Abstract
Current approaches to human cognition often take a strong nativist stance based on Western adult performance, backed up where possible by neonate and infant research and almost never by comparative research across the Hominidae. Recent research suggests considerable cross-cultural differences in cognitive strategies, including relational thinking, a domain where infant research is impossible because of lack of cognitive maturation. Here, we apply the same paradigm across children and adults of different cultures and across all nonhuman great ape genera. We find that both child and adult spatial cognition systematically varies with language and culture but that, nevertheless, there is a clear inherited bias for one spatial strategy in the great apes. It is reasonable to conclude, we argue, that language and culture mask the native tendencies in our species. This cladistic approach suggests that the correct perspective on human cognition is neither nativist uniformitarian nor “blank slate” but recognizes the powerful impact that language and culture can have on our shared primate cognitive biases.
Keywords: cognitive evolution, cultural differences, great apes
Cognitive psychology has been centrally concerned with the nature of human adult cognition and its ontogenetic development and has largely treated this process as the emergence of a universal cognitive structure from innate sources that can be glimpsed in infancy. Many of these processes have been traditionally thought to be discontinuous with our nearest primate cousins.
This picture needs to be corrected in two main directions. First, adult cognitive strategies diverge according to expertise and culture in some quite fundamental domains such as color (1), number (2, 3), or spatial cognition (4–6). Language seems to play an important role in this divergent specialization of the intellect. Innate biases are thus masked by cultural and linguistic divergence. Although neonate and infant research might throw light on these biases, many cognitive abilities, for example, those involved in relational reasoning, are not fully developed before cultural effects take hold. Second, continuities with our primate cousins should be presumed, and efforts should be made to track them (7–9). The overall picture that then emerges is, we argue, one in which human infants inherit many of the same cognitive preferences and biases as our primate cousins but then go on to build cognitive structures that may diverge in various ways from this primate base under the influence of language and culture (10).
In this article, we focus on the cognition of spatial relations, which shares the relational characteristics of many higher cognitive processes (11). First, in part 1, we explore human cognition for spatial relations in two cultures, examining both adults and children. As predicted by earlier work, we find major divergence in the two cultural groups, parallel to linguistic coding strategies. Such a result is compatible with a “blank-slate” view of human cognition but need not imply it. Spatial cognition is vital to all foraging species and is served by phylogenetically conservative neural systems (12), so there are good reasons to suppose an inherited substrate. The standard approach would be to look for preferences in human infants, but relational thinking is a domain where it is difficult or impossible to acquire insight into innate biases by infant research, because the relevant cognitive skills do not mature until well after children learn language and, with it, all the baggage of culture. In experiments 2 and 3, therefore, in addition to looking at European preschool children, we looked at mature apes of all of the other great ape genera to establish the inherited primate baseline and, moreover, gain insight into the evolutionary history of spatial cognition.
Part 1: Cross-Cultural Variation
Spatial relations provide basic framing structures for the encoding of events (13), and relational thought forms the basis for propositional structure, predication, and understanding analogy and metaphor (11, 14). Therefore, spatial memory, and the relational learning it requires, is central to human cognition. Children acquire relational thought relatively late in ontogeny, coeval with the acquisition of the relevant linguistic expressions (11). Because of this coemergence of cognitive and linguistic concepts of spatial relations in children, it has been argued that the ontogeny of relational thought is tightly interwoven with, or perhaps even dependent on, relational language (11).
Spatial relational language follows coordinate systems or frames of reference (FoRs), which serve to specify the directional relationships between objects in space, in reference to a shared referential anchor (15). Extensive field research in >20 languages analyzing natural and elicited conversation has revealed that, in language, just three FoRs seem to be used but that languages vary in the repertoire they code and also in the habitual usage of FoRs (16). Some languages mainly use a relative, viewpoint-dependent FoR with terms like front, back, left, and right: “The ball is to the left of the tree” (from my point of view). Some languages mainly use an intrinsic FoR, which makes reference to faceted objects, e.g., “The ball is at the front of the house.” Some languages mainly use a third, so-called absolute FoR in which linguistic descriptions use cardinal-direction type systems such as our North, South, East, and West: “The hot water is in the northern tap.” Although most languages have several FoRs in their repertoire, relative constructions are predominant in European languages, whereas the absolute FoR is dominant, for example, in several indigenous languages of Australia, Papua New Guinea, Mexico, Nepal, and south West Africa (16, 17).
Continuing investigations into the cognition of speakers of absolute languages suggest that they prefer absolute (geocentric) to relative (egocentric) strategies in simple nonlinguistic spatial memory tasks, whereas the reverse is found in European speakers of predominantly relative languages, such as English or Dutch (4, 5, 16–19); for a critique, see refs. 20 and 21. In other words, language difference covaries with differences in cognitive strategy for nonlinguistic tasks.
Here, we investigate spatial relational learning in two distinct cultural communities: a Dutch village representing a typical western European, postindustrial culture and ≠Akhoe Hai‖om, a Khoisan hunter–gatherer community in Namibia (see Methods). Both Dutch and ≠Akhoe Hai‖om (referred to hereafter as Hai‖om) languages make at least residual use of all three FoRs (relative, intrinsic, and absolute) in natural conversation. However, these groups differ in their language usage patterns. Speakers of Dutch almost exclusively use relative constructions to describe small-scale spatial relations (4, 16). Hai‖om speakers, in principle, have a relative FoR available, but they almost always use absolute spatial descriptions (22). In experiment 1, we tested children at the age of approximate emergence of the relevant spatial relational terms [7–11 years of age; (23, 24)] in a feedback-learning paradigm, with minimal verbal instructions to minimize cross-culturally variant translations and interpretations (20). We also tested adults in both cultures to see whether differences were not only initial variations of an emerging cognitive skill but were actually stable across the life span. On the basis of earlier results (16), we predicted that consultants from the two distinct cultures who vary in their linguistic expression of spatial relations would also vary in their habitual cognitive coding of spatial relations and that cognitive preferences would match the linguistic preference. Hence, Dutch speakers should prefer egocentric to geocentric cognitive strategies, whereas Hai‖om speakers should show the reverse pattern.
Experiment 1.
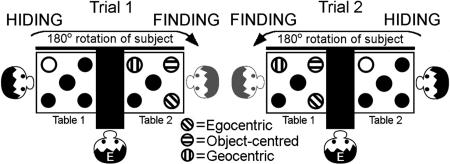
We used a nonlinguistic spatial relational learning paradigm to test whether the preferred linguistic FoR in a given language would predict the preferred cognitive strategy. As subjects, we used four groups: Dutch children, Dutch adults, Hai‖om children, and Hai‖om adults (see Methods). Two small tables were placed next to each other with a solid opaque screen in between to separate them visually. Five identical cups were placed on each table in a dice-five constellation (Fig. 1). Participants were instructed in their first language to find a hidden target when prompted (see Methods). At the beginning of a session, the participant was positioned in front of table 1 facing the screen. They watched an experimenter (E, Fig. 1) place a target under one of the five cups (Fig. 1, HIDING). Then the participants were directed to table 2, again facing the screen, thus shifting their orientation 180°. Here, they were prompted to indicate the cup under which they judged the target would be found (Fig. 1, FINDING). After their response, the experimenter turned over their cup of choice and, in case of an incorrect choice (choosing any cup without a hidden target), turned over the correct cup to allow participants to adjust their behavior in order to maximize the hit rate. We scored the container selected by subjects based on videotapes and/or in situ notes. Trial 2 started with a new hiding at table 2, after which participants moved back to table 1 for finding. This procedure was iterated for a total of 30 trials (three blocks of 10). After two correct responses, with the target in the central position as a training criterion, targets were hidden by following three rules (Fig. 1) as described below.
Fig. 1.
Experiment 1: Experimental setup in two consecutive example trials. Ten exactly identical cups were placed on two tables (five cups on each table). Participants were watching while a target was hidden under the cup depicted as white (HIDING). Then the participants moved to the other table and indicated where they thought a second target might be hidden (FINDING). The three differently striped cups show the different contingencies rewarded in the three consecutive blocks of trials.
Egocentric condition.
The hiding and finding cups maintained position relative to the viewpoint of the participant. If the hiding cup was close to her on her left-hand side, the finding cup was again the close one on the left-hand side after she rotated into her new position at the other table.
Object-centered condition.
The hiding and finding cups maintained position in relation to a salient landmark between the two tables, namely the screen or the experimenter. If the hiding cup was, for example, the one diagonally across from the experimenter, the finding cup was again diagonally across from him after the participant rotated into her new position at the other table.
Geocentric condition.
The hiding and finding cups maintained position Relative to the larger, surrounding environment. If the hiding cup was the northwestern cup, the finding cup was again the northwestern one after the participant rotated into her new position at the other table.
Fig. 1 makes clear the distinct position of the finding cup in each condition. The three different conditions were administered to each individual in three consecutive blocks of 10 randomized trials, counterbalanced for order across subjects. The transition between the blocks was unmarked; thus, the prior winning strategy no longer worked, and a new one had to be learned. Randomly intermixed within all three blocks were two trials each in which the center cup was the finding cup (middle condition). In these middle trials, all three rules lead to the same solution.
Results.
We conducted a mixed ANOVA with the within-subject factor condition (egocentric/object-centered/geocentric/middle) and the between-subject factors language (Dutch/Hai‖om) and age group (adults/children).
These and all further descriptive statistics are percentages. Analysis of the percentage of correct scores revealed a main effect of condition (F(3,132) = 38.13, P < 0.001; egocentric: M = 51.27, SD = 5.1; object-centered M = 51.31, SD = 4.3; geocentric M = 57.57, SD = 4.3; middle M = 93.75, SD = 2.1). A Bonferroni–Holm post hoc test showed that, over all, subjects performed better in the middle condition than in any other (egocentric vs. object-centered: t(47) = −0.01, P > 0.05; object-centered vs. geocentric: t(47) = −0.99, P > 0.05; geocentric vs. middle: t(47) = −7.52, P < 0.01).
We also found a main effect of language (F(1,44) = 65.48, P < 0.001; Dutch: M = 76.58, SD = 8.1; Hai‖om: M = 50.37, SD = 14.4). Dutch participants outperformed Namibian participants, most likely because of more advanced formal schooling. We found no significant effect for age group (P = 0.08).
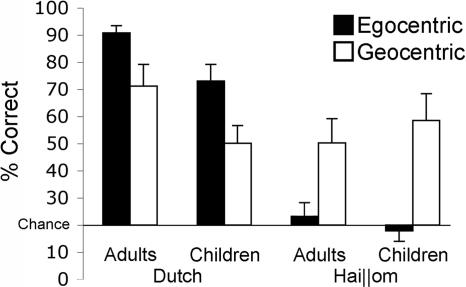
The ANOVA also revealed an interaction of condition × language (F(3,132) = 14.23, P < 0.001; Dutch, egocentric: M = 82.0; SD = 18.4; object-centered: M = 64.4; SD = 25.6; geocentric: M = 60.7; SD = 26.9; middle: M = 99.3; SD = 3.5; and Hai‖om, egocentric: M = 20.6; SD = 16.0; object-centered: M = 38.3; SD = 28.6; geocentric: M = 54.4; SD = 32.1; middle: M = 88.2; SD = 19.3). For the condition × language interaction, we predicted a priori that the preferred linguistic FoRs would also constitute the preferred cognitive strategy in comparison with the infrequently used one. So Dutch subjects should perform better in the relative than in the absolute condition, and the reverse should be true for the Hai‖om. Bonferroni–Holm post hoc tests indeed reveal this to be the case [Dutch, egocentric vs. geocentric: t(23) = 3.76, P < 0.01; and Hai‖om, egocentric vs. geocentric: t(23) = −4.08, P < 0.01] (Fig. 2).
Fig. 2.
Mean percentage correct (±SE) for the egocentric and geocentric conditions for both adults and children in the Dutch and Hai‖om communities. Means are plotted against chance level (20%, one of five cups).
Discussion.
In this experiment, we trained three response options in a spatial relational learning task, which match the threefold relative–intrinsic–absolute discrimination of FoRs in natural language. Our data show a correlation between the habitual linguistic strategy and the preferred cognitive strategies to process spatial relations: Both children and adults were more accurate (made fewer errors) and were faster to learn the finding pattern that matched the FoR dominant in their language. This correlation is fully robust by age 8 and persists into adulthood. In sum, Dutch and Hai‖om subjects varied in their preferred cognitive strategy to solve a spatial relational learning task, and their preference matched the preferred mode of description in their respective language.
Clearly, human cognitive competence encompasses all three FoRs, and indeed special neurocognitive systems seem to support each of them (12). Cross-cultural differences in spatial cognition therefore concern preference and proficiency and not absolute ability. Many things might hypothetically fuel cross-cultural variation of spatial cognition in this sense. Several potential sources have been proposed, such as group cohesion or lifestyle (21), context (21, 25), and language (16, 17). The largest and strongest body of evidence supports the latter theory (4, 5, 16, 18, 19), which proposes that cognitive categories and concepts are not necessarily universal but potentially variable and seem to align with cross-linguistically variable semantics. To communicate about space, in a way appropriate within a linguistic community, cognitive representations need to be aligned with habitual linguistic categories so that information is coded appropriately for later linguistic use. Like other expertise effects, frequent use of a particular language will train the cognitive system in the necessary underlying processing.
Whatever the right combination of factors might be that ultimately explains the variation of spatial strategies across human groups, it will, in one way or another, be part of what we loosely call “culture.” However, cultural variation in cognition does not, of course, exclude a rich inherited basis, even in the variables in question. It is therefore reasonable to ask what the input or cognitive default is in this domain for humans. Is the default spatial relational strategy unset (the blank slate view), or is it preset but malleable enough to be overridden by cultural preferences?
Part 2: Phylogenetic Inheritance
Because relational cognition fully develops only late in ontogeny, there is no infant data that can shed light on a default strategy preference. There is, however, an alternative source of information from comparative cognitive science (7, 9): If all genera of a phylogenetic family (in our case the Hominidae) exhibit the same behavioral tendencies or cognitive biases, this finding would suggest inheritance from the common ancestor shared by all genera (in our case Pongo, Gorilla, Pan, and Homo). It is also reasonable to assume that any such tendencies shared by all nonhuman great ape genera and any human population is most likely part of the primate inheritance shared by all humans. In this second part of the article, we apply this cladistic reasoning to investigate inherited preferences for coding spatial relations in FoRs.
There has been a great deal of speculation about the inherited structure of spatial relational thought (26). Immanuel Kant argued that the human body provides the source of our basic intuitions about the nature of space (27). In agreement, many cognitive scientists hold the assumption that spatial cognition is fundamentally egocentric [(28–30); but see refs. 31 and 32]. However, there are some reasons to doubt this assumption. It is true that infants initially, before they are fully mobile agents, display a quite inflexible egocentric bias (33). However, as soon as they have become proficiently mobile and competent navigators [≈16 months of age (34)], they successfully use nonegocentric (allocentric) cognitive strategies. If the two types of strategies are immediately compared, English-speaking children, at least between 3 and ≈5 years of age, are better at allocentric strategies than at egocentric ones (35, 36). Moreover, children learning an absolute language acquire the relevant linguistic expressions at least as early as children learning a relative language (see refs. 24 and 37 for relative and refs. 18, 23, and 38 for absolute languages).
Relevant data from other species is sparse; there is only scant evidence for a preference for egocentric vs. allocentric cognitive strategies, although what there is mostly suggests an allocentric advantage [Chimpanzee (39); rats (40); cats (41); but see dogs (42) and a gorilla infant (43)]. However, none of these experiments, with infant or animal, used a strictly relational paradigm. Because prior research suggests that at least 4-year olds (11) and chimpanzees (44, 45) can process relational information, we set out, in experiments 2 and 3, to determine whether there is a background preference for egocentric or allocentric coding of spatial relations through all great ape genera to see whether they share a cognitive “wild type” inherited from a common ancestor.
Experiment 2.
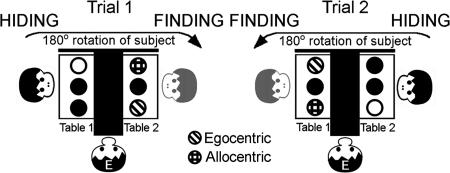
This experiment is precisely analogous to experiment 1, conducted on adults and children in two communities. But to adapt it to the shorter attention span of our nonhuman participants, and because of their known limitations with respect to abstract reasoning (46), we have simplified the conditions as follows. In contrast to experiment 1, we presented only three instead of five cups on each table. As a result, the object-centered and geocentric conditions are collapsed (Fig. 3). The three identical cups in a straight line offer only two alternative strategies: The egocentric one and an allocentric one, which could be based on either object-centered or geocentric cues. The two conditions were administered in two consecutive blocks of 12 randomized trials for each individual, counterbalanced for order across subjects. The transition between the blocks was unmarked, as before. Randomly intermixed within blocks were four trials each in which the center cup was the finding cup (middle condition). In these middle trials, both rules lead to the same solution. With this design, we tested all nonhuman great ape genera and European preschool children.
Fig. 3.
Experiment 2: Experimental setup in two consecutive example trials. Six exactly identical cups are placed on two tables (three cups on each table). Participants are watching while a target is hidden under the cup depicted as white (HIDING). Then the participants move to the other table and indicate where they think a second target might be hidden (FINDING). The two differently striped cups show the different contingencies rewarded in one of two consecutive blocks of trials.
Results.
We used a mixed ANOVA to analyze the effect of the within-subject factor condition (egocentric/allocentric/middle) and the between-subject factor genus (Pongo/Gorilla/Pan/Homo) on the percentage of trials in which subjects found the reward.
The ANOVA using percentage of correct scores revealed a significant main effect of condition (F(2,42) = 13.96; P < 0.001; egocentric: M = 27.5; SD = 16.5; allocentric: M = 49.5; SD = 21.8; middle: M = 67.0; SD = 21.0).
The planned simple comparison between the egocentric vs. allocentric conditions was conducted by using a paired sample t test, and the P value was corrected for multiple comparisons according to Bonferroni–Holm. Participants performed better when the finding container maintained the hiding container's spatial relations to the surrounding environment than to the participants' own body axis (egocentric vs. allocentric: t(24) = 4.07; P < 0.001).
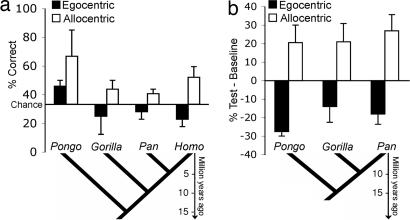
We detected no significant main effect of genus (P = 0.67) and no interaction (P < 0.25). Trends in the descriptive statistics show a similar pattern across all groups (Fig. 4a).
Fig. 4.
Results of experiments 2 and 3. (a) Experiment 2: Mean percentage correct (±SE) for the egocentric and allocentric conditions for all great ape genera. Means are plotted against chance levels (33%, one of three cups). (b) Experiment 3: Difference in choice of egocentric and allocentric cups between baseline and test trials. Below are plotted phylogenetic trees displaying the evolutionary relationships between Hominid genera (Pongo, Gorilla, Pan, Homo). All five extant species of Hominids participated in the reported studies: Orangutan (Pongo pygmaeus), gorilla (Gorilla gorilla), bonobo (Pan paniscus), chimpanzee (Pan troglodytes), and human (Homo sapiens). Here, we assume the Hominidae to include all of the great apes including humans but not the Hylobatidae or small apes.
Discussion.
Processing small-scale spatial relations between objects, apes and European 4-year olds deploy environmental layout more readily than self. Despite common expectations, this data indicate that Hominid spatial cognition is at least not always primarily egocentric.
Experiment 3.
Although experiment 2 shows that nonhuman great apes are able to solve spatial relational tasks, their performance was at a low level. Previous literature has shown that abstract rules in general put considerable constraints on nonhuman great apes' performance (46). In experiment 3, we used the same physical setup as in experiment 2 but used a design in which there is no necessity for complex abstract rule learning. We induced a strong spatial response bias for one of three identical cups by training apes to pick one particular cup from a lateral array of three. We then investigated how this response bias would manifest itself when the subject is rotated 180°. Suppose the training induces an expectation of reward under the leftmost cup. When the animal is rotated, if the bias has been conceived of in egocentric terms, it should choose the leftmost cup; if, on the other hand, the animal has conceived of the array using allocentric coordinates, it should choose the rightmost cup (Fig. 5c). In this way, rather than having to learn an abstract rule, the animal simply had to express its interpretation of the training bias.
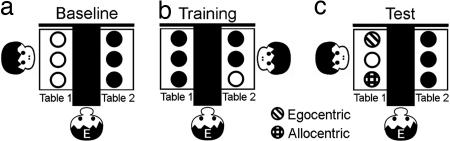
Fig. 5.
Setup and procedure in experiment 3: When the animal entered the testing room it was directed to table 1. Here, it had 10 trials in which all three cups were rewarded (a, Baseline). Then the animal was directed to table 2 and the experimenter started rewarding only one of the three cups until the animal would pick this particular cup 10 of 12 times in a row (b, Training). When the animal hit criterion, it was directed back to table 1 and again chose 10 times with all three cups rewarded in all trials (c, Test). We compared choice distributions between test and baseline trials.
Every animal went through three test sessions on three different days. Within each session, we followed a before–after design to isolate effects of training on every animal's individual baseline performance: When the animal entered the testing room, it was directed to table 1 (Fig. 5). Here, the animal had 10 trials in which all three cups were rewarded (Baseline, Fig. 5a). We recorded the distribution of choices across the three containers. We scored the container selected by subjects based on video tapes and/or in situ notes. After the initial 10 trials, the ape was directed to table 2, where again for 10 trials, all three cups were rewarded to avoid two different game contexts for the two tables. Then, still at table 2, the experimenter started rewarding only one of the three identical cups for all trials to come, until the animal would pick this particular cup 10 of 12 times in a row (Training, Fig. 5b). Every animal participated in one session for each of the three potential training cups on table 2. The order of sessions was counterbalanced across subjects. When the ape hit criterion (10 of 12 correct choices in a row) it was directed back to table 1, thus undergoing 180° rotation and, again, chose 10 times with all three cups rewarded in all trials (Test, Fig. 5c). Again, we recorded the distribution of choices across the containers.
Results.
For statistical analysis, we subtracted baseline- from test-choice percentages for each cup to isolate effects of training. As a manipulation control, we first analyzed the session in which the center cup was the training cup on table 2 (middle sessions). If any training bias translated from table 2 back to table 1, the percentage of trials in which animals pick the center cup should increase from baseline to test trials in middle sessions and therefore test − baseline > 0. A one-sample t test against zero revealed a significant increase in percentage of center-cup choices on table 1 after it was repeatedly rewarded on table 2 (t(14) = 4.17; P < 0.001; M = 22.00; SD = 20.42).
To further see whether apes preferred a particular strategy, we analyzed sessions in which one of the side cups was rewarded during training on table 2 (side sessions). We subtracted the percentage of choices on baseline from those on test trials for those cups, which preserved either the egocentric or the allocentric characteristics of the trained cup. We computed the average value across the two side sessions and conducted a mixed ANOVA with the within-subject factor FoR (egocentric/allocentric) and the between-subject factor genus (Pongo/Gorilla/Pan). The ANOVA revealed a main effect of FoR (F(1,13) = 11.1; P < 0.01; egocentric: M = −15.63; SD = 22.9; allocentric: M = 21.25; SD = 17.5), no main effect of genus (P = 0.74), and no interaction (P = 0.86). Post hoc one-sample t test against zero (Bonferroni–Holm-corrected) revealed that animals chose the egocentric cup significantly less in test than in baseline trials (t(15) = −2.73; P < 0.005), whereas they chose the allocentric cup significantly more often in test than in baseline trials (t(15) = 4.87; P < 0.001). This combination of results indicates an allocentric translation of response bias. Trends in the descriptive statistics show a similar pattern across all three genera (Fig. 4b).
Discussion.
Experiment 3 replicated nonhuman great apes' preference to use environmental cues in contrast to self in a less demanding task. Taking experiment 2 and 3 together, all great ape genera prefer to process spatial relations based on environmental cues and not self. The standard methods of comparative cognition thus suggest a common phylogenetic inheritance of a preference for allocentric spatial strategies from the ancestor shared by all four existing genera of Hominidae (Pongo, Gorilla, Pan, and Homo). Based on this result, we argue that, at least for small-scale spatial relations, the inherited cognitive mode of operation is not, as argued by Kant and others, egocentric but preferably deploys environmental cues as common reference between objects.
General Discussion.
In this article, we combine research on intrahuman variability and inter-Hominidae continuities to understand human cognition in its roots and variability. We compared humans with different cultural backgrounds and nonhuman great apes in a domain, spatial relations, accessible and highly relevant to all tested species. Experiment 1 showed that human spatial relational learning varies cross-culturally and that habitual cognitive preferences covary with habitual usage patterns in natural spatial language. This correlation is fully robust by age 8 and persists in adulthood.
In experiments 2 and 3, we tracked the functional signature of spatial relational learning through all great ape genera, i.e., right across the whole Hominidae family, including representatives of Pongo, Gorilla, Pan, and Homo (European 4 year olds). All genera prefer environment- to self-centered processing of spatial relations. The standard methods of comparative cognition suggest a common phylogenetic inheritance of a preference for allocentric over egocentric spatial strategies from the ancestor shared by all four genera. This conclusion upsets the Kantian assumption of the priority of egocentric spatial reasoning, but it does so on firm empirical grounds.
This inherited bias toward the allocentric coding of spatial relations can be overridden by cultural preferences, as in our own preference for egocentric or relative spatial coding. This override is not a rare or typically European phenomenon. Relative languages have been documented in industrial and indigenous cultures all over the globe (17), including for example the speakers of Kgalagadi, a Bantu language, who live a mere few-hundred kilometers from the Hai‖om language area (5). Nevertheless, overriding the bias might be expected to incur some costs; thus, the theory makes predictions about the relatively greater difficulty of acquiring a predominantly egocentric coding system. First, some individuals might be expected to have some special difficulties; prima facie evidence comes from lifelong difficulties with “left” and “right” that some adults evidence (47). Second, the relevant linguistic spatial relational constructions may be expected to be learned later by children. Again, the evidence suggests that this is correct: Children in cultures where absolute coding is predominant seem to master this system as early as 4 and certainly by 7 years of age (18, 23, 38), whereas children in relative-coding cultures do not seem to master full use of the left/right systems until ≈11 years of age (24, 37).
The model for human cognition that we propose has a rich, inherited primate basis, which may be masked by language and culture. Our primary access to these underlying defaults is through the study of our nearest primate cousins. The model does not suppose that language and culture can necessarily build cognitive structures entirely de novo; in the domain of spatial relations, at least, all three frames of reference have clear neural substrates [egocentric, posterior parietal cortex (48); object-centered, supplementary eye fields (49); and geocentric, hippocampus (50) and entorhinal cortex (51)], and these perhaps exhaust the available alternatives. The model makes predictions about differential human performance in the conditions where culture overrides an inherited default strategy and places cladistic reasoning at the heart of an evolutionary psychology.
We hope this perspective will supercede the very limited rhetoric of the controversies (52–55) that pit a simple nativist account of human cognition, admitting no cross-cultural variation, against a naïve blank-slate approach, which admits no strong phylogenetic inheritance behind human cognition.
Materials and Methods
Cultures.
The Netherlands and Germany are postindustrial Western European nations with a mixed rural and urban lifestyle, inhabiting densely populated landscapes. Dutch and German speakers predominantly use relative spatial relational descriptions but also deploy intrinsic constructions. Cardinal directions are used only for large-scale spatial reference (“Amsterdam is north of The Hague”) but never for tabletop space. The Dutch research site for this study is a village called Millingen aan de Rijn with ≈6,000 inhabitants. The German research site is Leipzig, a city with ≈500,000 inhabitants.
TheHai‖om are a group of hunter–gatherers living in the savanna of northern Namibia. Their language is part of the Khoekhoe cluster within the Central Khoisan language family. Despite political and economical marginalization, many aspects of Hai‖om traditional culture have been maintained, including an absolute linguistic system for spatial relations. Besides the dominant absolute system in the language, the speakers have an intrinsic and a rarely used relative system with left–right–front–behind terms (22). The research site for this study is a camp called Farm 6 in Mangetti West, with some 200 Hai‖om. An ethnographic description of the Hai‖om can be found in ref. 56.
Experiment 1.
Participants.
The sample consisted of 12 adults and 12 children from both the Dutch and the Hai‖om communities. (Dutch adults: 6 male and 6 female, mean age = 23 years 1 month, range = 18–34 years, SD = 4 years 6 months; Hai‖om adults: 3 male and 9 female, mean age = 21 years 10 months, range = 15–40 years, SD = 6 years 7 months; Dutch children: 8 male and 4 female, mean age = 8 years 6 months, range = 8–10 years, SD = 9 months; Hai‖om children: 8 male and 4 female, mean age = 8 years 10 months, range = 7–11 years, SD = 1 year 7 months). All 48 volunteers received rewards for participation, and teachers and parents gave their informed consent for Dutch and Hai‖om children. Participants that did not at least perform 50% correct on middle trials (cup in central position) were excluded from the final analysis (three excluded). In all side trials (all but the middle condition), participants had a two in five chance of picking a finding cup that was not related to the hiding cup by following any of the three rules mentioned above (relative, intrinsic, and absolute). Subjects who did so significantly below chance level (binomial test: <6 errors in 24 trials) were also excluded from the final analysis (12 excluded).
Apparatus and materials.
Two small tables were placed next to each other with an ≈30-cm gap between them. A solid opaque screen separated the two tables visually. Five identical cups were placed on each table. The containers were arranged in a dice-five constellation (Fig. 1). The setups varied only slightly in size across groups. All participants except three Hai‖om adults were tested in similar indoor contexts. The three adults were tested outdoors close to their home village. The experimenters were D.B.M.H. and C.J.R., who interfaced with the teachers in both communities in English and with participants through native-speaker video instructions.
Instructions.
“Here, you see a set of cups on a table. You will watch Daniel hide this block under one of them. Then you will go to another table with another set of cups, where you can search for a block. The game is to find the hidden block.” (abbreviated translation into English).
Experiment 2.
Participants.
Twelve German preschool children (6 male and 6 female, mean age = 4 years 10 months, range = 4 years 10 months to 4 years 11 months), three orangutans (Pongo pygmaeus), two gorillas (Gorilla gorilla), three bonobos (Pan paniscus), and five chimpanzees (Pan troglodytes) participated in this experiment. Among nonhuman great apes, there were four males and nine females ranging from 8 to 28 years of age (M = 14 years 2 months; SD = 6 years 9 months). All nonhuman great apes were housed at the Wolfgang Köhler Primate Research Center at Zoo Leipzig (Leipzig, Germany), lived in social groups with conspecifics, and had access to indoor and outdoor areas. During testing, the apes were fed according to their daily routine four times a day on a diet of fruit, vegetables, and monkey chow; water was at their disposal at all times. Participants that did not perform at least 50% correctly on middle-trials (cup in central position) were excluded from the final analysis (three excluded). In all side trials (all but the middle condition), participants had a one in three chance of picking a finding cup that was not related to the hiding cup by following a relative or nonrelative rule. Subjects who did so 50% of the time or more were also excluded from the final analysis (eight excluded).
Apparatus and materials.
Apparatus and materials are similar to experiment 1, with the exception that the number of cups on each table was reduced to three. The cups were arranged equidistantly in a straight from left to right of the participant (Fig. 3). The experimenter was D.B.M.H. There were no instructions beyond the request to move to the other table and an invitation to search.
Experiment 3.
Participants.
The final sample included two orangutans (Pongo pygmaeus), five gorillas (Gorilla gorilla), four bonobos (Pan paniscus), and six chimpanzees (Pan troglodytes). There were 6 males and 11 females ranging from 8 to 29 years of age (M = 14 years 10 months; SD = 7 years 10 months). All apes were part of the same population described above. Of the original sample, four animals were excluded because they chose the same cup in ≥90% of all trials across all sessions, and one animal was excluded because of experimenter error. If, in the training section of a session, an animal did not choose the training cup 10 of 12 times in a row within 60 trials, the session was terminated and excluded from the analysis. Of a total of 51 sessions, 4 had to be excluded because of a failed criterion and three because of experimenter error.
Apparatus and materials.
Apparatus and materials are the same as in experiment 2. The experimenter was D.B.M.H.
Acknowledgments
We thank the ≠Akhoe Hai‖om, Linda Uises, Marianne Kheimses, and Thomas Widlok, the children and teachers at St. Martinus School (Millingen aan de Rijn, the Netherlands), and the keepers of Zoo Leipzig for their generous cooperation; Elena Rossi and Antonia Misch for support; and Brian Hare and Katja Liebal for advice. This work was funded by the Max Planck Society for the Advancement of Science.
Abbreviation
- FoR
frame of reference.
Footnotes
The authors declare no conflict of interest.
References
- 1.Roberson D, Davies I, Davidoff J. J Exp Psychol Gen. 2000;129:369–398. doi: 10.1037//0096-3445.129.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Pica P, Lemer C, Izard W, Dehaene S. Science. 2004;306:499–503. doi: 10.1126/science.1102085. [DOI] [PubMed] [Google Scholar]
- 3.Gordon P. Science. 2004;306:496–499. doi: 10.1126/science.1094492. [DOI] [PubMed] [Google Scholar]
- 4.Pederson E, Danziger E, Wilkins D, Levinson SC, Kita S, Senft G. Language. 1998;74:557–589. [Google Scholar]
- 5.Neumann S, Widlok T. In: The Construal of Space in Language and Thought. Puetz M, Dirven R, editors. Berlin: Mouten de Gruyter; 1996. pp. 345–373. [Google Scholar]
- 6.Chua HF, Boland JE, Nisbett RE. Proc Natl Acad Sci USA. 2005;102:12629–12633. doi: 10.1073/pnas.0506162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne RW. The Thinking Ape. New York: Oxford Univ Press; 1995. [Google Scholar]
- 8.Hauser MD, Spelke E. In: The Cognitive Neurosciences III. Gazzaniga M, editor. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 9.Haun DBM, Call J, Janzen G, Levinson SC. Curr Biol. 2006;16:1736–1740. doi: 10.1016/j.cub.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Vygotsky L. Thought and Language. Cambridge, MA: MIT Press; 1962. [Google Scholar]
- 11.Gentner D. In: Language in Mind. Gentner D, Goldin-Meadow S, editors. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- 12.Burgess N, Jeffery KJ, O'Keefe J. The Hippocampal and Parietal Foundations of Spatial Cognition. New York: Oxford Univ Press; 1999. p. xi, 490. [Google Scholar]
- 13.Burgess N. Q J Exp Psychol. 2002;55:A1057–A1080. [Google Scholar]
- 14.Tomasello M. Constructing a Language. Cambridge, MA: Harvard Univ Press; 2003. [Google Scholar]
- 15.Levelt WJM. In: Language and Space. Bloom P, Peterson M, Nadel L, Garrett M, editors. Cambridge, MA: MIT Press; 1996. pp. 77–108. [Google Scholar]
- 16.Levinson SC. Space in Language and Cognition. Cambridge, UK: Cambridge Univ Press; 2003. [Google Scholar]
- 17.Majid A, Bowerman M, Kita S, Haun DBM, Levinson SC. Trends Cogn Sci. 2004;8:108–114. doi: 10.1016/j.tics.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Wassmann J, Dasen PR. J R Anthropol Inst. 1998;4:689–711. [Google Scholar]
- 19.Levinson SC, Kita S, Haun DBM, Rasch BH. Cognition. 2002;84:155–188. doi: 10.1016/s0010-0277(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 20.Bloom P, Keil FC. Mind Language. 2001;16:351–367. [Google Scholar]
- 21.Li P, Gleitman L. Cognition. 2002;83:265–294. doi: 10.1016/s0010-0277(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 22.Widlok T. J R Anthropol Inst. 1997;3:317–332. [Google Scholar]
- 23.Brown P, Levinson SC. In: Culture, Thought, and Development. Nucci L, Saxe G, Turiel E, editors. Mahwah, NJ: Erlbaum; 2000. pp. 167–197. [Google Scholar]
- 24.Piaget J. Judgment and Reasoning in the Child. London: Ruttledge & Kegan Paul; 1928. [Google Scholar]
- 25.Gallistel CR. Trends Cogn Sci. 2002;6:321–322. doi: 10.1016/s1364-6613(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 26.Newcombe NS, Huttenlocher J. Making Space. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 27.Kant I. In: The Philosophy of Right and Left. van Cleve J, Frederick RE, editors. The Netherlands: Kluwer, Dordrecht; 1768. [1991] [Google Scholar]
- 28.Miller GA, Johnson-Liard PN. Language and Perception. Cambridge, MA: Harvard Univ Press; 1976. [Google Scholar]
- 29.Halligan PW, Fink GR, Marshall JC, Vallar G. Trends Cogn Sci. 2003;7:125–133. doi: 10.1016/s1364-6613(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Spelke E. Trends Cogn Sci. 2002;6:376. doi: 10.1016/s1364-6613(02)01961-7. [DOI] [PubMed] [Google Scholar]
- 31.Burgess N, Spiers HJ, Paleologou E. Cognition. 2004;94:149–166. doi: 10.1016/j.cognition.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Waller D, Hodgson E. J Exp Psychol Learn Mem Cogn. 2006;32:867–882. doi: 10.1037/0278-7393.32.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremner G. Dev Psychol. 1978;14:346–355. [Google Scholar]
- 34.Acredolo LP. In: Spatial Cognition. Bellugi U, Stiles-Davies J, Kritchevsky M, editors. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 35.Allen GL. Spat Cogn Comput. 1999;1:413–429. [Google Scholar]
- 36.Nardini M, Burgess N, Breckenridge K, Atkinson J. Cognition. 2006;101:153–172. doi: 10.1016/j.cognition.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Rigal R. Percept Mot Skills. 1994;79:1259–1278. doi: 10.2466/pms.1994.79.3.1259. [DOI] [PubMed] [Google Scholar]
- 38.de Leon L. Linguistics. 1994;32:857–884. [Google Scholar]
- 39.Menzel EW. Science. 1973;182:943–945. doi: 10.1126/science.182.4115.943. [DOI] [PubMed] [Google Scholar]
- 40.Ray ED, Heyes CM. Anim Cogn. 2002;5:245–252. doi: 10.1007/s10071-002-0154-7. [DOI] [PubMed] [Google Scholar]
- 41.Fiset S, Dore FY. J Exp Psychol Anim Behav Process. 1996;22:420–437. doi: 10.1037//0097-7403.22.4.420. [DOI] [PubMed] [Google Scholar]
- 42.Fiset S, Gagnon S, Beaulieu C. J Comp Psychol. 2000;114:315–324. doi: 10.1037/0735-7036.114.4.315. [DOI] [PubMed] [Google Scholar]
- 43.Visalberghi E. In: Current Perspectives in Primate Social Dynamics. Taub DM, King FA, editors. New York: Van Nostrand Reinholt; 1986. pp. 445–452. [Google Scholar]
- 44.Kuhlmeier VA, Boysen ST, Mukobi KL. J Comp Psychol. 1999;113:396–402. doi: 10.1037/0735-7036.113.4.396. [DOI] [PubMed] [Google Scholar]
- 45.Oden DL, Thompson RKR, Premack D. Child Dev. 1990;61:621–631. [PubMed] [Google Scholar]
- 46.Call J. J Comp Psychol. 2004;118:232–241. doi: 10.1037/0735-7036.118.2.232. [DOI] [PubMed] [Google Scholar]
- 47.Elze C. J Neurol. 1926;90:146–151. [Google Scholar]
- 48.Cohen YE, Andersen RA. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- 49.Olson CR, Gettner SN. Science. 1995;269:985–988. doi: 10.1126/science.7638625. [DOI] [PubMed] [Google Scholar]
- 50.O'Keefe J, Burgess N. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 51.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 52.Pinker S. The Language Instinct. New York: Morrow; 1994. [Google Scholar]
- 53.Pinker S. The Blank Slate. New York: Viking; 2002. [Google Scholar]
- 54.Duchaine B, Cosmides L, Tooby J. Curr Opin Neurobiol. 2001;11:225–230. doi: 10.1016/s0959-4388(00)00201-4. [DOI] [PubMed] [Google Scholar]
- 55.Tooby J, Cosmides L. In: The Adapted Mind. Barkow L, Cosmides L, Tooby J, editors. New York: Oxford Univ Press; 1992. [Google Scholar]
- 56.Widlok T. Living on Mangetti. Oxford: Oxford Univ Press; 1999. [Google Scholar]