Abstract
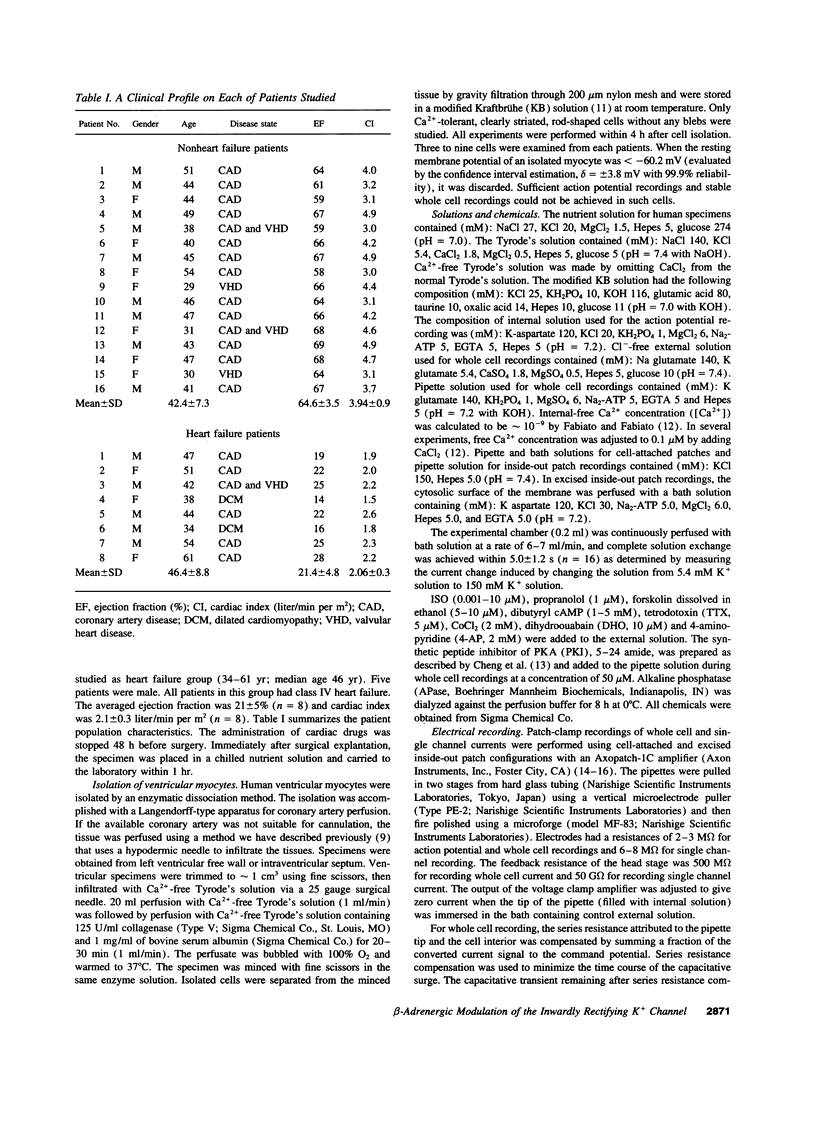
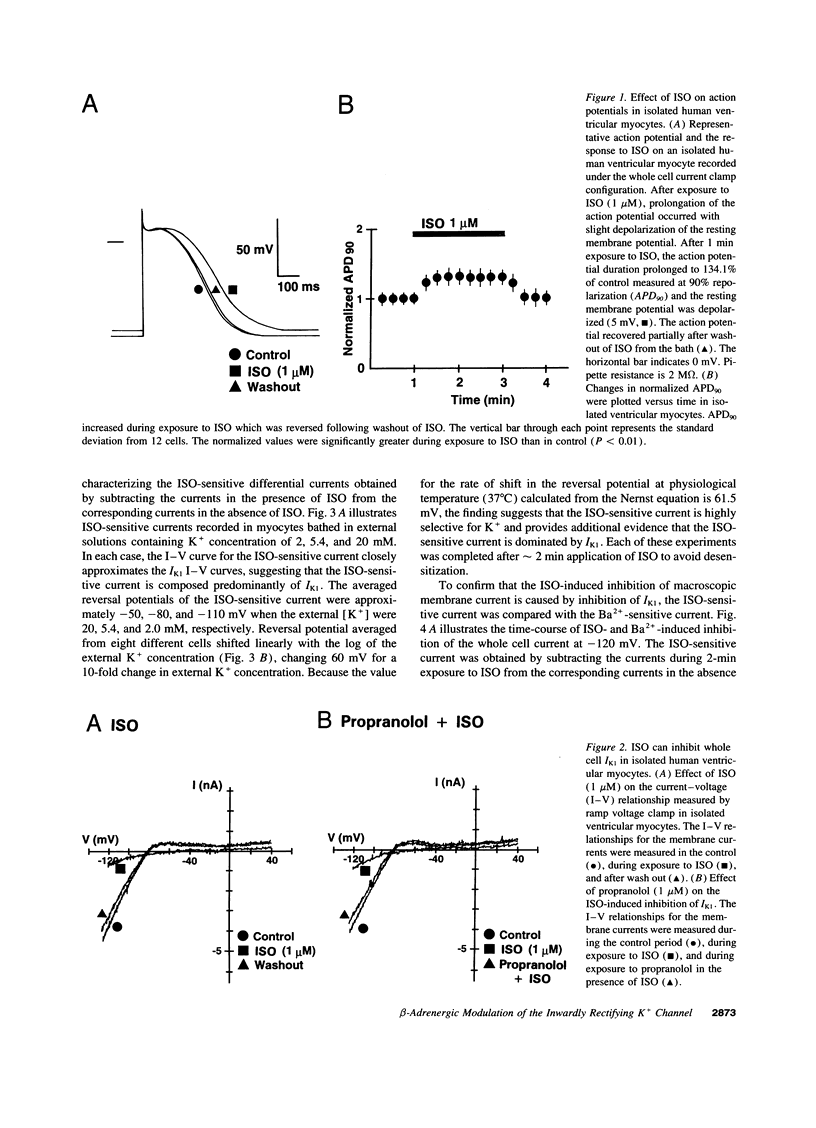
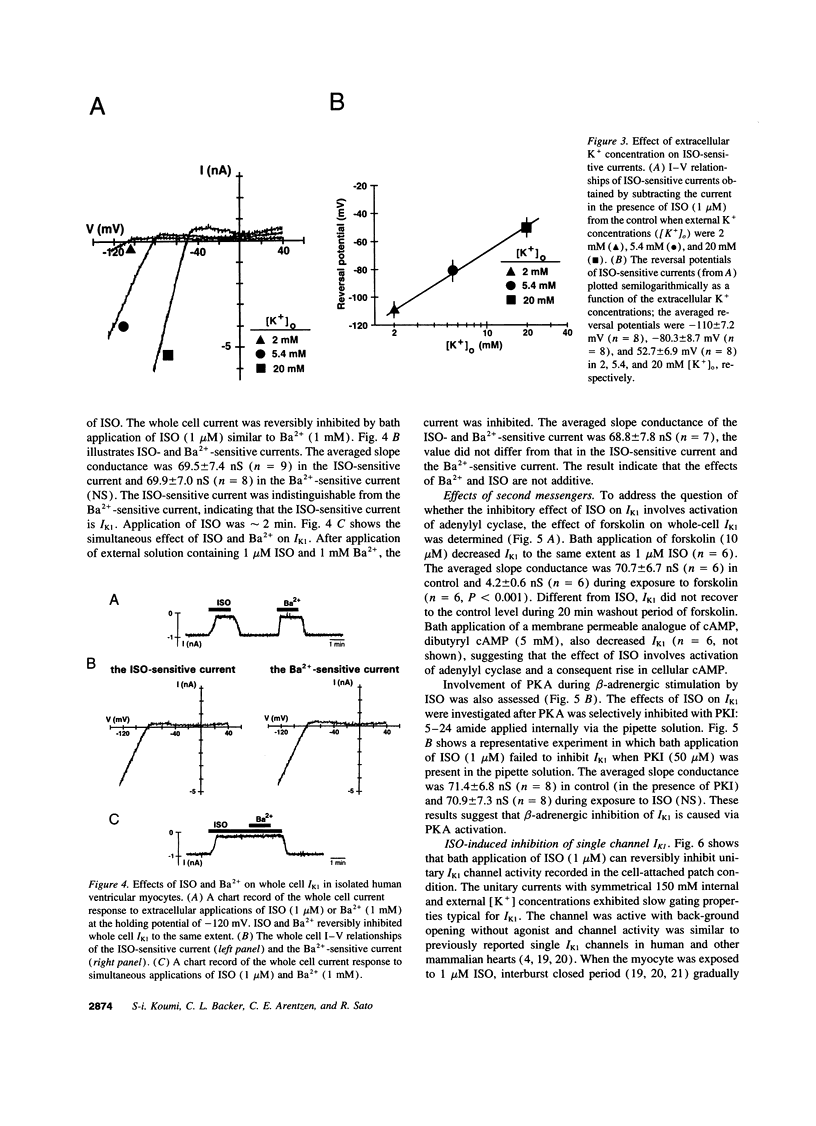
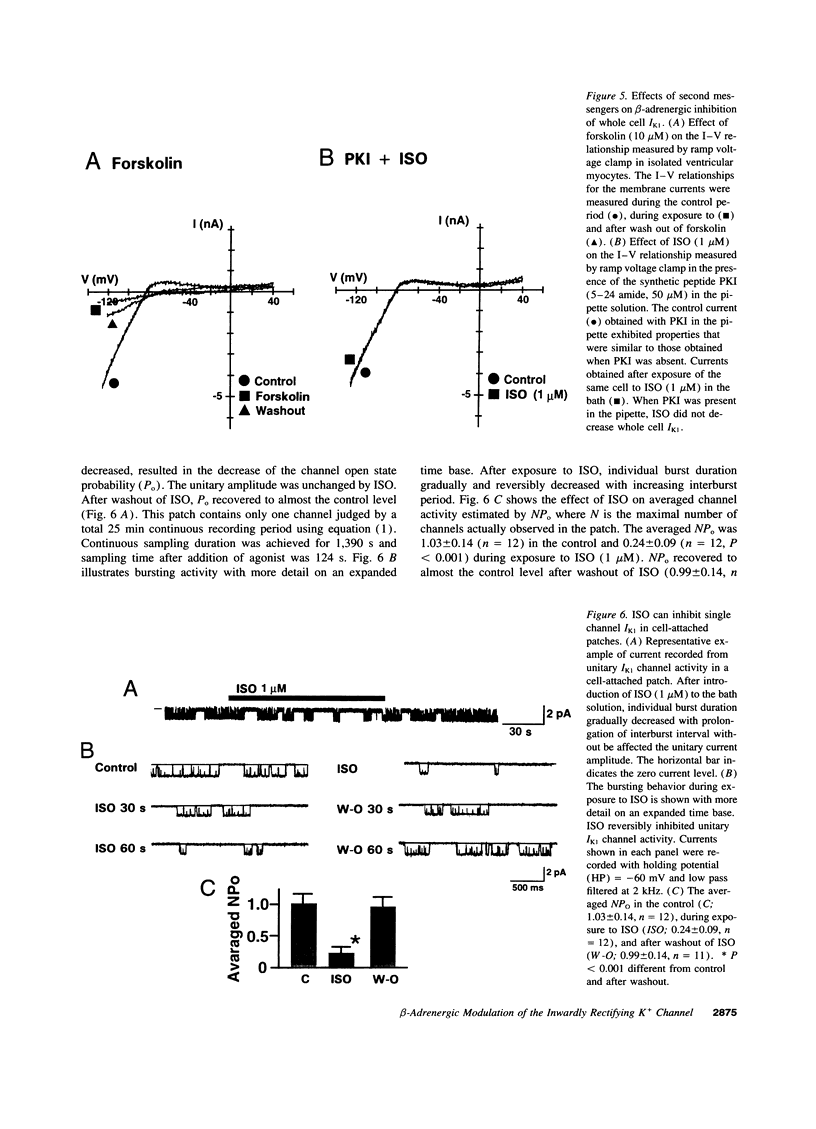
The beta-adrenergic modulation of the inwardly-rectifying K+ channel (IK1) was examined in isolated human ventricular myocytes using patch-clamp techniques. Isoproterenol (ISO) reversibly depolarized the resting membrane potential and prolonged the action potential duration. Under the whole-cell C1- -free condition, ISO applied via the bath solution reversibly inhibited macroscopic IdK1. The reversal potential of the ISO-sensitive current was shifted by approximately 60 mV per 10-fold change in the external K+ concentration and was sensitive to Ba2+. The ISO-induced inhibition of IK1 was mimicked by forskolin and dibutyrl cAMP, and was prevented by including a cAMP-dependent protein kinase (PKA) inhibitor (PKI) in the pipette solution. In single-channel recordings from cell-attached patches, bath applied ISO could suppress IK1 channels by decreasing open state probability. Bath application of the purified catalytic sub-unit of PKA to inside-out patches also inhibited IK1 and the inhibition could be antagonized by alkaline phosphatase. When beta-adrenergic modulation of IK1 was compared between ventricular myocytes isolated from the failing and the nonfailing heart, channel response to ISO and PKA was significantly reduced in myocytes from the failing heart. Although ISO inhibited IK1 in a concentration-dependent fashion in both groups, a half-maximal concentration was greater in failing (0.12 microM) than in nonfailing hearts (0.023 microM). These results suggest that IK1 in human ventricular myocytes can be inhibited by a PKA-mediated phosphorylation and the modulation is significantly reduced in ventricular myocytes from the failing heart compared to the nonfailing heart.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bennett P., McKinney L., Begenisich T., Kass R. S. Adrenergic modulation of the delayed rectifier potassium channel in calf cardiac Purkinje fibers. Biophys J. 1986 Apr;49(4):839–848. doi: 10.1016/S0006-3495(86)83713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Characteristics of calcium-current in isolated human ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1991 Aug;23(8):929–937. doi: 10.1016/0022-2828(91)90135-9. [DOI] [PubMed] [Google Scholar]
- Boyden P. A., Cranefield P. F., Gadsby D. C. Noradrenaline hyperpolarizes cells of the canine coronary sinus by increasing their permeability to potassium ions. J Physiol. 1983 Jun;339:185–206. doi: 10.1113/jphysiol.1983.sp014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986 Sep;59(3):297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Nargeot J. Electrophysiology of human cardiac cells. Cardiovasc Res. 1993 Oct;27(10):1713–1725. doi: 10.1093/cvr/27.10.1713. [DOI] [PubMed] [Google Scholar]
- Delmar M., Ibarra J., Davidenko J., Lorente P., Jalife J. Dynamics of the background outward current of single guinea pig ventricular myocytes. Ionic mechanisms of hysteresis in cardiac cells. Circ Res. 1991 Nov;69(5):1316–1326. doi: 10.1161/01.res.69.5.1316. [DOI] [PubMed] [Google Scholar]
- Denniss A. R., Marsh J. D., Quigg R. J., Gordon J. B., Colucci W. S. Beta-adrenergic receptor number and adenylate cyclase function in denervated transplanted and cardiomyopathic human hearts. Circulation. 1989 May;79(5):1028–1034. doi: 10.1161/01.cir.79.5.1028. [DOI] [PubMed] [Google Scholar]
- Eckberg D. L., Drabinsky M., Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971 Oct 14;285(16):877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Falk R. T., Cohen I. S. Membrane current following activity in canine cardiac Purkinje fibers. J Gen Physiol. 1984 May;83(5):771–799. doi: 10.1085/jgp.83.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. D., Copelas L., Gwathmey J. K., Phillips P., Warren S. E., Schoen F. J., Grossman W., Morgan J. P. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987 Feb;75(2):331–339. doi: 10.1161/01.cir.75.2.331. [DOI] [PubMed] [Google Scholar]
- Fermini B., Wang Z., Duan D., Nattel S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am J Physiol. 1992 Dec;263(6 Pt 2):H1747–H1754. doi: 10.1152/ajpheart.1992.263.6.H1747. [DOI] [PubMed] [Google Scholar]
- Fowler M. B., Laser J. A., Hopkins G. L., Minobe W., Bristow M. R. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation. 1986 Dec;74(6):1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Giles W., Nakajima T., Ono K., Shibata E. F. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J Physiol. 1989 Aug;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg R., Bristow M. R., Billingham M. E., Stinson E. B., Schroeder J. S., Harrison D. C. Study of the normal and failing isolated human heart: decreased response of failing heart to isoproterenol. Am Heart J. 1983 Sep;106(3):535–540. doi: 10.1016/0002-8703(83)90698-1. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Vereecke J., Carmeliet E. Three different potassium channels in human atrium. Contribution to the basal potassium conductance. Circ Res. 1990 May;66(5):1277–1286. doi: 10.1161/01.res.66.5.1277. [DOI] [PubMed] [Google Scholar]
- Higgins C. B., Vatner S. F., Eckberg D. L., Braunwald E. Alterations in the baroreceptor reflex in conscious dogs with heart failure. J Clin Invest. 1972 Apr;51(4):715–724. doi: 10.1172/JCI106865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. On the ionic mechanism underlying adrenergic-cholinergic antagonism in ventricular muscle. J Gen Physiol. 1982 Jan;79(1):69–86. doi: 10.1085/jgp.79.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Koumi S., Arentzen C. E., Backer C. L., Wasserstrom J. A. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994 Nov;90(5):2213–2224. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- Koumi S., Backer C. L., Arentzen C. E. Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation. 1995 Jul 15;92(2):164–174. doi: 10.1161/01.cir.92.2.164. [DOI] [PubMed] [Google Scholar]
- Koumi S., Sato R., Aramaki T. Characterization of the calcium-activated chloride channel in isolated guinea-pig hepatocytes. J Gen Physiol. 1994 Aug;104(2):357–373. doi: 10.1085/jgp.104.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S., Sato R., Hayakawa H. Modulation of the delayed rectifier K+ current by apamin in guinea-pig heart. Eur J Pharmacol. 1994 Aug 11;261(1-2):213–216. doi: 10.1016/0014-2999(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Koumi S., Sato R., Horikawa T., Aramaki T., Okumura H. Characterization of the calcium-sensitive voltage-gated delayed rectifier potassium channel in isolated guinea pig hepatocytes. J Gen Physiol. 1994 Jul;104(1):147–171. doi: 10.1085/jgp.104.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S., Wasserstrom J. A. Acetylcholine-sensitive muscarinic K+ channels in mammalian ventricular myocytes. Am J Physiol. 1994 May;266(5 Pt 2):H1812–H1821. doi: 10.1152/ajpheart.1994.266.5.H1812. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca modulates outward current through IK1 channels. J Membr Biol. 1990 Jun;116(1):41–45. doi: 10.1007/BF01871670. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DiFrancesco D. Intracellular Ca modulates K-inward rectification in cardiac myocytes. Pflugers Arch. 1989 Jan;413(3):322–324. doi: 10.1007/BF00583549. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Rasmussen H. H., Singer D. H., Ten Eick R. E. Inhibition of Na-K pump current in guinea pig ventricular myocytes by dihydroouabain occurs at high- and low-affinity sites. Circ Res. 1989 Jun;64(6):1063–1069. doi: 10.1161/01.res.64.6.1063. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Fozzard H. A. Adrenergic modulation of the transient outward current in isolated canine Purkinje cells. Circ Res. 1988 Jan;62(1):162–172. doi: 10.1161/01.res.62.1.162. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R., Hisatome I., Wasserstrom J. A., Arentzen C. E., Singer D. H. Acetylcholine-sensitive potassium channels in human atrial myocytes. Am J Physiol. 1990 Dec;259(6 Pt 2):H1730–H1735. doi: 10.1152/ajpheart.1990.259.6.H1730. [DOI] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Shah A., Cohen I. S., Rosen M. R. Stimulation of cardiac alpha receptors increases Na/K pump current and decreases gK via a pertussis toxin-sensitive pathway. Biophys J. 1988 Aug;54(2):219–225. doi: 10.1016/S0006-3495(88)82950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Drury T., Refsum H., Aldrete V., Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989 Dec;257(6 Pt 2):H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Sorota S., Siegal M. S., Hoffman B. F. The isoproterenol-induced chloride current and cardiac resting potential. J Mol Cell Cardiol. 1991 Oct;23(10):1191–1198. doi: 10.1016/0022-2828(91)90207-3. [DOI] [PubMed] [Google Scholar]
- Sorota S., Siegal M. S., Hoffman B. F. The isoproterenol-induced chloride current and cardiac resting potential. J Mol Cell Cardiol. 1991 Oct;23(10):1191–1198. doi: 10.1016/0022-2828(91)90207-3. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Knoth K. M., Walbridge J. A., Kroemer H., Roden D. M., Glover D. M. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991 Mar 1;5(3):331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- Tromba C., Cohen I. S. A novel action of isoproterenol to inactivate a cardiac K+ current is not blocked by beta and alpha adrenergic blockers. Biophys J. 1990 Sep;58(3):791–795. doi: 10.1016/S0006-3495(90)82422-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. A combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 1990 Oct;417(2):142–148. doi: 10.1007/BF00370691. [DOI] [PubMed] [Google Scholar]





