Abstract
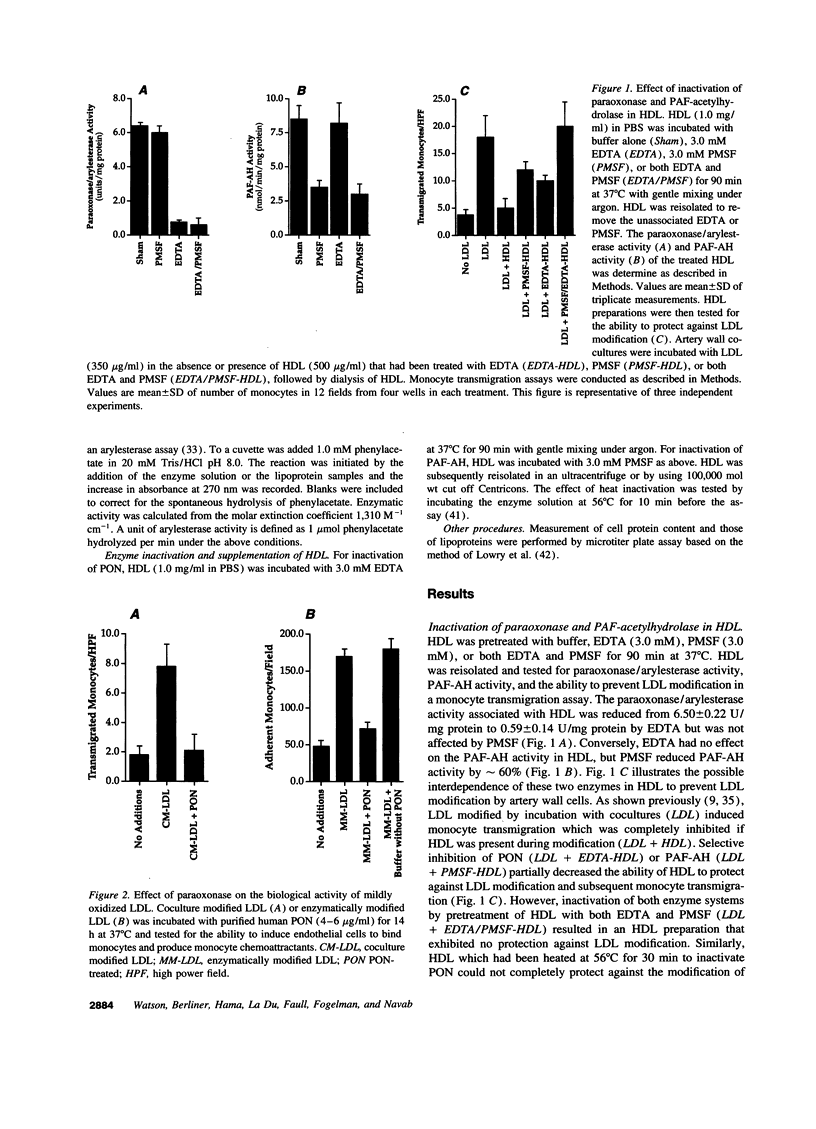
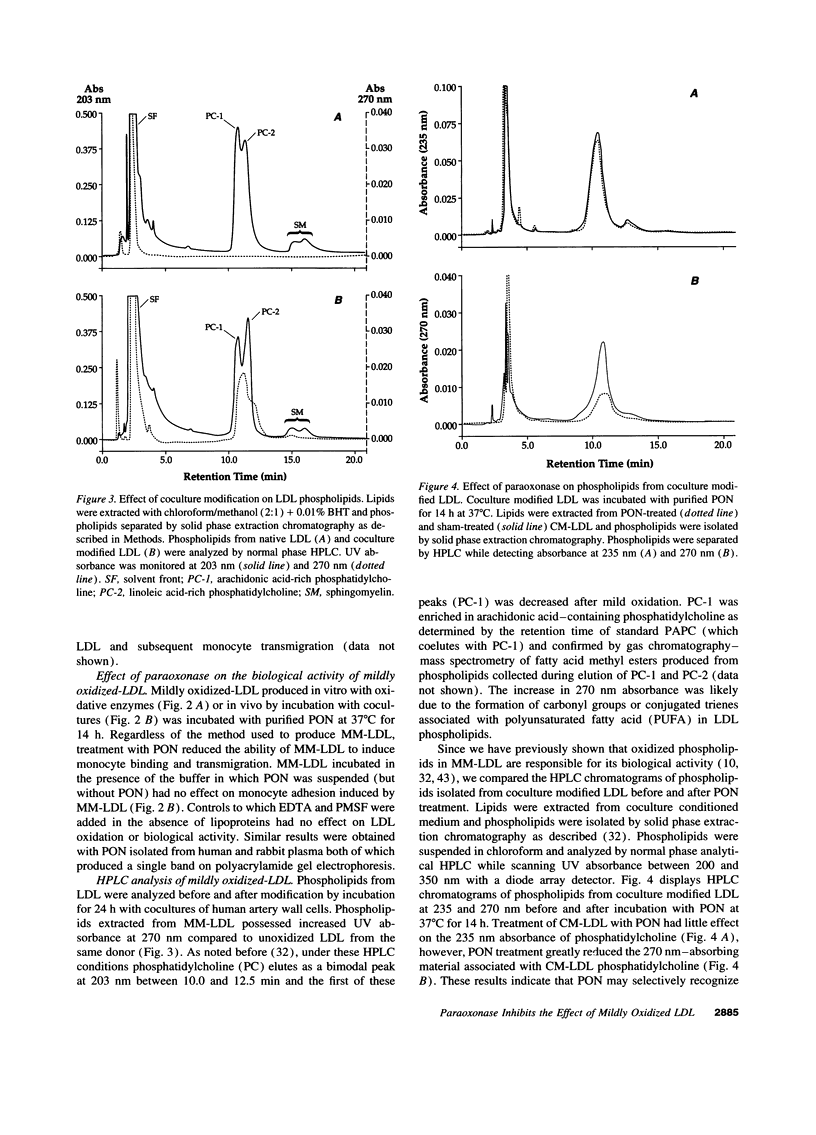
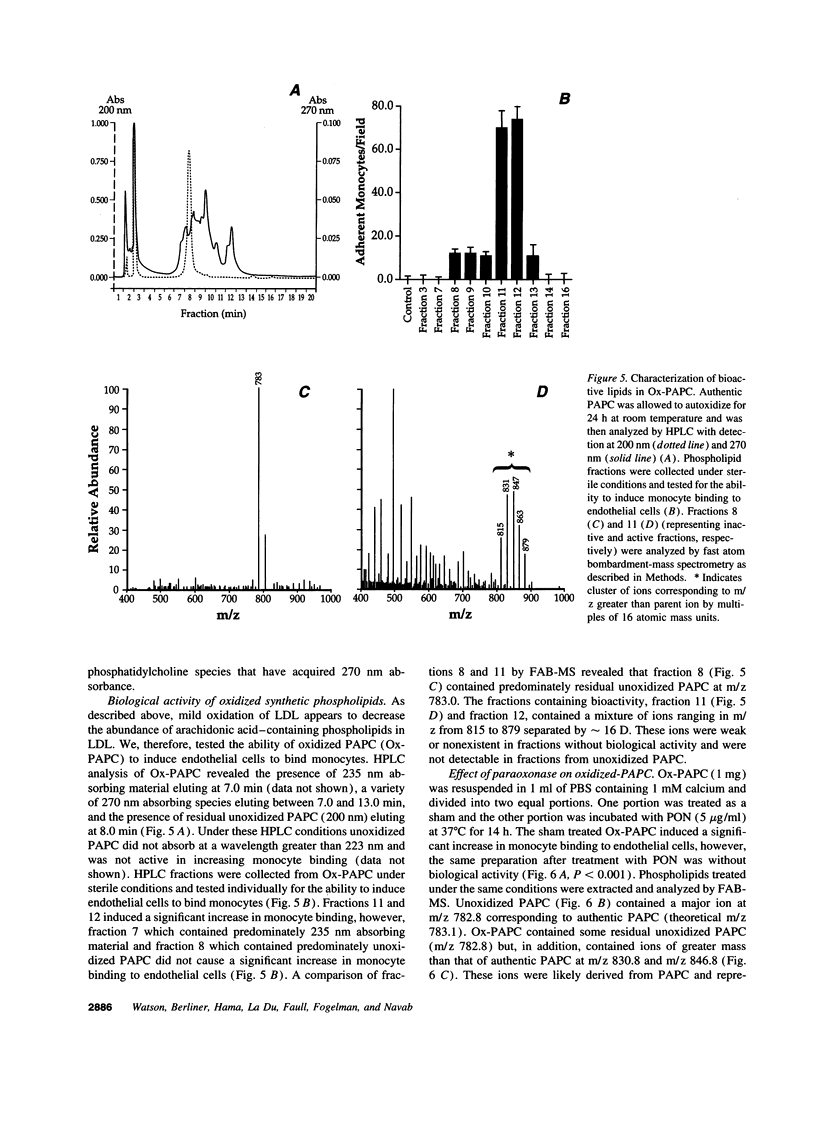
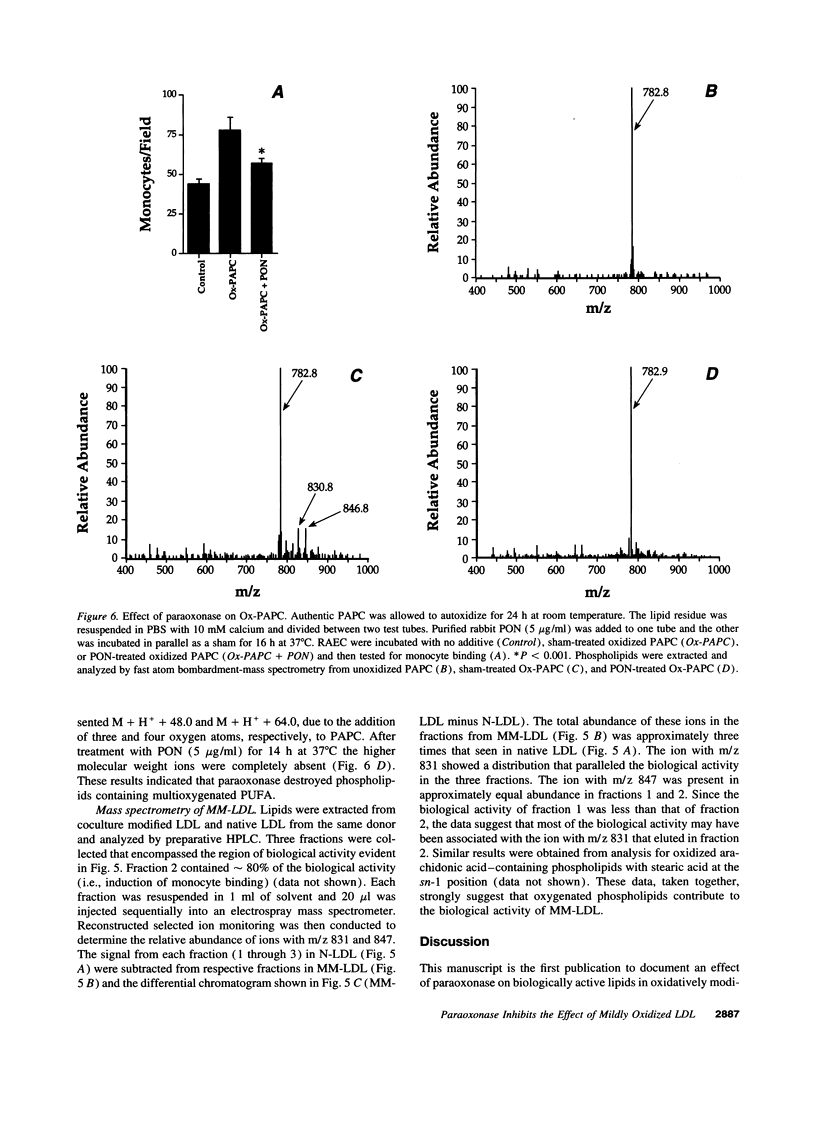
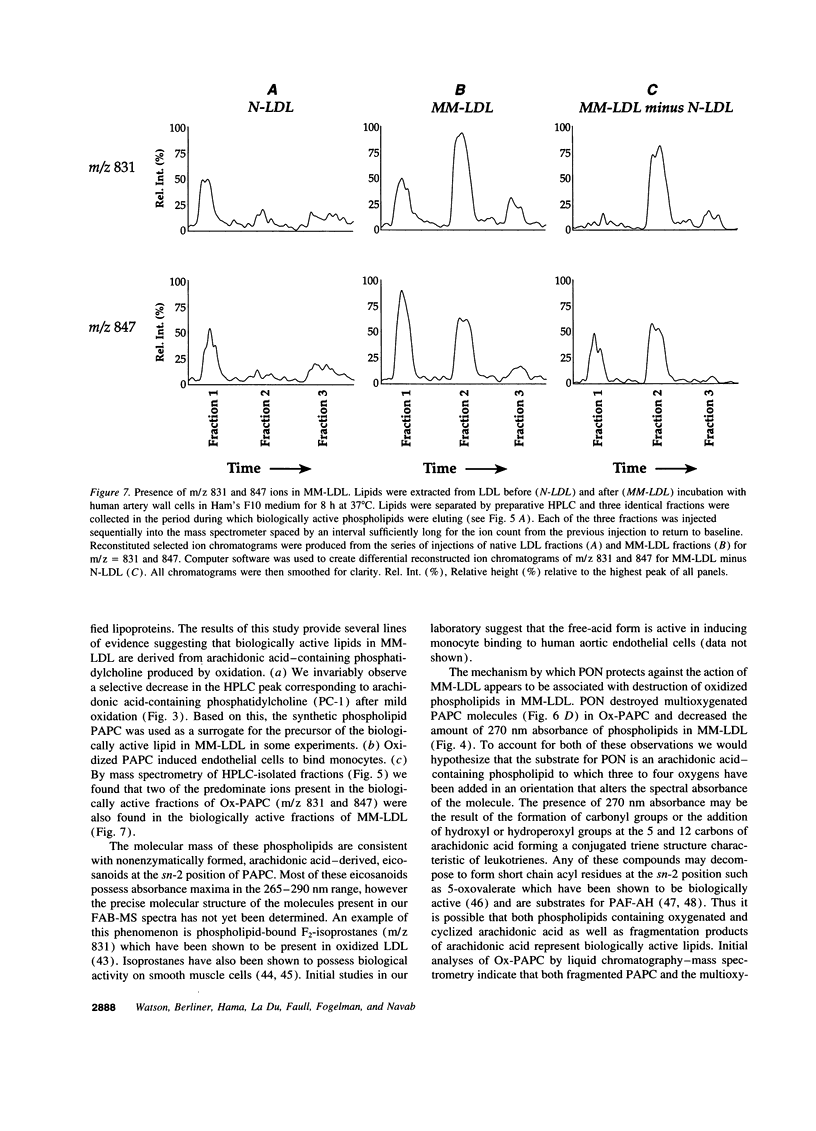
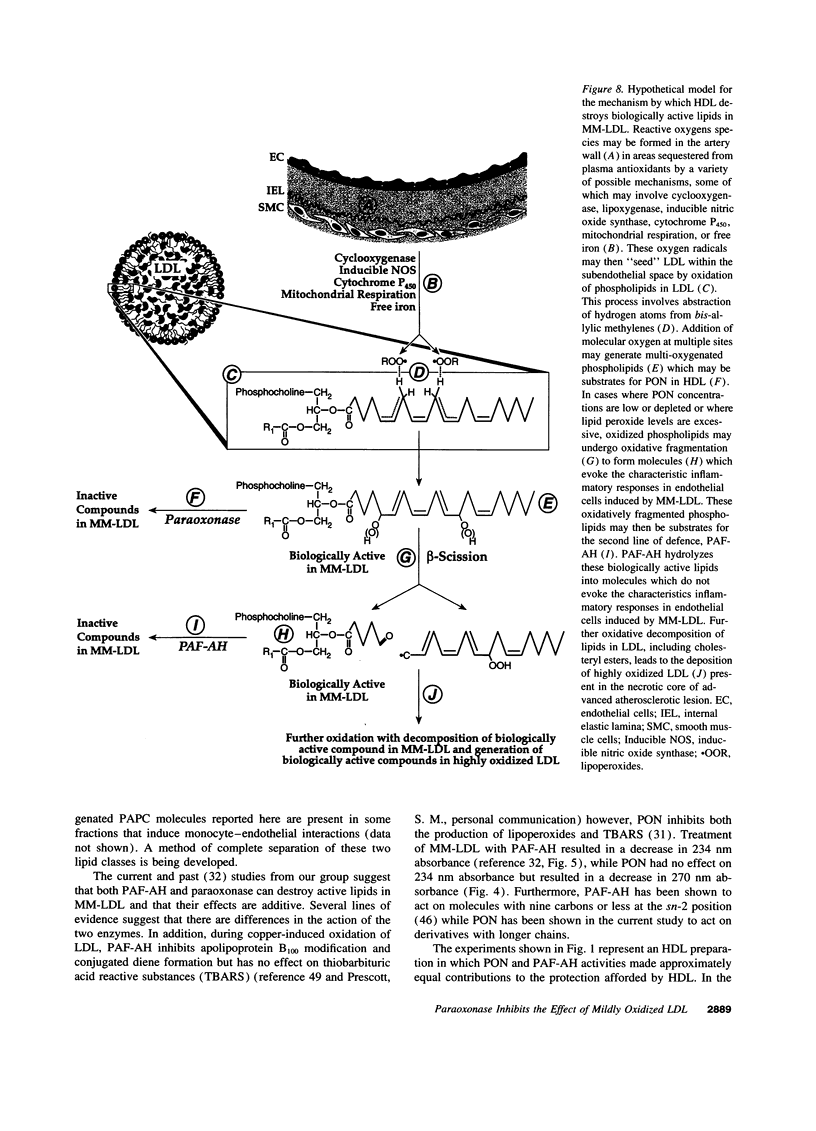
Our group has previously demonstrated that oxidized phospholipids in mildly oxidized LDL (MM-LDL) produced by oxidation with lipoxygenase, iron, or cocultures of artery wall cells increase monocyte-endothelial interactions and this sequence of events is blocked by HDL. To obtain further insight into the mechanism by which HDL abolishes the activity of MM-LDL we investigated the effect of the HDL-associated ester hydrolase paraoxonase (PON). Treatment of MM-LDL with purified PON significantly reduced the ability of MM-LDL to induce monocyte-endothelial interactions. Inactivation of PON by pretreating HDL with heat or EDTA reduced the ability of HDL to inhibit LDL modification. HPLC analysis of phospholipids isolated from MM-LDL before and after treatment with purified PON showed that the 270 nm absorbance of phospholipids was decreased, while no effect was observed on 235 nm absorbance. Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (Ox-PAPC) and specific fractions of Ox-PAPC isolated by HPLC induced the same monocyte-endothelial interactions as did MM-LDL. Biologically active and inactive HPLC fractions of Ox-PAPC were compared by fast atom bombardment-mass spectrometry which revealed that active fractions possessed ions with a mass to charge [correction of change] ratio greater than native PAPC by multiples of 16 D suggesting the addition of 3 and 4 oxygen atoms to PAPC. Comparison of Ox-PAPC by fast atom bombardment-mass spectrometry before and after PON treatment showed that PON destroyed these multi-oxygenated molecules found in biologically active fractions of Ox-PAPC. These results suggest that PON in HDL may protect against the induction of inflammatory responses in artery wall cells by destroying biologically active lipids in mildly oxidized LDL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banerjee M., Kang K. H., Morrow J. D., Roberts L. J., Newman J. H. Effects of a novel prostaglandin, 8-epi-PGF2 alpha, in rabbit lung in situ. Am J Physiol. 1992 Sep;263(3 Pt 2):H660–H663. doi: 10.1152/ajpheart.1992.263.3.H660. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Atherosclerosis. Scavenging for receptors. Nature. 1990 Feb 8;343(6258):508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Chisolm G. M. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991 Jan;32(1):63–70. [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989 Mar 15;142(6):1963–1969. [PubMed] [Google Scholar]
- Chemnitius J. M., Losch H., Losch K., Zech R. Organophosphate detoxicating hydrolases in different vertebrate species. Comp Biochem Physiol C. 1983;76(1):85–93. doi: 10.1016/0742-8413(83)90048-8. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Elahi F., Sykes K., Van Lenten B. J., Territo M. C., Berliner J. A. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988 Sep;29(9):1243–1247. [PubMed] [Google Scholar]
- Fukunaga M., Takahashi K., Badr K. F. Vascular smooth muscle actions and receptor interactions of 8-iso-prostaglandin E2, an E2-isoprostane. Biochem Biophys Res Commun. 1993 Sep 15;195(2):507–515. doi: 10.1006/bbrc.1993.2075. [DOI] [PubMed] [Google Scholar]
- Gan K. N., Smolen A., Eckerson H. W., La Du B. N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991 Jan-Feb;19(1):100–106. [PubMed] [Google Scholar]
- Gopaul N. K., Nourooz-Zadeh J., Mallet A. I., Anggård E. E. Formation of F2-isoprostanes during aortic endothelial cell-mediated oxidation of low density lipoprotein. FEBS Lett. 1994 Jul 18;348(3):297–300. doi: 10.1016/0014-5793(94)00628-8. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989 Jan;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Abbott C. A., Durrington P. N. Is paraoxonase related to atherosclerosis. Chem Biol Interact. 1993 Jun;87(1-3):161–171. doi: 10.1016/0009-2797(93)90038-z. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Durrington P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991 Jul 29;286(1-2):152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Walker C. H., Carlson L. A. Low A-esterase activity in serum of patients with fish-eye disease. Clin Chem. 1987 Apr;33(4):587–588. [PubMed] [Google Scholar]
- Maier J. A., Barenghi L., Pagani F., Bradamante S., Comi P., Ragnotti G. The protective role of high-density lipoprotein on oxidized-low-density-lipoprotein-induced U937/endothelial cell interactions. Eur J Biochem. 1994 Apr 1;221(1):35–41. doi: 10.1111/j.1432-1033.1994.tb18712.x. [DOI] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Stevenson L. W., Drinkwater D. C., Laks H., Fogelman A. M. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J Clin Invest. 1988 Dec;82(6):1853–1863. doi: 10.1172/JCI113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F., Fang Z. T., Fogelman A. M., Andalibi A., Territo M. C., Berliner J. A. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993 Jul;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J., Fong L. G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990 May 22;1044(2):275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Saha N., Roy A. C., Teo S. H., Tay J. S., Ratnam S. S. Influence of serum paraoxonase polymorphism on serum lipids and apolipoproteins. Clin Genet. 1991 Oct;40(4):277–282. doi: 10.1111/j.1399-0004.1991.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Simeon V., Pavković E. Heat inactivation of paraoxonase and arylesterase activities in human and rabbit serum. Chem Biol Interact. 1993 Jun;87(1-3):103–107. doi: 10.1016/0009-2797(93)90030-3. [DOI] [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Prescott S. M. Platelet-activating factor acetylhydrolase from human plasma. Methods Enzymol. 1990;187:344–357. doi: 10.1016/0076-6879(90)87041-z. [DOI] [PubMed] [Google Scholar]
- Stafforini D. M., Zimmerman G. A., McIntyre T. M., Prescott S. M. The platelet-activating factor acetylhydrolase from human plasma prevents oxidative modification of low-density lipoprotein. Trans Assoc Am Physicians. 1992;105:44–63. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stremler K. E., Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem. 1991 Jun 15;266(17):11095–11103. [PubMed] [Google Scholar]
- Stremler K. E., Stafforini D. M., Prescott S. M., Zimmerman G. A., McIntyre T. M. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J Biol Chem. 1989 Apr 5;264(10):5331–5334. [PubMed] [Google Scholar]
- Watson A. D., Navab M., Hama S. Y., Sevanian A., Prescott S. M., Stafforini D. M., McIntyre T. M., Du B. N., Fogelman A. M., Berliner J. A. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995 Feb;95(2):774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L. The oxidation hypothesis of atherosclerosis. Lancet. 1994 Sep 17;344(8925):793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]



