Abstract
What is already known about this subject
Cytochrome P450 (CYP) 3A4 plays a prominent role in the metabolism of many drugs, which in turn implies, that changes in the activity of this enzyme caused by, for example, co-administered drugs, might result in clinically significant drug interactions.
Roflumilast, a targeted PDE4 inhibitor in clinical development for the treatment of COPD and asthma, is partly metabolized by CYP3A4, and thus may have the potential to inhibit its activity.
What this study adds
The results of this study show that therapeutic steady-state concentrations of roflumilast and its active metabolite roflumilast N-oxide do not alter the disposition of the CYP3A substrate midazolam.
Therefore, roflumilast treatment has a low susceptibility to alter the clearance of drugs metabolized by CYP3A4.
Aims
The aim of this study was to investigate the effects of roflumilast, an investigational PDE4 inhibitor for the treatment of COPD and asthma, on the pharmacokinetics of the CYP3A probe drug midazolam and its major metabolites.
Methods
In an open, randomized (for midazolam treatment sequence) study, 18 healthy male subjects received single doses of midazolam (2 mg oral and 1 mg i.v., 1 day apart) alone, repeated doses of roflumilast (500 µg once daily for 14 days) alone, and repeated doses of roflumilast together with single doses of midazolam (2 mg oral and 1 mg i.v., 1 day apart).
Results
A comparison of clearance and peak and systemic exposure to midazolam following administration of roflumilast indicated no effect of roflumilast dosed to steady state on the pharmacokinetics of midazolam. Point estimates (90% CI) were 0.97 (0.84, 1.13) for the AUC of i.v. midazolam and 0.98 (0.82, 1.17) for that of oral midazolam with and without roflumilast.
Conclusions
Therapeutic steady state concentrations of roflumilast and its N-oxide do not alter the disposition of the CYP3A substrate midazolam in healthy subjects. This finding suggests that roflumilast is unlikely to alter the clearance of drugs that are metabolized by CYP3A4.
Keywords: CYP3A substrate, drug–drug interaction, midazolam, PDE4 inhibitor, pharmakokinetics, roflumilast
Introduction
Roflumilast is a new chemical entity, which is currently under development for the treatment of chronic obstructive pulmonary disease (COPD) and asthma [1]. Roflumilast is a highly potent and targeted inhibitor of phosphodiesterase 4 (PDE4), which exerts its anti-inflammatory properties by amplifying the intracellular cAMP signalling and which attenuates the inflammatory response mediated by various cells [2, 3]. Oral, once-daily doses of roflumilast 500 µg were shown to be well tolerated and clinically effective in the treatment of COPD and asthma [4–8].
Roflumilast is rapidly and almost completely absorbed after oral administration. The mean absolute bioavailability of a 500 µg immediate-release tablet is 79% and the terminal plasma half-life (t1/2) is 17 h [9]. In humans, a major pathway of metabolism of roflumilast is N-oxidation by cytochrome P450 (CYP) 3A4 (CYP3A4) and CYP1A2 to the pharmacologically active metabolite roflumilast N-oxide, which has similar intrinsic PDE4 inhibitory activity to the parent drug in vivo [2]. The t1/2 of roflumilast N-oxide is about 27 h and its area under the plasma concentration-time curve (AUC) exceeds that of the parent drug by about 10-fold [9]. Therefore, roflumilast N-oxide is considered to be the major contributor to the overall PDE4 inhibitory activity of the drug. The mean peak plasma concentrations (Cmax) of roflumilast and roflumilast N-oxide are reached at 1 h and 4 h, respectively, after drug intake.
Midazolam is a short-acting imidazo-benzodiazepine used as an anxiolytic and sedative/hypnotic before surgery and for induction of general anaesthesia. Midazolam is primarily metabolized through 1-hydroxylation and 4-hydroxylation by CYP3A4 and CYP3A5 and is widely used as a marker substrate for the activity of CYP3A [10–12].
Owing to its broad substrate specificity, inhibition and induction of CYP3A frequently leads to drug–drug interactions [13].
The aim of the present study was to assess the effect of steady-state roflumilast on CYP3A activity by evaluating the pharmacokinetics of midazolam when administered intravenously (i.v.) and orally.
Methods
Subjects and study design
This study was performed in healthy males using an open-label, randomized design and consisted of three periods. Period 1 (midazolam alone) consisted of 4 days with a randomized, single-dose administration of midazolam (2 mg oral and 1 mg i.v.) on days 1 and 3. Period 2 (roflumilast alone) consisted of 14 days of oral roflumilast at a dose of 500 µg given once daily. During period 3 (4 days), roflumilast was continued without a washout period for an additional 3 days and co-administered with midazolam as in period 1.
The administration of oral and i.v. midazolam was used to estimate the contribution of intestinal CYP3A to metabolism. Dose selection was based on that used in previous CYP3A interaction studies and on the overall tolerability reported for both dosage forms [14, 15]. In previous studies, a dose of roflumilast 500 µg given once daily was shown to be well tolerated and to be the anticipated recommended therapeutic dose [5].
The study was performed at the Clinical Unit of SocraTec R&D, Bad Berka, Germany. Healthy, male, nonsmoking, Caucasians were enrolled (n = 18, age range 19–44 years, median age 34 years, body weight 60–98 kg, median body weight 78 kg). All subjects completed the study according to the protocol. Before the start, each subject gave written informed consent to participate in the study. The study was approved by the Ethics Committee of the Chamber of Physicians Thüringen, Germany. The study conformed to the Declaration of Helsinki (Somerset West Amendment, 1996), Notes for Guidance on Good Clinical Practice (CGMP/ICH/135/95), and the German Drug Law (AMG).
Roflumilast was manufactured by ALTANA Pharma Oranienburg GmbH, Oranienburg, Germany. Midazolam (Dormicum® V 5 mg 5 ml−1) was purchased from Hoffmann-La Roche AG, Basel, Switzerland. The study drugs were administered in the morning between 07.00 h and 09.00 h. Tablets containing roflumilast 500 µg were swallowed with 240 ml water. For the oral administration of midazolam, 2 mg were taken from 5 ml ampoules containing 5 mg, dissolved in 5 ml NaCl 0.9% solution and swallowed with 240 ml water. For i.v. administration, 1 mg midazolam was taken from a 5 ml ampoule containing 5 mg, dissolved in 5 ml NaCl 0.9% solution, and infused over 1 min. Drugs were administered after the subjects had fasted overnight for at least 10 h.
Subjects reported to the clinical unit in the evening before the pharmacokinetic profiling days and remained there until completion of the 24 h period of blood sampling. On the following mornings, an indwelling cannula was inserted into a forearm vein to allow repeated blood sampling. Two hours after drug administration, the subjects were allowed water but were not allowed to eat until 4 h postdose. Subjects received standardized meals at specified times: lunch 4 h postdose (at about 12.00 h), snack 8 h postdose (at about 16.00 h), and dinner 12 h postdose (at about 20.00 h). During the study, the subjects were asked to avoid strenuous physical exercise and to go to bed and rise at their normal times. Subjects were asked not to ingest alcohol-containing food and beverages from 36 h prior to drug administration until the poststudy examination. A similar request was made with respect to xanthine-containing food and beverages such as chocolate, black and green tea, coffee, cola, chewing gum, and energy drinks from 36 h prior to the first period of the study until the last blood sample of the final treatment. Likewise, subjects were asked to avoid known dietary cytochrome P450 inhibitors and inducers such as grapefruit/pomelo, poppy seeds, Brussels sprouts, or broccoli from 7 days prior to the first drug administration until the last blood sample of the study [16–18] and to avoid fruit juice from time of confinement in the clinical unit until the last blood sample of the study.
Serial blood samples were collected at the following times: predose, 5 min, 10 min, 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 5 h, 6 h, 8 h, 10 h, 12 h, 16 h, and 24 h after midazolam administration. On the morning of days 11, 12, and 13 of roflumilast administration (period 2), blood was collected predose for the determination of trough plasma concentrations of roflumilast and roflumilast N-oxide and to monitor the achievement of steady-state.
Blood samples were collected in monovettes containing lithium-heparinate and were centrifuged within 20–30 min following blood withdrawal (1600 g, 15 min, +4 °C). Plasma was transferred into polypropylene plastic tubes and frozen at −20 °C or below within 60 min of collection.
Drug and metabolite analysis
Plasma concentrations of midazolam, 1-hydroxy midazolam, and 4-hydroxy midazolam, were determined using a high performance liquid chromatography assay with tandem mass spectrometry (HPLC-MS/MS) detection. The measurements were performed using an API3000 System (MDS Sciex, Concord ON, Canada).
In brief, 0.5 ml plasma was spiked with the internal standard (flurazepam for midazolam and bromazepam for the metabolites) and incubated with 50 µl (U) β-glucuronidase/arylsulphatase (Roche Diagnostics, Mannheim, Germany) in 0.5 ml sodium acetate, pH 5.0 at 37 °C for 3 h to cleave the conjugates of the midazolam metabolites. After the addition of 0.4 ml of 1 m sodium carbonate and 6 ml hexane : trichloromethane (2 : 1), the samples were shaken for 1 min and centrifuged. Before evaporation of the organic phase, 40 µl of DMSO were added. The residues were then reconstituted in 200 µl 50% (v : v) methanol : water. Analytes were separated using a Grom Saphir110 C18, 125 × 2 mm column with water : acetonitrile (76 : 24) containing 1% formic acid as the mobile phase. Midazolam was monitored in positive ion mode with the transition of m/z 326.1 to m/z 291.2, 1-hydroxy and 4-hydroxy midazolam with the transition of m/z 342.1 to m/z 324.1 and m/z 342.1 to m/z 325.1, respectively. The lower limit of quantification (LLOQ) of midazolam was 0.50 ng ml−1 and that for both midazolam metabolites was 0.25 ng ml−1. For midazolam a total of 13 batches, each containing at least three replicate quality controls at three different concentrations (1.25, 7.49, and 85.0 ng ml−1), were analyzed, yielding an accuracy ranging from 100.6 to 108.8% with an imprecision from 4.30 to 6.74%. For 1-hydroxy and 4-hydroxy midazolam a total of 12 batches, each containing at least three replicate quality controls at three different concentrations (0.625, 3.74, and 42.5 ng ml−1), were analyzed, yielding an accuracy ranging from 95.6 to 101.2% with an imprecision ranging from 3.76 to 8.35%.
Clinical assessment
Clinical assessment included monitoring of adverse events, clinical laboratory evaluation (blood chemistry, haematology, urine analysis), physical examination, monitoring of vital signs (blood pressure, pulse rate), and resting standard 12-lead electrocardiogram (ECG). Adverse events were monitored throughout the study. Laboratory evaluations were performed at the screening and poststudy examination visits. A complete physical examination including vital signs and a 12-lead ECG was performed at the screening and poststudy examinations. Vital signs and 12-lead ECG examinations were performed after the conclusion of each pharmacokinetic profiling day (about 24 h after study drug administration). ECG readings were taken and measurements of vital signs were performed after 5 min resting in supine position. The pulse rate was measured using an automatic blood pressure device (BOSO-medicus). The ECG parameters (PR, QRS, QT, QTc, HR) were obtained from the ECG printout and evaluated manually.
Data analysis
Pharmacokinetic parameter estimates were obtained using a noncompartmental analysis approach using WinNonlin professional, version 4.0.1 (Pharsight, Mountain View, CA, USA).
Plasma Cmax and tmax were obtained directly from the data. The slope of the terminal log-linear portion (λz) of each individual plasma concentration-time curve was determined by regression analysis. The apparent terminal plasma t1/2 was calculated from the expression ln(2)/λz. Estimates of AUC0–last were obtained using the linear trapezoidal rule up to the last sampling point. Estimates of total AUC (AUC0–∞ were derived from the expression AUC0–last + Clast/λz, where Clast is the last quantifiable plasma concentration. Clearance (CL) was calculated using the equation CL = dose/AUC(0,∞).
To compare AUC, Cmax, and CL between treatments, an analysis of variance (anova) on the ratios of the least-squares means (LSM) was performed using linear mixed effects modelling yielding point estimates (geometric means) with 90% confidence intervals (CI) (WinNonlin (version 4.0.1); Bioequivalence Wizard). A lack of interaction was concluded if the 90% CI were entirely within the standard bioequivalence range of 0.80–1.25 for AUC and CL and within the extended equivalence range of 0.70–1.43 for Cmax.
Data from the clinical assessments were analyzed descriptively.
Results
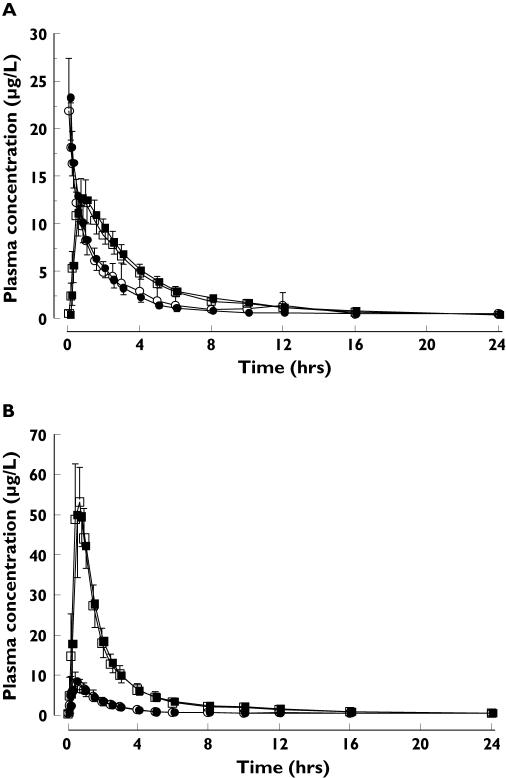
The plasma concentration-time profiles (mean ± SD) of midazolam and its 1-hydroxy metabolite after a single 1 mg i.v. dose of midazolam or a single oral 2 mg dose did not differ when given alone or with oral roflumilast 500 µg dosed to steady-state (Figure 1). Accordingly, the pharmacokinetic parameters for midazolam were comparable between the study treatments (Table 1). AUC, Cmax and tmax for 1-hydroxy midazolam and 4-hydroxy midazolam were also similar after administration of midazolam (i.v. and oral) alone and with roflumilast (data not shown).
Figure 1.
Plasma concentration-time profiles (mean ± SD) of midazolam (○, •) and 1-hydroxy midazolam (□, ▪) after a single i.v. dose of 1 mg (A) and a single oral dose of 2 mg midazolam (B) administered alone (open symbols) and with roflumilast 500 µg dosed to steady-state (closed symbols); n = 18 healthy male subjects
Table 1.
Pharmacokinetic data of midazolam after single doses (1 mg i.v. or 2 mg oral), administered alone (Reference), and concomitantly with roflumilast 500 µg dosed to steady-state (Test) as well as point estimates and 90% confidence interval (CI) for the Test : Reference ratios of AUC, CL and Cmax
| Midazolam i.v. (n = 18) | Midazolam oral (n = 18) | |||||
|---|---|---|---|---|---|---|
| Alone | With roflumilast | Ratio Test : Reference (90% CI) | Alone | With roflumilast | Ratio Test : Reference (90% CI) | |
| AUC0–∞ (µg l−1 h) | 36.3 | 35.2 | 97.0 | 17.9 | 17.6 | 97.8 |
| 26.1, 50.7 | 29.6, 42.0 | (83.5, 112.6) | 12.8, 25.2 | 12.9, 23.8 | (81.6, 117.4) | |
| AUC0–last (µg l−1 h) | 33.6 | 32.9 | 97.8 | 16.1 | 15.9 | 98.7 |
| 24.6, 45.9 | 27.3, 39.5 | (84.6, 113.0) | 11.3, 23.1 | 11.6, 21.8 | (81.6, 119.3) | |
| CL (l h−1) | 27.5 | 28.4 | 103.1 | 111.5 | 114.0 | 102.2 |
| 19.7, 38.4 | 23.8, 33.8 | (88.8, 119.8) | 79.5, 156.5 | 83.9, 154.7 | (85.2, 122.6) | |
| Cmax (µg l−1) | 22.0 | 22.9 | 104.1 | 9.0 | 8.7 | 97.2 |
| 17.4, 27.8 | 19.0, 27.6 | (92.4, 117.3) | 6.6, 12.2 | 6.4, 11.9 | (81.6, 115.7) | |
| tmax (h) | 0.1 | 0.1 | – | 0.5 | 0.5 | – |
| 0.1, 0.3 | 0.1, 0.2 | 0.3, 1.0 | 0.3, 1.0 | |||
| t1/2 (h) | 2.4 | 2.4 | – | 1.7 | 1.6 | – |
| 1.7, 3.5 | 1.7, 3.4 | 1.2, 2.4 | 1.2, 2.2 | |||
Data are presented as geometric mean and 68% range, tmax : median and min, max
The point estimates and 90% CI for the ratios of the AUC0–∞, AUC0–last, CL, and Cmax for midazolam were within the standard bioequivalence acceptance range of 0.80–1.25 (Table 1).
Sixteen subjects reported a total of 50 adverse events, which were either mild or moderate in intensity. During administration of midazolam alone, a total of eight adverse events were reported, six of which occurred after i.v. midazolam (dizziness, headache, dysarthria) and two after oral midazolam (headache and fatigue). During administration of roflumilast alone for 14 days, a total of 38 adverse events were reported. The most frequent ones were headache (8), myalgia (5), diarrhoea (4), fatigue (3), and frequent bowel movements (3). These symptoms represent known adverse effects of PDE4 inhibitors and are part of the expected adverse event profile of roflumilast. During concomitant administration of roflumilast and midazolam, four adverse events were reported (myalgia, peripheral neuropathy, sunburn, and fatigue). Overall, the most frequently observed symptom was headache with 11 events (22%) reported by eight (44%) subjects. All adverse events resolved completely and none resulted in withdrawal from the study. The results of the laboratory tests did not show any clinically relevant changes between the screening and poststudy examinations. Blood pressure, pulse rate, and standard ECG were also unaffected by the drug treatments.
Discussion
Roflumilast and its pharmacologically active metabolite roflumilast N-oxide are highly potent and targeted inhibitors of PDE4 with favourable pharmacokinetic properties. Current clinical experience illustrates that once-daily oral dosing with roflumilast 500 µg improves pulmonary function and markers of airway inflammation in patients with COPD and asthma [4, 6, 19, 20].
The primary objective of the present study was to assess the potential effects of roflumilast dosed to steady-state on CYP3A4, using midazolam as a marker substrate. Steady-state plasma concentrations of roflumilast and roflumilast N-oxide are achieved within 4 and 6 days, respectively, of once daily administration (ALTANA Pharma, unpublished data on file). Therefore, the selected 14-day treatment period was adequate to ensure the achievement of steady-state for both compounds.
Because midazolam has been shown to be cleared by hepatic and intestinal CYP3A, we investigated the effect of roflumilast on the pharmacokinetics of midazolam after both i.v. and oral administration.
Because midazolam is a drug with a medium to high hepatic extraction ratio, its systemic clearance is a function not only of CYP3A activity but also of hepatic blood flow [21]. Therefore, on the days when midazolam pharmacokinetics were determined, we standardized those factors that could have triggered substantial changes in liver blood flow, such as the amount, composition, and timing of food intake, as well as body position. The observed interindividual variability in the clearance of midazolam administered alone in the present study was comparable with previously reported data [11].
No effect of roflumilast on the disposition of midazolam was detected when it was given both i.v. and orally. The findings indicate that there was no change in hepatic or intestinal extraction of midazolam after co-administration of roflumilast. The pharmacokinetic parameters for midazolam when given alone were similar to those reported previously [22, 23].
In conclusion, the results of this study have shown that therapeutic steady-state concentrations of roflumilast and roflumilast N-oxide do not alter the disposition of the CYP3A substrate midazolam in healthy males. This finding suggests that roflumilast has a low susceptibility to alter the clearance of drugs metabolized by CYP3A4 (for example, corticosteroids).
Acknowledgments
The study was sponsored by ALTANA Pharma AG, Konstanz, Germany.
The authors thank Mrs Maria Anschütz (SocraTec R&D GmbH, Oberursel, Germany) for excellent organizational support during the study and pharm-analyt Labor GmbH, Baden, Austria for determination of plasma concentrations of midazolam and its hydroxy-metabolites. The authors thank Dr Angela Schilling and Dr Kathy B. Thomas (Department of Medical Writing, ALTANA Pharma AG, Konstanz, Germany) for helpful suggestions during the prepaaration of the manuscript.
References
- 1.Reid P. Roflumilast Altana Pharma. Curr Opin Invest Drugs. 2002;3:1165–70. [PubMed] [Google Scholar]
- 2.Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297:280–90. [PubMed] [Google Scholar]
- 3.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
- 4.Bousquet J, Aubier M, Sastre J, Izquierdo JL, Adler LM, Hofbauer P, Rost KD, Harnest U, Kroemer B, Albrecht A, Bredenbroker D. Comparison of roflumilast, an oral anti-inflammatory, with beclomethasone dipropionate in the treatment of persistent asthma. Allergy. 2006;61:72–8. doi: 10.1111/j.1398-9995.2005.00931.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–75. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 6.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 7.Timmer W, Leclerc V, Birraux G, Neuhäuser M, Hatzelmann A, Bethke T, Wurst W. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. J Clin Pharmacol. 2002;42:297–303. doi: 10.1177/00912700222011328. [DOI] [PubMed] [Google Scholar]
- 8.van Schalkwyk E, Strydom K, Williams Z, Venter L, Leichtl S, Schmid-Wirlitsch C, Bredenbroker D, Bardin PG. Roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, attenuates allergen-induced asthmatic reactions. J Allergy Clin Immunol. 2005;116:292–8. doi: 10.1016/j.jaci.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 9.David M, Zech K, Seiberling M, Weimar C, Bethke TD. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavailability. J Allergy Clin Immunol. 2004;113:S220–S221. [Google Scholar]
- 10.Kim JS, Nafziger AN, Tsunoda SM, Choo EE, Streetman DS, Kashuba AD, Kulawy RW, Beck DJ, Rocci ML, Jr, Wilkinson GR, Greenblatt DJ, Bertino JS., Jr Limited sampling strategy to predict AUC of the CYP3A phenotyping probe midazolam in adults: application to various assay techniques. J Clin Pharmacol. 2002;42:376–82. [PubMed] [Google Scholar]
- 11.Rogers JF, Rocci ML, Jr, Haughey DB, Bertino JS., Jr An evaluation of the suitability of intravenous midazolam as an in vivo marker for hepatic cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:153–8. doi: 10.1067/mcp.2003.23. [DOI] [PubMed] [Google Scholar]
- 12.Streetman DS, Kashuba AD, Bertino JS, Jr, Kulawy R, Rocci ML, Jr, Nafziger AN. Use of midazolam urinary metabolic ratios for cytochrome P450 3A (CYP3A) phenotyping. Pharmacogenetics. 2001;11:349–55. doi: 10.1097/00008571-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Metabolism and Transport. Drug Interaction Database. University of Washington; 2006. http://www.druginteractioninfo.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tateishi T, Watanabe M, Nakura H, Asoh M, Shirai H, Mizorogi Y, Kobayashi S, Thummel KE, Wilkinson GR. CYP3A activity in European American and Japanese men using midazolam as an in vivo probe. Clin Pharmacol Ther. 2001;69:333–9. doi: 10.1067/mcp.2001.115447. [DOI] [PubMed] [Google Scholar]
- 15.Thummel KE, O’Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, Wilkinson GR. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 16.Goho C. Oral midazolam–grapefruit juice drug interaction. Pediatr Dent. 2001;23:365–6. [PubMed] [Google Scholar]
- 17.Kall MA, Clausen J. Dietary effect on mixed function P450 1A2 activity assayed by estimation of caffeine metabolism in man. Hum Exp Toxicol. 1995;14:801–7. doi: 10.1177/096032719501401004. [DOI] [PubMed] [Google Scholar]
- 18.Kall MA, Vang O, Clausen J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: evaluation of caffeine, oestrone and chlorzoxazone metabolism. Carcinogenesis. 1996;17:793–9. doi: 10.1093/carcin/17.4.793. [DOI] [PubMed] [Google Scholar]
- 19.Grootendorst DC, Gauw SA, Sterk PJ, Bethke TD, Hospers JJ, Hiemstra PS, Rabe KF. Treatment with the PDE4 inhibitor roflumilast reduces sputum neutrophil and eosinophil numbers in patients with COPD. Proc Am Thorac Soc. 2005;2:A543. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hargreave FE, Boulet LP, Cartier A, Efhimiadis A, Wenzel SE, Teichmann P, Hospers JJ, Knoerzer D, Bethke TD. The effect of roflumilast on sputum eosinophils in patients with asthma. Ann Allergy Asthma Immunol. 2005;96:203–4. [Google Scholar]
- 21.Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 22.Floyd MD, Gervasini G, Masica AL, Mayo G, George ALJ, Bhat K, Kim RB, Wilkinson GR. Genotype–phenotype associations for common CYP3A4 and CYP3A5 variants in the basal and induced metabolism of midazolam in European-and African-American men and women. Pharmacogenetics. 2003;13:595–606. doi: 10.1097/00008571-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wandel C, Witte JS, Hall JM, Stein CM, Wood AJJ, Wilkinson GR. CYP3A activity in African American and European American men: Population differences and functional effect of the CYP3A4*1B 5′–promoter region polymorphism. Clin Pharmacol Ther. 2000;68:82–91. doi: 10.1067/mcp.2000.108506. [DOI] [PubMed] [Google Scholar]