Abstract
What is already known about this subject
As of now, no population-based data exist on the incidence of pregnancy, elective abortions, and birth defects while on isotretinoin.
From surveys or interventional studies, it is known that pregnancy rates while on isotretinoin are comparable to the baseline rate, and that birth defect rates associated with first-trimester exposure are 10 times greater than in the general population.
Given that women decide to terminate pregnancies based on the available data, population-based estimates are needed.
What this study adds
This first non-interventional population-based study on the risk of pregnancy while being exposed to isotretinoin has shown that the rate of pregnancy is four times greater than what has been published to date (32.7/1000 person-years).
Any pregnancy while using isotretinoin is the result of a failure of pregnancy prevention strategies, hence, thus far, pregnancy prevention programmes have failed.
The rate of elective abortions is much higher than previously reported (84%).
Women of a lower socio-economic level and high users of healthcare services are more likely to become pregnant while on isotretinoin, suggesting that high use of health services increase the opportunity of having a prescription for isotretinoin or of having a pregnancy diagnosis.
Aims
To estimate the population-based incidence rates of pregnancy, spontaneous and elective abortions, and birth defects associated with isotretinoin use, and to determine predictors of pregnancy while on isotretinoin.
Methods
Using the RAMQ (medical and pharmaceutical data), MED-ECHO (hospitalizations) and ISQ (births and deaths) databases for the period 1984–2002, a cohort of 8609 women between 13 and 45 years of age and with a first prescription for isotretinoin (date of entry in the cohort) was identified. Women were eligible if they were insured by RAMQ for their medications at least 12 months before entry in the cohort and until 1 month after the end of their isotretinoin treatment. Pregnancies, spontaneous and elective abortions, and birth defects were identified using procedure codes and medical diagnoses.
Results
Of the 8609 women included, 90 became pregnant, an annual incident pregnancy rate during isotretinoin treatment of 32.7 per 1000 person-years of treatment (95% confidence interval 26.6, 40.1). Of the 90 women who became pregnant while on the drug, 76 terminated the pregnancy (84%), three had a spontaneous abortion (3%), two had trauma during delivery resulting in neonatal deaths (2%) and nine had a live birth (10%). Among the live births, only one had a congenital anomaly of the face and neck (11%). Adjusting for potential confounders, predictors of becoming pregnant while on isotretinoin were lower socio-economic level, one or more visits to the doctor or to the emergency department, or one or more hospitalization while on isotretinoin; concomitant isotretinoin and oral contraceptive use had a preventive effect.
Conclusions
This first non-interventional population-based study generated incidence rates of pregnancy while on isotretinoin four times greater than what has been reported in the literature thus far; elective abortion rates were also much higher in our study. This shows the importance of using population-based data for public health purposes.
Keywords: 19-year perspective, abortions, birth defects, isotretinoin use, population-based study, pregnancies
Introduction
Since the thalidomide disaster of the 1960s, physicians and the population as a whole have developed an increased awareness of the potential side-effects of drug exposure during pregnancy. Nevertheless, it remains that known teratogens are still being prescribed without proper physician surveillance. Indeed, this is the case with isotretinoin, approved in 1982 for the treatment of cystic, recalcitrant acne. Isotretinoin is by far more cost-effective than the other antiacne medications for the treatment of severe and scarring acne, but has been shown to be teratogenic when used during pregnancy [1]. In recent years, it has also been used for mild to moderate forms of acne, hence increasing the likelihood of exposed pregnancies and birth defects. Indeed, in a population-based study, 64% of first isotretinoin prescriptions were given to patients who did not have a history of other antiacne medication use [2]. Isotretinoin is used 90% of the time in young people between 13 and 45 years of age [3] and 50% of isotretinoin prescriptions are for women [2]. It is the most broadly prescribed teratogenic drug in the USA and Canada [2, 4]. In Canada it has been estimated that 3/10 000 women between 13 and 45 years old are using or have used isotretinoin [2]; the prevalence in the USA is 10 times greater at 3/1000 women of reproductive age [4]. Tragically, despite clear labelling of isotretinoin as contraindicated during pregnancy, birth defects consequent to in utero exposure are still reported soon after dispensing of the drug [5–7]. The risk of becoming pregnant while on isotretinoin treatment, as reported in voluntary surveys, is estimated at 8/1000 person-years of treatment [5], which is likely to be an underestimate given the fact that not all women exposed to isotretinoin are participating. The baseline risk of malformations in the population is 3–5%, but it has been reported to increase to almost 30% in women exposed to isotretinoin during the first trimester of pregnancy in analysis of spontaneous report databases or self-report surveys [5, 8]. Congenital malformations associated with isotretinoin-exposed pregnancies include serious craniofacial, cardiovascular, thymic and central nervous system malformations.
Since 1983, when the teratogenicity of isotretinoin was first documented in humans [9], the Dermatologic and Ophthalmic Drugs Advisory Committee of the US Food and Drugs Administration (FDA) has met frequently to make recommendations to balance the needs of patients with severe cystic acne to receive isotretinoin with the need to protect fetuses from exposure [10]. Central to these efforts has been a sponsor-developed Pregnancy Prevention Program (PPP) that has since also been introduced in Canada [6]. Even after the implementation of the PPP, the number of new isotretinoin prescriptions to reproductive-aged women tripled, from around 70 000/year in 1989 to estimates of nearly 210 000 in 1999 [2, 11]. Since the drug was approved in 1982 and until 2000, the manufacturer has documented 1995 isotretinoin-exposed pregnancies, which we believe is an under-representation of the total number of exposed pregnancies as the enrolment in the PPP has been low. Therefore, in 2000, the FDA's Advisory Committee recommended new restrictions on prescribing and dispensing, and implemented the new risk management programme: System to Manage Accutane® Related Teratogenicity™ (SMART); this programme has not been implemented in Canada. Given that generic isotretinoin compounds entered the market in 2002 and that risk management programmes have not been centralized thus far, the FDA has launched an integrated isotretinoin risk management programme for all isotretinoin compounds (iPLEDGE, 2006).
Isotretinoin use in women of childbearing age is a very important public health issue because of the risk of spontaneous and elective abortions, and children with major malformations that will require continuous healthcare services throughout their lifetime; it is an even more important problem due to the fact that it is preventable. Thus far, there are no population-based risk estimates of pregnancy, abortion, miscarriage or birth defect while on isotretinoin. Although other investigators have studied the use and impact of isotretinoin in young women, it has been solely done in restricted populations with low participation rates, small sample sizes, self-reports or retrospective data collection schemes, and is thus prone to various biases including selection bias, and leading to unknown absolute pregnancy exposure rates in the population. In addition, nobody has looked at trends of isotretinoin-related pregnancy rates since its inception time on the market, and nobody has identified women at risk of becoming pregnant while on isotretinoin in order to develop appropriate interventions and management strategies. Given the fact that cheaper generic forms of isotretinoin have now been introduced on the market, it is pivotal to assess fully the association between isotretinoin use and pregnancy or birth defects, and to identify women at risk of becoming pregnant while on the drug.
Therefore, the objectives of this study were to estimate population-based incidence rates of pregnancy, elective abortion, miscarriage and birth defects while on isotretinoin over a 19-year period, and to identify and quantify predictors of becoming pregnant while on the drug. Predictors of having an elective abortion after being exposed to isotretinoin in the first trimester of pregnancy were also explored.
Methods
Study design
This was a retrospective cohort study.
Study population
A cohort of women exposed to isotretinoin was formed using data from the medication database of the Régie de l'assurance maladie du Québec [RAMQ-Rx; data on name (brand and generic) of medication dispensed, dosage, formulation, duration of the prescription, calendar date of dispensation, and whether it is a refill or a new prescription). In order to characterize healthcare utilization, pregnancy and birth outcomes, the cohort was linked to the administrative databases of (i) the universal healthcare insurance programme of the RAMQ [diagnoses according to the International Classification of Diseases, ninth revision (ICD-9) [12], healthcare services, physician and emergency department (ED) visits, patient and healthcare provider characteristics]; (ii) MED-ECHO (gestational age, and hospitalization data in Quebec); and (iii) the Institut de la statistique du Québec (ISQ; patient and newborn socio-demographic data, and birth and mortality data in Quebec). The database linkage between RAMQ and MED-ECHO was done using patients' ‘Numéro d’assurance maladie' (NAM), which is a unique identifier provided to all legal residents in the province of Quebec. The ISQ database was used to gather information on the newborn at birth such as weight, gestational age and socio-economic status of the mother and father. The mother–child linkage was possible using the unique identifier which links each baby born in Quebec to his/her mother in the RAMQ databases. Although the ISQ does not have a NAM, the mother–child linkage was possible using the name, surname and date of birth of both the mother and child. The linkage of the RAMQ, MED-ECHO and ISQ data by the investigators was possible using the unique scrambled confidential ID numbers generated by the three organizations. The RAMQ medication database has been shown to be valid and reliable [13].
Women covered by the medication plan of the RAMQ (RAMQ-Rx: social assistance beneficiaries before 1 January 1997 and drug plan and social assistance beneficiaries after 1 January 1997) at any time between 1 January 1984 and 31 December 2002 were considered for this cohort, and determined the study population. To be included in this cohort, women had to (i) have at least one prescription for isotretinoin [drug identification numbers (DIN): 582344 (10 mg) and 582352 (40 mg)] between 1 January 1984 and 31 December 2002, (ii) be between 13 and 45 years of age at the time of the first prescription for isotretinoin between 1 January 1984 and 31 December 2002, and (iii) be covered by the RAMQ-Rx plan for at least 12 months prior to the date of the first isotretinoin prescription and until 1 month after the end of the isotretinoin treatment regimen. There was the possibility that women could have had more than one isotretinoin course during the 19-year study period. However, given the fact that previous analyses have shown that 75% of women in the cohort had only one course between 1984 and 2002 [2], only the first isotretinoin treatment course was considered and hence eligible for analyses. Therefore, within the first course, all isotretinoin prescription durations were multiplied by 125% when calculating the overall duration of a regimen to take into account late renewals. Follow-up of subjects started at the time of the first prescription for isotretinoin during the study period [date of entry in the cohort (DE)] and ended (i) 1 month after the end of the treatment regimen [any time between 1 month (one prescription without any refills) and any number of cumulative months with constant prescription renewals] if no pregnancy occurred between DE and 1 month after isotretinoin discontinuation, or (ii) at the date of an elective or a spontaneous abortion if a pregnancy occurred while on isotretinoin or within 1 month after isotretinoin discontinuation with an elective abortion/miscarriage, or (iii) at the date of a stillbirth if a pregnancy occurred while on isotretinoin or within 1 month after isotretinoin discontinuation without abortion and without a live birth, or (iv) 3 years after the end of pregnancy if a pregnancy occurred while on isotretinoin or within 1 month after isotretinoin discontinuation with a live birth, whichever applied.
Cohort variables
Outcome variables
Pregnancy, abortion (spontaneous or elective) and congenital malformation were the outcome variables.
Only pregnancies that occurred while on the first isotretinoin course between 1984 and 2002 were considered and analysed. Pregnancies were identified using the RAMQ and MED-ECHO administrative databases; ICD-9 codes (236.1, 630.0–648.9, 650.0–650.9, 760.0–779.9) along with pregnancy-related procedure codes (V22.0–V22.2, V23.1–V23.2, V23.4–V23.5, V23.8–V23.9, V61.5–V61.7, V724) were used to identify a pregnancy. To ascertain whether a pregnancy was incident during the treatment, the above-mentioned ICD-9 and procedure codes could not have been present in the 40 weeks prior to their first appearance during the treatment. The ISQ database was searched to identify any additional births that occurred in cohort subjects during the treatment regimen. Using data on gestational age present in MED-ECHO, we further identified pregnancies which occurred during the isotretinoin treatment. Therefore, the beginning of pregnancy [index date (ID)] was defined as the calendar time of the first day of the last menstrual period. The ID of subjects with no identified pregnancies was defined by generating a random calendar date between the DE and the last day of the isotretinoin regimen.
End of pregnancy was identified in the RAMQ or MED-ECHO databases using ICD-9 codes for live births (650.0–650.9), data on death certificates for neonatal deaths or stillbirths, and in the MED-ECHO database using ICD-9 codes for elective abortions (632.0–632.9, 635.0–635.9, 636.0–636.9, 637.0–637.9, 638.0–638.9, 779.6), spontaneous abortions (634.0–634.9, 761.8) or ectopic pregnancies, breech delivery/extraction, use of forceps or vacuum extractor, C-section (633.0–633.9, 639.8, 652.2, 669.5–669.7).
All congenital malformations that have already been associated with isotretinoin use in the literature were considered using ICD-9 codes present in the RAMQ and MED-ECHO databases: (i) any mental retardations including encephalocele, microcephalus and hydrocephalus (742, 742.0–742.5, 742.8, 742.9); (ii) lack of ears (anotia) and malformations of the ear (external, middle, internal) (744, 744.0–744.5, 744.8, 744.9); (iii) facial malformations (754, 754.0, 754.1); (iv) ophthalmoplegia (367.52, 378.5, 378.55, 378.56, 378.8, 378.86); (v) maxillary bone malformations (754.0, 756.0); (vi) teeth malformations (520.0–520.9); (vii) cardiac malformations (various) (746, 746.0–746.9); (viii) limb reduction defects (755, 755.2–755.4); (ix) skeletal hyperostosis (733.3); and (x) cleft palate malformations (749, 749.0–749.2).
Predictors of pregnancy while on isotretinoin
In the 12 months preceding the DE in the cohort: physician and ED visits, hospitalizations, overall medication use and, more specifically, antiacne medication and oral contraceptive (OC) use.
At DE: specialty of the physician that wrote the isotretinoin prescriptions [dermatologist vs. other (general practitioners or other nondermatologists)], calendar time of isotretinoin use [1984–1988 (before the PPP), 1988–1994 (introduction of the PPP in 1988), 1995–1999 (guideline changes in 1995 – isotretinoin should be used only after two to three other antiacne medications had failed), 2000–2002 (addition of depression in the list of adverse events in 2000, and introduction of generic compounds on the market in 2002)].
Between DE and ID (first day of the pregnancy): OC use, concomitant antiacne medication use, average daily dosage of isotretinoin use (mg day−1), isotretinoin prescribed by more than one physician, overall medication use (excluding isotretinoin and OC use), number of physician visits, ED visits and hospitalizations.
At ID: maternal age, RAMQ-Rx status (social assistance or medication plan beneficiaries), place of residence (urban or rural dwellers), OC use, and whether the isotretinoin prescription was made during a physician visit or whether it was an automatic refill without a physician visit (given that only the PPP was implemented in Canada, refills were possible, and thus prescribed).
Predictors of having an elective abortion after first trimester exposure to isotretinoin
This was done amongst women who had a pregnancy while using isotretinoin. For these, the index date (ID) of subjects was defined as the calendar time of the elective abortion or by generating a random calendar date between the first day of pregnancy and date of birth for those who did not have an elective abortion. Predictors of having an elective abortion were:
In the 12 months preceding DE in the cohort: number of physician or ED visits, number of hospitalizations, overall medication use and, more specifically, antiacne medication and OC use.
At DE: specialty of the physician who wrote the isotretinoin prescriptions [dermatologist vs. other (general practitioners or other nondermatologists)], calendar time of isotretinoin use [1984–1988 (before the PPP), 1988–1994 (introduction of the PPP in 1988), 1995–1999 (guideline changes in 1995 – isotretinoin should only be used after two to three other antiacne medications had failed), 2000–2002 (addition of depression in the list of adverse events in 2000, and introduction of generic compounds on the market in 2002)].
Between DE and first day of the pregnancy: OC use, concomitant antiacne medication use, average daily dosage of isotretinoin (mg day−1), isotretinoin prescribed by more than one physician, overall medication use (excluding isotretinoin and OC use), number of physician visits, ED visits, and hospitalizations.
At ID: maternal age, RAMQ-Rx status (social assistance or medication plan beneficiaries), place of residence (urban or rural dwellers) and whether the patient was treated by a dermatologist or not at the time the pregnancy was diagnosed.
Statistical analysis
Data on medication use were based on filled prescriptions. The mean number of days per isotretinoin treatment regimen and the mean number of days between DE and the first day of pregnancy were calculated. The annual incidence rate of pregnancy and the overall rates of elective abortions, miscarriages and congenital malformations while on isotretinoin therapy were estimated. The pregnancy rate per 20-week isotretinoin regimen (suggested treatment duration – product labelling [14]) was also calculated.
Predictors of pregnancy while on isotretinoin were identified and quantified using univariate and multivariate logistic regression models. Predictors of abortions after first-trimester exposure to isotretinoin were also identified and quantified using univariate and multivariate logistic regression models.
Sensitivity analyses were done including data on pregnancies that occurred within 1 month of isotretinoin discontinuation, given the fact that guidelines suggest monitoring pregnancies until 1 month after the drug intake is stopped.
All statistical analyses were conducted using SAS version 8.2 [15] (SAS Inc., Cary, NC, USA).
Ethics
This study was approved by the Ethics Committee of Ste-Justine's Hospital, Montreal, QC, Canada. The Commission d'accès à l'information du Québec also approved the confidential linkage of the three administrative databases (RAMQ, MED-ECHO, ISQ).
Results
Characteristics of the cohort
Eight thousand six hundred and nine female isotretinoin users met eligibility criteria and were thus included in the cohort. They were on average 26 years of age (SD 8 years), urban dwellers (79%) and welfare recipients (52%). Only 34% of women had received antiacne medications other than isotretinoin before their first isotretinoin prescription, and the majority were treated by dermatologists (58%).
Trends and rates of pregnancy during 1984–2002
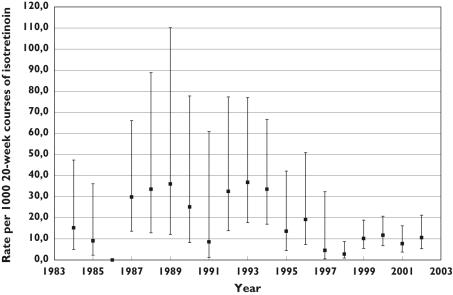
Of the 8609 women included in this cohort, 210 were pregnant at any given point in time during their isotretinoin regimen: 68 were already pregnant at the time they started their first isotretinoin prescription (32%), 90 became pregnant while taking isotretinoin (43%) and 52 became pregnant within 1 month of isotretinoin discontinuation (25%) (Table 1). Using only newly diagnosed pregnancies that occurred while on isotretinoin (n = 90), the overall annual incident pregnancy rate while on isotretinoin was 32.7 per 1000 person-years of treatment [95% confidence interval (CI) 26.6, 40.1] and the overall incident pregnancy rate per 1000 20-week courses of isotretinoin (as suggested by the manufacturer) was 12.6 (95% CI 10.2, 15.4). The pregnancy rate was stable over time and there was no statistically significant trend or any effect of the PPP or of other guidelines/label changes during this 19-year period (P> 0.05 for trend) (Figure 1). In women who did not become pregnant, the mean isotretinoin treatment duration was 111.6 days (SD 65.3) and in those who became pregnant (n = 90) the mean time from treatment initiation (DE) and pregnancy was 60.5 days (SD 54.3).
Table 1.
Number of incident pregnancies at the beginning of the isotretinoin treatment, during treatment, and 1 month after treatment discontinuation
| Time period | n (%) |
|---|---|
| At the beginning of treatment | 68 (32) |
| During treatment | 90 (43) |
| Within 1 month of treatment discontinuation | 52 (25) |
| Total | 210 (100) |
Figure 1.
Incident pregnancy rates during isotretinoin treatment from 1984 to 2002. (P> 0.05 for trend)
Pregnancy outcomes
Of the 90 women who became pregnant while exposed to isotretinoin, 76 terminated the pregnancy (elective abortions: 84.4%), three had a spontaneous abortion (3.3%), two had trauma during delivery resulting in neonatal deaths (2.2%) and nine had a live birth (10.0%) (Table 2). No ectopic pregnancies, stillbirths, breech deliveries/extractions, use of forceps or C-sections were identified (Table 2).
Table 2.
Pregnancy outcomes in women who became pregnant while being exposed to isotretinoin
| Outcomes (ICD-9 codes) | n (%) |
|---|---|
| Live births (650.0–650.9) | 9 (10.0) |
| Planned abortions (632.0–632.9, 635.0–635.9, 636.0–636.9, 637.0–637.9, 638.0–638.9, 779.6) | 76 (84.4) |
| Spontaneous abortions (634.0–634.9, 761.8) | 3 (3.3) |
| Obstructed or trauma during delivery resulting in neonatal death (death certificate) | 2 (2.2) |
| Stillbirths (death certificate) | 0 (0.0) |
| Ectopic pregnancies, breech delivery/extraction, use of forceps or vacuum extractor, C-section (633.0–633.9, 639.8, 652.2, 669.5–669.7) | 0 (0.0) |
| Total | 90 (100.0) |
Congenital malformations
Among the nine live births, only one had a congenital anomaly of the face and neck (11%; 95% CI 0.3, 48.2) diagnosed at 3 years old; the remaining babies had no congenital malformations reported during the first 3 years of life. Given the 19-year period of our cohort, we were able to follow the children for up to 7 years after birth, and no additional congenital anomalies or deaths were detected. No congenital malformations were detected in babies of women who were already pregnant at the time of their first isotretinoin prescription.
Predictors of pregnancy while on isotretinoin
Table 3 presents the characteristics of women who became pregnant while exposed to isotretinoin during the study period. In multivariate analyses, predictors of becoming pregnant while on isotretinoin were lower socio-economic level [welfare recipients (yes/no) relative risk (RR) 1.93, 95% CI 1.03, 3.61], one or more visits to the doctor while on isotretinoin (RR 9.51, 95% CI 5.06, 17.80), one or more ED visit while on treatment (RR 2.44, 95% CI 1.28, 4.66), or one or more hospitalization while on treatment (RR 32.0, 95% CI 17.5, 58.6), and concomitant isotretinoin/OC use had a preventive effect (RR 0.27, 95% CI 0.10, 0.78); being treated by a dermatologist, and calendar time of the treatment had no influence on the incidence of pregnancy.
Table 3.
Predictors of becoming pregnant while being exposed to isotretinoin for the first time
| Pregnancy (n = 90) | No pregnancy (n = 8519) | Crude RR (95% CI) | Adjusted RR (95% CI) | |
|---|---|---|---|---|
| At index date* | ||||
| Age of patients, years (mean, SD) | 27.3 (6.3) | 26.3 (7.7) | 1.02 (0.99, 1.04) | 1.00 (0.97, 1.03) |
| Urban dwellers (n, %)† | 73 (85.9%) | 6233 (78.4%) | 1.67 (0.91, 3.09) | 1.79 (0.91, 3.49) |
| Welfare recipients (n, %) | 65 (72.2%) | 4448 (52.2%) | 2.38 (1.50, 3.78) | 1.93 (1.03, 3.61) |
| OC use (n, %) | 8 (8.9%) | 1476 (17.3%) | 0.47 (0.23, 0.96) | 0.27 (0.10, 0.78) |
| Isotretinoin prescription was an automatic refill (n, %) | 30 (33.3%) | 3110 (36.5%) | 0.87 (0.56, 1.35) | 0.63 (0.38, 1.03) |
| Between date of entry in the cohort‡ and index date | ||||
| OC use (n, %) | 17 (18.9%) | 2038 (23.9%) | 0.74 (0.44, 1.26) | 0.98 (0.40, 2.38) |
| Concomitant antiacne medications (n, %) | 7 (7.8%) | 496 (5.8%) | 1.36 (0.63, 2.97) | 1.04 (0.45, 2.41) |
| Average daily dosage of isotretinoin (mg day−1) (mean, SD) | 39.3 (14.4) | 41.9 (18.9) | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.01) |
| Isotretinoin prescribed by ≥ 2 physicians (n, %) | 4 (4.4%) | 334 (3.9%) | 1.14 (0.42, 3.13) | 0.88 (0.30, 2.58) |
| Other medication use (other than isotretinoin and OC) (n, %) | 50 (55.6%) | 3755 (44.1%) | 1.59 (1.04, 2.41) | 0.36 (0.21, 0.62) |
| At least one visit to the physician (other than treating physician for isotretinoin) (n, %) | 74 (82.2%) | 3129 (6.7%) | 7.97 (4.63, 13.70) | 9.51 (5.06, 17.80) |
| At least one visit to the emergency department (n, %) | 16 (17.8%) | 474 (5.6%) | 3.67 (2.12, 6.35) | 2.44 (1.28, 4.66) |
| At least one hospitalization (n, %) | 31 (34.4%) | 102 (1.2%) | 43.4 (26.90, 69.80) | 32.0 (17.50, 58.60) |
| In the 12 months immediately prior to date of entry in the cohort | ||||
| Anti-acne medication use (n, %) | 32 (35.6%) | 2933 (34.4%) | 1.05 (0.68, 1.62) | 1.04 (0.64, 1.69) |
| Other medication use (other than isotretinoin and OC) (n, %) | 79 (87.8%) | 7107 (83.4%) | 0.70 (0.37, 1.32) | 0.80 (0.40, 1.62) |
| OC use (n, %) | 28 (31.1%) | 2402 (28.2%) | 1.15 (0.73, 1.80) | 1.65 (0.90, 3.03) |
| At least one visit to the physician (n, %) | 89 (98.9%) | 8246 (96.8%) | 2.95 (0.41, 21.2) | 1.21 (0.16, 9.04) |
| At least one visit to the ED (n, %) | 30 (33.3%) | 2212 (26.0%) | 1.43 (0.92, 2.22) | 0.90 (0.53, 1.51) |
| At least one hospitalization (n, %) | 19 (21.1%) | 1241 (14.6%) | 1.57 (0.94, 2.61) | 0.79 (0.43, 1.47) |
| At date of entry in the cohort | ||||
| Treated by a dermatologist (n, %)§ | 50 (55.6%) | 4909 (57.8%) | 0.91 (0.60, 1.39) | 0.97 (0.60, 1.55) |
| Time period (n, %) | ||||
| 1 January 1984 to 31 September 1988 | 15 (16.7%) | 1228 (14.4%) | 1.00 (Reference) | 1.00 (Reference) |
| 1 October 1988 to 31 December 1994 | 27 (30.0%) | 1231 (14.5%) | 1.80 (0.95, 3.39) | 1.27 (0.58, 2.79) |
| 1 January 1995 to 31 December 1999 | 21 (23.3%) | 3101 (36.4%) | 0.55 (0.29, 1.08) | 0.57 (0.24, 1.33) |
| 1 January 2000 to 31 December 2002 | 27 (30.0%) | 2959 (34.7%) | 0.75 (0.40, 1.41) | 1.06 (0.44, 2.57) |
OC, Oral contraceptive; RR, relative risk; CI, confidence interval; SD, standard deviation; ED, emergency department.
Index date is the date of the first day of gestational age for women with a pregnancy, and a random calendar date between date of entry in the cohort and last day of isotretinoin treatment for women without a pregnancy.
Proportions based on 8032 patients with available data; 85 with a pregnancy, 7947 with no pregnancy.
Date of entry in the cohort is the first day of the first isotretinoin treatment between 1984 and 2002.
Proportions based on 8583 patients with available data; 90 with pregnancy, 8493 with no pregnancy.
Predictors of having an elective abortion following first trimester exposure to isotretinoin
Given the fact that we could not determine whether women who had a miscarriage would have decided to continue their pregnancy, we analysed predictors of having an elective abortion (n = 76) vs. a live birth or a delivery which resulted in a neonatal death (n = 11). No patient or prescriber characteristics, nor any healthcare utilization variables such as physician or ED visits and hospitalizations were significantly associated with the decision to abort the pregnancy. In addition, there was no trend over time in the likelihood to have an elective abortion following first-trimester exposure to isotretinoin.
Sensitivity analyses
Including pregnancies in the month following isotretinoin discontinuation, 52 additional incident pregnancies occurred in the 30 days following isotretinoin discontinuation. Of these, 14 resulted in a live birth (27%), one in a spontaneous abortion (2%) and 37 in elective abortions (71%). Of the 14 live births, none had a diagnosis of congenital malformations at 3 years old; no further malformations were detected in children where follow-up data were available for up to 7 years of age. Including all pregnancies which occurred during isotretinoin treatment and within 30 days of isotretinoin discontinuation, predictors of becoming pregnant were the same as those previously reported in Table 3.
Discussion
This first non-interventional population-based study of the risk of pregnancy while being exposed to isotretinoin has shown that the rate of pregnancy is four times greater than what has been published thus far; the rate of elective abortions is much higher than previously reported, and the risk of major malformations associated with first-trimester exposure to isotretinoin is smaller than reported earlier. This study is the first to examine trends and rates of pregnancy while on isotretinoin since the time the drug came on the Canadian market until the time generic compounds were introduced – 1984–2002.Results based on valid and reliable data on medication use and pregnancy diagnosis have shown that guidelines to increase awareness of the teratogenicity of the drug have had no significant impact on pregnancy rates over time. In addition, predictors of becoming pregnant and of having an elective abortion after first-trimester isotretinoin exposure have been identified, hence helping public health officials in defining appropriate risk management strategies.
Our study population was 26 years of age on average and comparable to other published cohorts in this regard [5]. Although the majority of our cohort was treated by dermatologists, the proportion was much lower than what has been reported in the USA (58% vs. 92% [5]). This could be explained partly by the nature of the healthcare system in Quebec, which makes it difficult to consult a specialist in a timely manner. In addition, only one-third of women had received antiacne medications before their first isotretinoin prescription.
Overall, 158 pregnancies were detected while using isotretinoin. Sixty-eight pregnancies (43%) had began before the start of the isotretinoin therapy and 90 (57%) occurred during isotretinoin treatment; an additional 52 pregnancies were diagnosed in the 1 month following isotretinoin discontinuation. Of those who became pregnant while on isotretinoin (n = 90), 76 (84%) had elective abortions, three (3%) had spontaneous abortions and nine (10%) had live births; no stillbirths occurred, but two (2%) live births resulted in neonatal deaths. Although most pregnancies occurred while on isotretinoin, as was seen in the study of Mitchell et al.[5], the proportion was much lower (57% vs. 88%). In addition, the elective abortion rate found in our study is much higher, and the rate of miscarriage much lower than what has been previously reported [4, 5, 8]. However, our rate of live births (10%) is comparable to that in the literature. This can be explained by the nature of the data in our cohort, who did not rely on women's self-report for determining whether an abortion was elective or spontaneous but a diagnosis or a procedure code. Therefore, it is likely that women that had an elective abortion reported it as a miscarriage in other published studies. We found a rate of major malformations of 11%, which is 2/3 smaller than estimates previously reported in the literature (28–30%) [4, 5, 8]. Although it is likely that our methodology could partly explain the discrepancy between studies (population-based cohort vs. survey/spontaneous reports databases/small sample sizes), it remains that our estimate is based on only one case and is thus not stable or robust. Nevertheless, we were able to follow the children for up to 7 years after birth, and no additional congenital anomalies or deaths were detected. Like other authors, we were not able to examine aborted fetuses, and thus we cannot exclude the possibility that birth defects could have been present amongst those fetuses.
Adjusting for maternal age, place of residence, being treated by a dermatologist, healthcare utilization before the initiation of treatment and calendar time of the treatment, predictors of becoming pregnant while on isotretinoin were lower socio-economic level and high use of healthcare services while on isotretinoin (MD visits, ED visits and hospitalizations); concomitant isotretinoin/OC use prevented from becoming pregnant. Within the context of our cohort, high use of health services can be viewed as a marker of good or bad management or of disease state. We agree that all women on isotretinoin should be targeted for pregnancy prevention education, but perhaps women in groups identified here as at high-risk of pregnancy while on isotretinoin should not be prescribed isotretinoin until they have demonstrated adherence to birth control for perhaps two or three menstrual cycles. Although based on a small sample size, no patient or prescriber characteristics, or healthcare utilization variables such as physician or ED visits and hospitalizations were significantly associated with the decision to abort the pregnancy. In addition, there was no trend over time in the likelihood to have an elective abortion following first-trimester exposure to isotretinoin. No impact of any guidelines was seen on pregnancy rates over this 19-year period by looking at pregnancy rates over time or by looking at the effect of different time periods on the likelihood of becoming pregnant in multivariate analyses. This result agrees with other studies [2, 4]. Given the fact that few pregnancies occurred during the 19-year study period, it was impossible for us to apply time-series modelling techniques, but we were able to see that the probability of becoming pregnant did not change over time by including different time periods in our multivariate logistic regression model.
Administrative databases offer many advantages over field studies, such as a well-defined population, a large population-based sample of subjects, accurate data on filled prescriptions, lack of recall bias for both drug exposures and diagnoses, and the possibility to analyse multiple medication classes and outcomes at the same time. For these reasons, administrative databases are often used in epidemiology and, more specifically, in pharmacoepidemiology [16–23], and the RAMQ, MED-ECHO and ISQ databases have been used already for the study of medication utilization [24–26]. Moreover, data on prescription fillings in the RAMQ database have been shown to be valid and comprehensive [13]. Hence, in our study, data on medication use in terms of dosages, duration of use and calendar time of all filled prescriptions were accurate, since all filled prescriptions to Quebecers insured by the RAMQ for their medications are automatically computerized at the time the medication is dispensed in any given pharmacy. This is an advantage over other studies published thus far that relied on women's self-report or spontaneous reports databases, where a significant number of female isotretinoin users choose not to participate or not to report adverse events, or report medication use in a retrospective manner after a severe adverse event has occurred such as a birth with congenital malformations. Given the fact that our study, within a stratum of the Quebec population, is population based, the denominator of our cohort includes all women exposed to isotretinoin between 1984 and 2002, and the numerator includes all incident pregnancies which occurred while on isotretinoin. Indeed, we were able to determine, using gestational age, the first day of all pregnancies, and thus appropriately categorize pregnancies that began previously to an isotretinoin course, during the course, and after the end of treatment. Therefore, the difference between our results on pregnancy rates and those published thus far could partly be explained by our methodology, and by the fact that we did not include pregnancies that were diagnosed before starting an isotretinoin therapy since we were interested in calculating the incident pregnancy rate while on the drug. Nevertheless, we found that over this 19-year period, the annual incident pregnancy rate during isotretinoin treatment was 32.7/1000 person-years of treatment (95% CI 26.6, 40.1), or 12.6/1000 20-weeks courses of isotretinoin (95% CI 10.2, 15.4). This is four times greater than what has been reported by Mitchell et al.[5], who found an annualized rate of pregnancy of 8.8/1000 person-years of exposure, and a pregnancy rate of 3.4/1000 20-weeks courses in the Accutane Survey between 1989 and 1993. Data collected in the Accutane Survey were self-reported by women, interventional in nature, and it was estimated that only 50% of female isotretinoin users in the USA participated in the survey [4], explaining in part the lower pregnancy rate. Even if our pregnancy rate was found to be much higher than in other studies, we believe that it remains an underestimate, given the fact that we did not consider drug sharing or pregnancies that occurred while using left-over prescriptions. Indeed, Robertson et al.[27] found that a significant number of pregnancies occurred while using isotretinoin from a friend's prescription or from a residual prescription. Finally, our study is based on medication fillings and not on actual medication intake. However, given that people insured by the RAMQ for their medications cannot have more than 30 days' supply at a time, they have to return to the pharmacy to get additional pills – the only reason why someone would return to a pharmacy to get their prescription refilled is that they have taken the medicines and need more. In a previous study on the same cohort [2], we showed that isotretinoin users continuously returned to their pharmacist for refills over a mean duration of 4 months, indicating that they were taking the drug during their isotretinoin therapy. More specifically, in the present study, isotretinoin users who became pregnant were on the drug for a mean duration of 60 days, and thus had the opportunity to have at least one refill, which gives us the assurance that they were taking their medication as prescribed. Furthermore, we were able to assess the exact first day of pregnancy using data on gestational age provided by MED-ECHO (hospital archive data), before the pregnancy was diagnosed, and before the women had the chance to discontinue taking their medication. This again indicates that they were exposed to isotretinoin at the time of the pregnancy diagnosis.
This study generated population-based estimates of the teratogenic risks associated with isotretinoin use. No such estimates of pregnancies, elective abortions and congenital malformations rates were previously available. Given the fact that generic forms of isotretinoin are now available on the market, which will be likely to increase the use and the risk of exposed pregnancies, such estimates were needed. Pregnancies while on isotretinoin are frequent in the population even if they are preventable, and identifying where the management could be improved is a major public health contribution, which could ultimately lead to the better health of young women and children. The findings of this study will, it is hoped, be used to target women who are at high risk of pregnancy while on isotretinoin, and lead to the development of more effective risk management strategies.
Acknowledgments
This study was funded by the Canadian Institutes of Health Research (CIHR) (grant no IHD – 67337). A.B. is the recipient of a career award from the CIHR/Health Research Foundation of Canada, and is on the endowment research chair of the Famille Louis-Boivin on ‘Medication, Pregnancy, and Lactation’ at the Faculty of Pharmacy of the University of Montreal; L.A. is the recipient of a PhD studentship from the Fonds de la recherche en santé du Québec (FRSQ); L.B is the recipient of a career award from the CIHR, and is on the AstraZeneca endowment research chair on respiratory health at the Faculty of Pharmacy of the University of Montreal; S.P. is the recipient of a career award from the FRSQ.
References
- 1.Holmes SC, Bankowska U, Mackie RM. The prescription of isotretinoin to women: is every precaution taken? Br J Dermatol. 1998;138:450–5. doi: 10.1046/j.1365-2133.1998.02123.x. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006;62:667–74. doi: 10.1007/s00228-006-0151-x. [DOI] [PubMed] [Google Scholar]
- 3.Moskop JC, Smith ML, De Ville K. Ethical aspects of teratogenic medications: the case of isotretinoin. J Clin Ethics. 1997;8:264–78. [PubMed] [Google Scholar]
- 4.Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of Accutanemd– exposed pregnancies. Teratology. 2001;64:142–7. doi: 10.1002/tera.1057. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AA, Van Bennekom CM, Louik C. A pregnancy-prevention program in women of childbearing age receiving isotretinoin. N Engl J Med. 1995;333:101–6. doi: 10.1056/NEJM199507133330206. [DOI] [PubMed] [Google Scholar]
- 6.Pastuszak A, Koren G, Rieder MJ. Use of the Retinoid Pregnancy Prevention Program in Canada: patterns of contraception use in women treated with isotretinoin and etretinate. Reprod Toxicol. 1994;8:63–8. doi: 10.1016/0890-6238(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.Atanackovic G, Koren G. Fetal exposure to oral isotretinoin: failure to comply with the Pregnancy Prevention Program. CMAJ. 1999;160:1719–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Dai WS, LaBraico JM, Stern RS. Epidemiology of isotretinoin exposure during pregnancy. J Am Acad Dermatol. 1992;26:599–606. doi: 10.1016/0190-9622(92)70088-w. [DOI] [PubMed] [Google Scholar]
- 9.Rosa FW. Teratogenicity of isotretinoin. Lancet. 1983;2:513. doi: 10.1016/s0140-6736(83)90538-x. [DOI] [PubMed] [Google Scholar]
- 10.Dermatologic and Opthalmic Drugs Advisory Committee. Briefing Information. 18-19 September 2004 Available at http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3639b1.htm(last accessed: 15 December 2004).
- 11.Jones KL, Adams J, Chambers CD, Erickson JD, Lammer E, Polifka J. Isotretinoin and pregnancy. JAMA. 2001;285:1022–5. doi: 10.1001/jama.285.16.2079-a. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. International Classification of Diseases. Geneva: World Health Organization; 1977. 9th Revision (ICD-9) [Google Scholar]
- 13.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 14.Physicians Desk Reference. Product Labeling for Isotretinoin. Montvale, NJ: Medical Economics Company, Inc.; 2001. [Google Scholar]
- 15.Statistical Analysis Software Institute Inc. Cary, NC: SAS Inc; Version 8.2. [Google Scholar]
- 16.Abrishamchian AR, Khoury MJ, Calle EE. The contribution of maternal epilepsy and its treatment to the etiology of oral clefts: a population based case–control study. Genet Epidemiol. 1994;11:343–51. doi: 10.1002/gepi.1370110404. [DOI] [PubMed] [Google Scholar]
- 17.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158:1091–5. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 18.Kallen B, Tandberg A. Lithium and pregnancy. A cohort study on manic-depressive women. Acta Psychiatr Scand. 1983;68:134–9. doi: 10.1111/j.1600-0447.1983.tb06991.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosa FW. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674–7. doi: 10.1056/NEJM199103073241006. [DOI] [PubMed] [Google Scholar]
- 20.Samren EB, Van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46:739–46. [PubMed] [Google Scholar]
- 21.Sorensen HT, Nielsen GL, Olesen C, Larsen H, Steffensen FH, Schonheyder HC, Olsen J, Czeizel AE. Risk of malformations and other outcomes in children exposed to fluconazole in utero. Br J Clin Pharmacol. 1999;48:234–8. doi: 10.1046/j.1365-2125.1999.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thulstrup AM, Sorensen HT, Nielsen GL, Andersen L, Barrett D, Vilstrup H, Olsen J. EuroMap Study Group. Fetal growth and adverse birth outcomes in women receiving prescriptions for acetaminophen during pregnancy. Am J Perinatol. 1999;16:321–6. doi: 10.1055/s-2007-993879. [DOI] [PubMed] [Google Scholar]
- 23.West R, Sherman GJ, Downey W. A record linkage study of valproate and malformations in Saskatchewan. Can J Public Health. 1985;76:226–8. [PubMed] [Google Scholar]
- 24.Garbe E, LeLorier J, Boivin JF, Suissa S. Risk of ocular hypertension or open-angle glaucoma in elderly patients on oral glucocorticoids. Lancet. 1997;350:979–82. doi: 10.1016/S0140-6736(97)03392-8. [DOI] [PubMed] [Google Scholar]
- 25.Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA. 1997;277:722–7. [PubMed] [Google Scholar]
- 26.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case–control study. Arch Intern Med. 2000;160:2363–8. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 27.Robertson JA, Martinez LP, Gallegos S, Leen-Mitchell MJ, Garcia V, Neuman J, Carey JC. Accutane cases: a teratogen information service's approach. Teratology. 2002;66:1–2. doi: 10.1002/tera.10035. [DOI] [PubMed] [Google Scholar]