treatment dramatically reduces this stress response. We could not comment on the cellular localization of HIF-1α because of diffuse labeling.
Fig. 2.
Colocalization of EpoR- and TdT-associated immunoreactivity with neuronal cells. (Left) CEpo treatment reduces the percentage of neuronal cells that express erythropoietin receptor (EPOR)-associated immunoreactivity. Cells with EpoR-associated immunoreactivity are stained red, NeuN-positive cells are stained blue. When merged, the combination of the two signals gives a bright purple signal. (Right) CEpo treatment has no significant effect on the subset of neuronal cells that become apoptotic after CH. Colocalization of cells with TdT-labeled nuclei with cells expressing NeuN, a specific marker for neuronal cells is shown. TdT-labeled cells are stained red, NeuN-positive cells are stained blue. When merged, the combination of the two signals gives a bright purple signal. (Scale bars: 20 μm.)
To determine whether TUNEL positivity, or apoptosis, was associated with neuronal cells, we performed the same procedure as described above, but we associated a red label with TdT-labeled nuclei and a blue label with NeuN (Fig. 2 Right). It appears that in CH brain samples only 35 ± 3% of neuronal cells colocalize with TdT-labeled nuclei. In CEpo-treated animals, this value is 25 ± 9% (P not significant), indicating that only a fraction of neuronal cells show signs of apoptosis, and that the antiapoptotic effect by CEpo is exerted at the levels of both neuronal and nonneuronal cells.
Myocardial biopsies were also obtained and frozen in liquid nitrogen within 2 min after the euthanization. Fig. 3 shows the expression level of the studied proteins and the results of the TUNEL measurements in all of the groups examined as explained for Fig. 1. A one-way ANOVA was significant only for apoptotic nuclei with 43 ± 9% in vehicle- and 20 ± 1.5% in CEpo-treated animals (P = 0.0006). Significantly, CEpo limited the CH-related increase in apoptosis to a value close to that measured during normoxia (16 ± 4%).
Fig. 3.
CEpo treatment moderates CH-induced myocardial apoptosis. Epo (A), EpoR (B), HIF-1α (C), and TUNEL (D) positivity are expressed as, respectively, protein level (pixel intensity from immunofluorescence images), no. of cells with EpoR-associated immunoreactivity, protein level (pixel intensity from immunofluorescence images) and no. of TdT-positive nuclei in myocardial tissue. All data (mean ± SEM) refer to 0.037-mm2 unit area. (Insets) P value of one-way ANOVA. §, P < 0.05 vs. respective value in normoxic animals; #, P < 0.05 vs. respective value in vehicle-treated animals (Bonferroni post hoc test).
Discussion
In this work, severe CH increased the blood Hb concentration as expected, and it produced an array of effects in cerebral tissue, including an increased number of apoptotic nuclei, an increased number of cells expressing EpoR-associated immunoreactivity, and an up-regulation of HIF-1α. Daily treatment of the animals with 10 μg of CEpo per kg of body weight did not change the blood Hb concentration, yet it significantly reduced the number of apoptotic nuclei, the number of cells expressing EpoR-associated immunoreactivity, and it lowered the level of HIF-1α expression. Colocalization studies enabled us to assess that the effects of CEpo are at least in part targeted directly to neuronal cells. In the myocardium, CH increased only apoptosis, but CEpo could significantly blunt the apoptotic signal even in myocardial cells.
To test for the effects of CEpo during CH in vivo, we maintained mice under a normobaric atmosphere containing 6.5% O2. This level of hypoxia is severe, corresponding to 8,500 m in altitude. Animals were maintained hypoxic without any exposure to room air, thereby preventing reoxygenation and thus minimizing the potential production of free radicals and oxidative stress. This issue is important because the half-life of HIF-1α in reoxygenated cell cultures is as short as few minutes (22, 23). In addition, certain phenotypes produced by CH are profoundly affected by even short reoxygenation episodes (19, 24–26). The selected experimental protocol thus represents a model for CH that may arise either from physiological situations as high altitude and airline flights or pathological conditions as congenital heart disease, anemia, carbon monoxide poisoning, or chronic lung diseases. In the design selected for this work, four groups of mice were tested to assess the separate and combined effects of CH and CEpo. We did not perform pharmacokinetic evaluation of CEpo treatment because it is known that CEpo distributes in the blood and in the cerebrospinal fluid, with peak concentrations 14 h after s.c. injection, and that the plasma concentration of CEpo remains >2 pM for >20 h when CEpo was injected at the dose of 44 μg/kg (14).
As expected, exposure to severe CH for 10 days has profound consequences in terms of survival rate, body weight loss, and blood Hb concentration. Administration of CEpo did not affect any of these parameters. Although a causal link between hypoxia and augmented RBC mass is beyond doubt, it is uncertain whether the increase was entirely the result of endogenous Epo or whether dehydration also played a role. We did not detect any relevant Epo increase in brain and myocardial tissue, which is not surprising because it is well known that Epo expression tends to normalize shortly after the onset of hypoxia (27), whereas the downstream gene expression is sustained even without the continued presence of Epo (6). The lack of Hb increase in either normoxic or CH mice treated with CEpo confirms the lack of erythropoietic effect (14).
HIF-1α, the major known molecular transducer of hypoxia, is synthesized independently of the cell oxygenation status, but its degradation is fast in the presence of O2, a substrate for prolyl hydroxylase that targets HIF-1α to ubiquitination and destruction (28). By contrast, HIF-1α is stabilized during hypoxia, enabling control of the expression of hundreds of downstream genes (4). In CH tissues in vivo, HIF-1α up-regulation can be sustained. However, this feature is not shared by all organs: whereas brain, muscle, and kidney cortex exhibit sustained HIF-1α response, HIF-1α is barely detectable in CH myocardium and liver (20), a pattern confirmed in this work. In the heart, it is likely that hypoxia-induced vasodilatation, e.g., produced by local metabolites (29), leads to increases in coronary flow, thereby maintaining adequate O2 delivery without a need to recruit hypoxia-related mechanisms. By contrast, in brain tissue, hypoxia-induced vasodilatation may not represent an important event as in the myocardium. This difference results in a sustained up-regulation of HIF-1α, especially in the brain (20).
In this work, we show that CEpo daily injections (10 μg/kg) depress hypoxia-induced HIF-1α up-regulation. This observation is consistent with a reduction of metabolic stress associated with CEpo administration. Similar observations have been obtained by using human ovarian cancer cells exposed to rhEpo, which inhibited HIF-1α stabilization and hypoxia-induced transcription of VEGF without affecting the growth rate of the tumor. This effect was presumably at the posttranscriptional level because HIF-1α mRNA levels were not affected (30). Several independent observations favor the hypothesis that CEpo acts directly by destabilizing HIF-1α. First, both rhEpo (15) and CEpo (16) limit mitochondrial permeability transition pore opening in cardiac myocytes, thereby preventing mitochondria swelling and release of substances such as reactive oxygen species and cytochrome c. Because mitochondria are considered O2 sensors, the increase in threshold for reactive oxygen species produced within mitochondria during hypoxia may destabilize HIF-1α (31, 32). Second, this hypothesis fits into the proposal that one of the signaling pathways involved in mediating Epo tissue protection is the activation of the survival kinases pathway (21), which controls the phosphorylation of prosurvival molecules, e.g., phosphatidylinositol-3 kinase/Akt and p42/p44 extracellular signal-regulated kinases (ERK1/2) (33). Because ERK1/2 is increased during chronic hypoxia (10% O2 in rats) (34), this mechanism may be one by which CEpo returns HIF-1α levels to near normoxic values during CH.
The specificity of the TUNEL method has been questioned because TUNEL positivity often reflects a range of cellular conditions rather than only apoptosis (35), and thus it may fail to discriminate among apoptosis, necrosis, and autolytic cell death (36). Also, although generally recognized as a hallmark of apoptosis, DNA fragmentation may be a late event that does not necessarily imply apoptosis, such that we cannot rule out that TUNEL positivity might also indicate necrosis. Despite these limitations, we report here that both hypoxic brain and myocardial tissue exhibited a strong apoptotic signal, which is markedly reduced by CEpo treatment. These data are only in part consistent with previously published data, which did not report an increased number of TdT-stained nuclei in the hypoxic brain (20). Perhaps the different model (mice vs. rats) and the more severe hypoxia conditions in the present study (6.5% vs. 10% O2) might account for the difference. In either case, we found that CEpo treatment markedly reduced TUNEL positivity, consistent with the claimed antiapoptotic effects of rhEpo and CEpo (for a review, see ref. 37). Indeed, rhEpo prevents apoptosis in cultured adult rat cardiac myocytes exposed to 3% O2 hypoxia for 28 h (8), and it decreases the number of TdT-positive neurons after focal cerebral ischemia and hypoxia-induced neuronal death through activation of ERK1/2 (6).
Two distinct receptors for Epo and CEpo exist. Whereas the homodimeric Epo receptor binds only Epo and mediates primarily its erythropoietic function, the heterodimer composed by the common β receptor (βcR) subunit also known as CD131 can bind both Epo and CEpo, and it is associated with tissue protection. The two receptors are coexpressed in Epo-sensitive cells, but CEpo signals only through βcR (38). In this work, we focused on EpoR-associated immunoreactivity because it is among the genes regulated by HIF-1α (4).
Binding of Epo or rhEpo to the EpoR initiates a variety of signaling pathways, including JAK2 (autophosphorylation of EpoR-associated immunoreactivity), STAT5 (gene expression), MAPK, NO production, and KATP+ channels, which turn out to mediate the erythropoietic effect of Epo in the bone marrow cells (39). In the present study, we observed that EpoR-associated immunoreactivity expression in brain tissue is up-regulated by hypoxia in vivo, consistently with the finding that Epo stimulates EpoR-associated immunoreactivity transcription factor in erythroid progenitor cells (40). In addition, we found that increased EpoR-associated immunoreactivity is specifically localized in neuronal cells. Although CEpo treatment does not result in any change of EpoR-associated immunoreactivity in normoxic cells, it blunts up-regulation of EpoR-associated immunoreactivity in CH neuronal cells.
In contrast, we found no up-regulation of EpoR-associated immunoreactivity in the myocardium, and consequently there was no effect of CEpo treatment on EpoR-associated immunoreactivity in this tissue. This observation is partly consistent with the lack of EpoR-associated immunoreactivity in adult hearts, although its presence during embryogenesis is critical for heart development (41). Nevertheless, CEpo treatment reduced apoptosis, in agreement with other observations that treatment with rhEpo reduced myocardial infarction and TdT-labeled nuclei in the myocardial area at risk (9, 16, 42).
In summary, treatment with CEpo prevents in part the effects induced by severe chronic hypoxia without further increasing hypoxia-induced polycythemia. Specifically, in the brain, CEpo prevents overexpression of HIF-1α- and EpoR-associated immunoreactivity, and it reduces DNA fragmentation. In the heart, HIF-1α- and EpoR-associated immunoreactivity do not appear to be a specific target of chronic hypoxia, but CEpo reduces DNA fragmentation nevertheless. These results suggest that further studies need to be performed to evaluate CEpo as an exogenous agent that stimulates preconditioning against hypoxia, a potentially lethal situation occurring in a great number of both physiological and pathological situations.
Experimental Procedures
Materials.
CEpo was provided by Warren Pharmaceuticals (Ossining, NY) as a 2.3 mg/ml stock solution. We used the following antibodies and dilutions: mouse anti-HIF-1α monoclonal Ab (diluted 1:400 in 1.5% normal goat serum; Chemicon International, Temecula, CA), rabbit anti-Epo polyclonal Ab (H-162, diluted 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-EpoR polyclonal Ab (H-194, diluted 1:100; Santa Cruz Biotechnology). Although a recent study has raised questions concerning the specificity of this last antibody for the EpoR per se, especially when used for immunocytochemistry (43), numerous studies (e.g., refs. 44–47) have noted that metabolically stressed cells increase expression of epitopes that are recognized by this antibody (as well as by other polyclonal and monoclonal antibodies raised again the EpoR). Further, the cytoprotective effects of Epo are neutralized by the same antibody (44). The cells that express those epitopes are in turn protected by Epo (as well as nonerythropoietic tissue-protective cytokines). Therefore, we will refer to the epitopes identified by this antibody as “EpoR-associated immunoreactivity.” To identify neuronal cells, we used mouse anti-NeuN monoclonal Ab (diluted 1:100; Chemicon International). The secondary antibodies included goat anti-mouse and anti-rabbit IgG fluorescein-conjugated Ab (diluted 1:200 in 1.5% normal goat serum; Santa Cruz Biotechnology) and anti-rabbit IgG rhodamine-conjugated Ab (diluted 1:200; Santa Cruz Biotechnology). To detect DNA fragmentation, we used the TUNEL ApopTag Red in situ apoptosis detection kit (Chemicon International).
Animals.
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (48). Male ICR CD-1 mice (5–6 weeks old, 30 g body weight at entry into the study) were randomly divided into four groups: normoxic, normoxic treated with CEpo, hypoxic, and hypoxic treated with CEpo. Animals exposed to CH were caged in a previously described hypoxic chamber (24) with minor modifications. To achieve the desired level of hypoxia, we used two separate cylinders containing air and N2. The flow from the cylinders was regulated independently by two precision glass tube flow meters (Key Instruments, Trevose, PA), then the outputs were mixed, and the O2 content was measured by an AERO2-MAT 4100 Clark electrode (Syland Scientific, Heppenheim, Germany). The flow meters were adjusted to 1.7 ± 0.1 and 0.8 ± 0.1 liters per min of N2 and air, respectively, to yield a final O2 content in the range of 6.5–6.8%, with total flow rate fixed at 2.5 ± 0.2 liters per min. Normoxic animals were caged in the same chamber as that described for hypoxic animals, except that the chamber was supplied with air (21% O2) alone. All mice had free access to water and conventional laboratory diet until 24 h before being euthanized. Room temperature was kept at 21 ± 2°C, and a 12-h light cycle was maintained.
Exposure to either normoxia or CH was for 10 days. For injection into mice, the stock CEpo solution was diluted in sterile PBS (1 μl of stock solution in 765 μl). One hundred microliters of this solution, equivalent to 10 μg of CEpo per kg of body weight, was injected s.c. once a day during the exposure to either normoxia or CH. To administer CEpo, mice were first transferred from the hypoxic environment into a procedure chamber that was maintained at the same O2 content as the hypoxic chamber. CEpo was then injected, and mice were transferred back to the hypoxic chamber without any exposure to room air. In control experiments, mice were injected with PBS alone. At the end of the experiment, CH mice were transferred into the procedure chamber, and they were anesthetized i.p. with sodium thiopental (10 mg per 100 g of body weight) plus heparin (200 units). Then blood was withdrawn by intracardiac puncture into heparinized syringes, and the animals were euthanized by cervical dislocation. After being euthanized, mice were quickly taken out of the procedure chamber, weighed, and dissected (<0.5 min). While one operator removed the brain, another one excised the heart. Blood was immediately assayed for total Hb concentration.
Sample Preparation.
Biopsies from the frozen organs were processed by embedding them in OCT (Optimum Cutting Temperature Compound; Leica Instruments, Nussloch, Germany), and serial 5-μm-thick sections were obtained by using a cryomicrotome (CM1510; Leica), and they were placed on silanized glass slides. The sections were dried at room temperature for 3 min, fixed in 4% buffered formalin for 45 min at 4°C, rinsed twice for 5 min in PBS, postfixed with ethanol/acetic acid 2:1 (vol/vol) at −20°C for 5 min, rinsed twice for 5 min in PBS, boiled for 10 min in 10 mM citrate buffer (pH 6.0), washed once in distilled water and three times in PBS, and finally used for immunofluorescence or DNA fragmentation staining.
Immunofluorescence.
The sections were immersed in 10% normal goat serum for 1 h with gentle agitation, incubated overnight at 4°C with the primary antibody, washed in PBS, and incubated at room temperature for 45 min with the secondary antibody. A negative control was prepared for each section by replacing the primary antibody with 1.5% normal goat serum. The slides were examined at a magnification of ×40 in an inverted fluorescence microscope (Axiovert 25 CFL; Zeiss, Göttingen, Germany), equipped with a filter for the detection of either fluorescein (filter set 9, excitation bandpass 450–490 nm, emission low-pass 515 nm) or rhodamine (filter set 15, excitation bandpass 546 ± 12 nm, emission low-pass 590 nm). The images were acquired by either a CCD camera (AxioCam czv CD 4.0; Zeiss) or a digital camera system for microscopy (DS-2Mv; Nikon, Tokyo, Japan), and stored in a personal computer. For the quantification of the signals, the images were analyzed by IPlab Software (Scanalytics, Fairfax, VA) and split into RGB channels. Either the green or the red channel was used to calculate the color intensity as the sum of the pixel intensity values. At least five random fields per section were selected, and the total color intensity was measured and subtracted from the signal detected by using the negative controls. The color intensity in the image is expressed as the sum of pixel intensity × 106/0.037 mm2.
In Situ TdT Assay for Detection of Apoptosis.
The TUNEL technique was used to identify apoptotic nuclei by the TdT-mediated dUTP-rhodamine nick end-labeling technique. After blocking, the sections were incubated for 60 min at 37°C with TdT to label free 3′ OH DNA termini with digoxigenin nucleotides, and the reaction was stopped by immersing the slide in stop/wash buffer for 10 min at room temperature. After three PBS washes, the sections were incubated with antidigoxigenin rhodamine-conjugated antibody in the dark for 15 min at 37°C and then for 15 min at room temperature, and finally they were washed four times in PBS. A negative control was established for each biopsy by replacing TdT with PBS. After the mounting as described above, images were acquired by using a rhodamine detection filter. Two operators counted the number of TdT-labeled nuclei by examining at least five random fields in a blinded procedure. Results are expressed as number of TdT-labeled nuclei/0.037 mm2.
Hemoglobin Concentration.
The Hb concentration (in mM) was determined by the Drabkin assay using the extinction coefficient 11.05 mM−1·cm−1.
Statistics.
All data are presented as mean ± SEM. To assess the significance of the differences among the various groups, we performed one-way ANOVA, followed by Bonferroni's multiple comparison test if significant (P < 0.05). To estimate the effect of CEpo on the survival rate, Fisher's exact test was used.
Acknowledgments
This work was supported in part by the Ministero Università e Ricerca, Rome, Italy (Grant 2004054720_002, PRIN2004 to M.S.) and by Fondazione Cariplo, Milan, Italy (Grant 5201001-55 to M.S.).
Abbreviations
- CEpo
carbamylated erythropoietin
- CH
chronic hypoxia
- Epo
erythropoietin
- EpoR
erythropoietin receptor
- HIF-1α
hypoxia-inducible factor 1α
- NeuN
neuronal nuclei
- rhEpo
recombinant human erythropoietin
- TdT
terminal deoxynucleotidyltransferase.
Footnotes
Conflict of interest statement: T.R.C., A. Cerami, and M.B. are employees of Warren Pharmaceuticals, which is developing tissue-protective cytokines for potential clinical use.
References
- 1.Hurtado A, Merino C, Delgado E. Arch Int Med. 1945;75:284–323. [Google Scholar]
- 2.Jelkmann W. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki R. Intern Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- 6.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, et al. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brines M, Ghezzi P, Keenan S, Agnello D, de Lanerolle N, Cerami C, Itri L, Cerami A. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Proc Natl Acad Sci USA. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Proc Natl Acad Sci USA. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 11.Baker JE. Vascul Pharmacol. 2005;42:233–241. doi: 10.1016/j.vph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 13.Reimer K, Murry C, Jennings R. Circulation. 1990;82:2266–2268. doi: 10.1161/01.cir.82.6.2266. [DOI] [PubMed] [Google Scholar]
- 14.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, et al. Science. 2004;305:239–242. [Google Scholar]
- 15.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K, Katschinski D, Hasegawa J, Schumacher D, Meller B, Gembruch U, Schramm U, Jelkmann W, Gassmann M, Fandrey J. Blood. 2001;97:536–542. doi: 10.1182/blood.v97.2.536. [DOI] [PubMed] [Google Scholar]
- 18.Ruschitzka F, Wenger R, Stallmach T, Quasching T, de Wit C, Wagner K, Labugger R, Kelm M, Noll G, Rulicke T, et al. Proc Natl Acad Sci USA. 2000;97:11609–11613. doi: 10.1073/pnas.97.21.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milano G, Corno A, Lippa S, von Segesser L, Samaja M. Exp Biol Med. 2002;227:389–397. doi: 10.1177/153537020222700604. [DOI] [PubMed] [Google Scholar]
- 20.Bianciardi P, Fantacci M, Caretti A, Ronchi R, Milano G, Morel S, von Segesser L, Corno A, Samaja M. Biochem Biophys Res Commun. 2006;342:875–880. doi: 10.1016/j.bbrc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Brines M, Cerami A. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B, Semenza G, Bauer C, Marti H. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 23.Wiesener M, Turley H, Allen W, William C, Eckardt K, Talks K, Wood S, Gatter K, Harris A, Pugh C, et al. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 24.Corno A, Milano G, Samaja M, Tozzi P, von Segesser L. J Thorac Cardiovasc Surg. 2002;124:105–112. doi: 10.1067/mtc.2002.121302. [DOI] [PubMed] [Google Scholar]
- 25.Milano G, Bianciardi P, Corno AF, Raddatz E, Morel S, von Segesser LK, Samaja M. Exp Biol Med. 2004;229:1196–1205. doi: 10.1177/153537020422901115. [DOI] [PubMed] [Google Scholar]
- 26.Corno AF, Milano G, Morel S, Tozzi P, Genton CY, Samaja M, von Segesser LK. J Thorac Cardiovasc Surg. 2004;127:1301–1308. doi: 10.1016/j.jtcvs.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt KU, Dittmer J, Neumann R, Bauer C, Kurtz A. Am J Physiol. 1990;258:F1432–F1437. doi: 10.1152/ajprenal.1990.258.5.F1432. [DOI] [PubMed] [Google Scholar]
- 28.Lando D, Peet D, Whelan D, Gorman J, Whitelaw M. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 29.Berne R. Circ Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- 30.Hale SA, Wong C, Lounsbury KM. Gynecol Oncol. 2006;100:14–19. doi: 10.1016/j.ygyno.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 31.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Chandel N, McClintock D, Feliciano C, Wood T, Melendez J, Rodriguez A, Schumacker P. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong SC. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Morel S, Milano G, Ludunge KM, Corno AF, Samaja M, Fleury S, Bonny C, Kappenberger L, von Segesser LK, Vassalli G. Basic Res Cardiol. 2006;101:336–345. doi: 10.1007/s00395-006-0596-1. [DOI] [PubMed] [Google Scholar]
- 35.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Cardiovasc Res. 2000;45:630–641. doi: 10.1016/s0008-6363(99)00349-1. [DOI] [PubMed] [Google Scholar]
- 36.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 37.Ghezzi P, Brines M. Cell Death: Differ. 2004;11(Suppl 1):S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 38.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, et al. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartesaghi S, Marinovich M, Corsini E, Galli CL, Viviani B. Neurotoxicology. 2005;26:923–928. doi: 10.1016/j.neuro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Development. Vol. 129. Cambridge,UK: 2002. pp. 505–516. [DOI] [PubMed] [Google Scholar]
- 42.Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, Doni M, Mengozzi M, Tonelli R, Ghezzi P, et al. Proc Natl Acad Sci USA. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, Spahr C, Um M, Van G, Begley CG. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 44.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- 45.Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. J Cereb Blood Flow Metab. 2005;25:1456–1465. doi: 10.1038/sj.jcbfm.9600142. [DOI] [PubMed] [Google Scholar]
- 46.Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 47.Spandou E, Papoutsopoulou S, Soubasi V, Karkavelas G, Simeonidou C, Kremenopoulos G, Guiba-Tziampiri O. Brain Res. 2004;1021:167–172. doi: 10.1016/j.brainres.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 48.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Natl Inst Health: Bethesda; 1985. pp. 85–23. DHHS Publ No (NIH) [Google Scholar]