Abstract
The amyloid precursor protein (APP) undergoes sequential cleavages to generate various polypeptides, including the amyloid-β protein (Aβ), which forms amyloid plaques in Alzheimer's disease (AD), secreted APPα (sAPPα) which enhances memory, and the APP intracellular domain (AICD), which has been implicated in the regulation of gene transcription and calcium signaling. The β-site APP cleaving enzyme 1 (BACE1) cleaves APP in an activity-dependent manner to form Aβ, AICD, and secreted APPβ. Because this neural activity was shown to diminish synaptic transmission in vitro [Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) Neuron 37:925–937], the prevailing notion has been that this pathway diminishes synaptic function. Here we investigated the role of this pathway in vivo. We studied transgenic mice overproducing APP that do not develop AD pathology or memory deficits but instead exhibit enhanced spatial memory. We showed enhanced synaptic plasticity in the hippocampus that depends on prior synaptic activity. We found that the enhanced memory and synaptic plasticity are abolished by the ablation of one or both copies of the BACE1 gene, leading to a significant decrease in AICD but not of any other APP cleavage products. In contrast to the previously described negative effect of BACE1-mediated cleavage of APP on synaptic function in vitro, our in vivo work indicates that BACE1-mediated cleavage of APP can facilitate learning, memory, and synaptic plasticity.
Keywords: transgenic, learning
Amyloid precursor protein (APP) undergoes proteolysis at α-, β-, γ-, and ε-secretase sites to release amino-terminal, internal, and carboxyl-terminal polypeptides, including APP intracellular domain (AICD) [another name for carboxyl-terminal fragment ε (CTFε)] (Fig. 1a). Activity-related regulation of APP cleavage has been proposed at the α- and β-secretase sites (1, 2), suggesting a physiological role of APP and APP cleavage in the brain. The activation of M1 and M3 muscarinic acetylcholine receptors stimulates α-secretase activity and releases secreted APPα (sAPPα) (1), which has been reported to enhance synaptic plasticity and memory (3, 4). Synaptic activity triggers the regulated cleavage of APP through another pathway, by way of β-site APP cleaving enzyme 1 (BACE1), followed by γ- and ε-secretase, releasing amyloid-β protein (Aβ) (2, 5) and AICD. Genetic ablation of APP, BACE1, or presenilin-1, the catalytic component of the γ- and ε-secretase complexes, profoundly reduces or eliminates Aβ and AICD production and can lead to impaired memory in mice (6–9). Although the molecular basis of impaired memory in mice lacking APP, BACE1, or presenilin-1 has not been defined, the ablation studies implicate a possible role for Aβ or AICD in normal brain function.
Fig. 1.
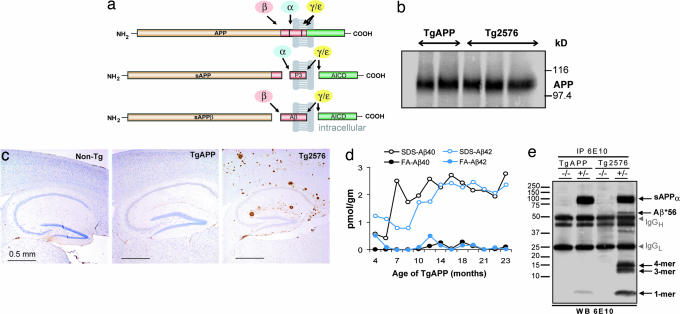
TgAPP mice do not accumulate Aβ or amyloid plaques with age. (a) Schematic diagram of APP, a transmembrane protein, and its major proteolytic products. Cleavage of APP by α-, β-, γ-, and ε-secretases produces secreted amino-terminal fragments, carboxyl-terminal polypeptides, and Aβ. (b) Western blot analysis of whole-brain homogenates probed with 6E10 antibodies shows ≈6 units of wild-type human APP in TgAPP brain, similar to the level of mutated human APP expressed in Tg2576 mice. (c) Immunohistology performed with 4G8 antibodies on hippocampal and overlying cortical brain areas shows no amyloid plaques in 19-month non-Tg or TgAPP mice, in contrast to abundant plaques in 18-month Tg2576 mice. (d) ELISAs of SDS-soluble (SDS) and SDS-insoluble, formic acid-soluble (FA) Aβ40 and Aβ42 species show low levels throughout life. (e) Immunoprecipitation (IP) with 6E10 antibodies and Western blotting (WB) with 6E10 antibodies of soluble extracellular-enriched brain extracts from 12-month TgAPP (+/−) and non-Tg (−/−) littermates and 13-month Tg2576 and non-Tg littermates shows Aβ*56 only in the Tg2576 mouse. IgGH and IgGL are the heavy and light chains of immunoglobulins, respectively. Arrows indicate bands comigrating with tetramers (4-mer), trimers (3-mer), and monomers (1-mer) of Aβ. Bars are means; error bars are SEM.
Here we address the neuronal function of APP and its cleavage products by characterizing transgenic mice that over express APP but do not develop Alzheimer-like pathology or memory deficits. In mice expressing different amounts of BACE1, we found that conditions that lead to elevated levels of AICD, but not Aβ or sAPPα, correlate with enhanced memory and the potentiation of “primed” forms of synaptic plasticity that depend on prior synaptic activity. The behavioral and synaptic phenotype observed in the APP transgenic mice was abolished by the elimination of one or both copies of the BACE1 gene. These data suggest the existence of a physiological mechanism underlying learning and memory that involves activity-dependent cleavage of APP by BACE1. In contrast to a previously described negative effect of BACE1-mediated cleavage of APP on synaptic function by using in vitro assays (2), our in vivo work indicates that BACE1-mediated cleavage of APP can exert salutary effects on brain and neuronal function. AICD correlates with the enhanced memory and plasticity and is therefore a potential mediator of the effects. However, the beneficial effects do not appear to be mediated by Aβ or sAPPβ.
Results
To investigate a potential physiological role of APP in the brain, we took advantage of the Tg5469 transgenic mouse line, called TgAPP here, which expresses ≈6 units (1 unit = level of endogenous murine APP) of the 695-aa isoform of wild-type human APP in the brain (Fig. 1b) and shows enhanced spatial reference memory in the Morris water maze (10). Aged TgAPP mice showed no amyloid plaques (Fig. 1c) and did not accumulate Aβ or Aβ*56, a 56-kDa Aβ assembly that impairs memory (11, 12) (Fig. 1 d and e), in contrast to Tg2576 mice (13), which express mutant human APP at levels similar to wild-type APP in TgAPP mice (Fig. 1 b, c, and e) and show memory deficits.
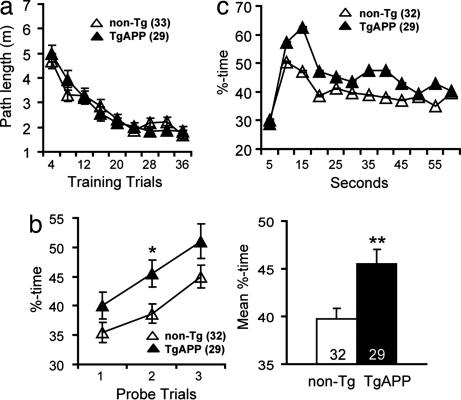
To confirm that TgAPP mice exhibit enhanced spatial reference memory, we extended our analysis of their behavior in the Morris water maze. TgAPP and nontransgenic (non-Tg) littermates found the submerged target platform equally quickly (Fig. 2a), indicating similar navigational abilities during the training period. To increase the memory burden of the testing procedure, we periodically removed the platform and observed the pattern of swimming for 60 seconds after an overnight delay. The occupancy of the target quadrant was significantly higher in TgAPP mice than in non-Tg littermates (Fig. 2b). The increase in target quadrant occupancy in TgAPP mice was not due to a reduction in extinction, because TgAPP and non-Tg mice showed similar decreases in target quadrant occupancy when the occupancy of the target quadrant was examined in 5-second intervals (Fig. 2c). Thus, TgAPP mice showed better retention of the target site location than non-Tg mice.
Fig. 2.
Enhanced spatial reference memory in TgAPP mice. (a) Hidden target. No difference in the acquisition of spatial memory in 5.8- to 12.4-month-old TgAPP and non-Tg littermates was found. (b) (Left) TgAPP mice show higher target quadrant occupancies during probe trials. (Right) The mean target quadrant occupancy is significantly greater in TgAPP mice. (c) Target quadrant occupancy. TgAPP and non-Tg mice show similar patterns of extinction during probe trials examined in 5-second intervals. Bars are means; error bars are SEM. Number of mice tested are shown in parentheses.
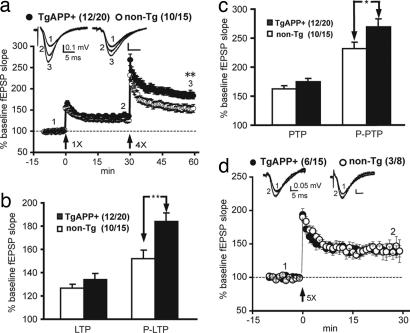
To ascertain whether enhanced spatial reference memory in TgAPP mice was associated with alterations in synaptic plasticity, we examined short- and long-lasting forms of synaptic plasticity in the Schaffer collateral pathway of the hippocampal formation. A specific form of long-term potentiation (LTP) was enhanced by ≈30% in TgAPP mice relative to non-Tg littermates (Fig. 3 a and b). This form of LTP was elicited by using a strong tetanus consisting of four trains of 10 pulses at 100 Hz preceded 30 min earlier by a weak tetanus consisting of one train of 10 pulses at 100 Hz, which served as a priming stimulus. We called this primed LTP (P-LTP). In addition, we found that primed posttetanic potentiation (P-PTP), a form of short-term synaptic plasticity, was enhanced in TgAPP mice by ≈20% (Fig. 3c). No changes in basal synaptic transmission or another form of short-term plasticity, paired-pulse facilitation, were found [supporting information (SI) Fig. 6 a and b].
Fig. 3.
Enhanced synaptic plasticity in TgAPP mice. (a) TgAPP mice show significantly enhanced P-LTP compared with non-Tg littermates at 7–18 months of age. ∗∗, P < 0.01, t test. (b) TgAPP mice show significantly increased P-LTP (one train followed by four trains) but not LTP (one train). ∗∗, P < 0.01, t test. (c) TgAPP mice show significantly increased P-PTP (one train followed by four trains) but not PTP (one train). (d) Enhanced synaptic plasticity depends on prior synaptic activity. A five-train high-frequency stimulation (HFS) induces similar LTP and PTP in 13- to 14-month TgAPP and non-Tg littermates. Bars are means; error bars are SEM. Number of mice/total number of slices tested is shown in parentheses.
To determine whether priming was necessary to reveal enhanced synaptic plasticity in TgAPP mice, we compared LTP elicited by using five trains to P-LTP produced with one train followed by four trains of tetanus. In contrast to our findings for P-LTP, we found no increase in LTP in TgAPP mice compared with non-Tg littermates by using the five-train protocol (Fig. 3d). The requirement for priming demonstrates that the facilitation of synaptic plasticity by APP is a dynamic process that depends on prior synaptic activation.
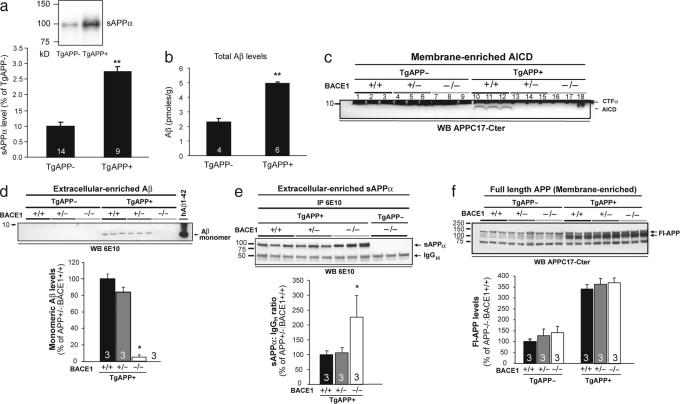
APP over expression leads to overproduction of its derivatives, and neuronal activity, which has been shown to modulate the secretion of Aβ and sAPPα (1, 2, 5, 14), may promote the production or activation of APP derivatives that enhance learning and plasticity. We therefore assayed the levels of sAPPα, Aβ, and AICD in the brains of TgAPP mice. The levels of sAPPα and Aβ were ≈2.9- and ≈2.1-fold higher, respectively, in TgAPP+ mice than in TgAPP− littermates (Fig. 4 a and b). Similarly, AICD levels were enhanced, because they were detected only in TgAPP+ mice (Fig. 4c, lanes 10–12 vs. lanes 1–3).
Fig. 4.
Ablation of one or both copies of BACE1 substantially reduces AICD in TgAPP mice. (a) sAPPα levels are ≈3-fold greater in 4- to 6-month TgAPP+ mice. ∗∗, P < 0.01, t test. (b) ELISA shows ≈2.1-fold more total Aβ in 6-month TgAPP+ mice. ∗∗, P < 0.01, t test. (c) In membrane-enriched fractions, which contain nuclear proteins, TgAPP+ mice overexpress AICD (lanes 10–12), which is not detectable in TgAPP− littermates (lanes 1–3). AICD decreases to below the level of detection in mice with one (+/−, lanes 13–15) or zero (−/−, lanes 16–18) copies of BACE1. (d) In soluble extracellular-enriched fractions, Aβ decreases significantly only in TgAPP mice lacking both copies of BACE1 (−/−). ∗, P < 0.05, t test. (e) Levels of sAPPα increase significantly only in TgAPP mice lacking both copies of BACE1 (−/−). ∗, P < 0.05, t test. (f) Levels of full-length APP do not change significantly as a function of BACE1 copy number. Bars are means; error bars are SEM. Numerals in bars indicate numbers of mice examined.
The elevation in the levels of all three molecules, Aβ, sAPPα, and AICD, in TgAPP mice led us to wonder whether one in particular was responsible for the enhanced memory and synaptic plasticity. To discriminate the molecule that corresponded most closely with the enhanced memory and synaptic plasticity in TgAPP mice, we genetically varied the levels of the three cleavage products separately. We inhibited the production of Aβ and promoted that of sAPPα by crossing TgAPP mice with TgBACE1-knockout (KO) mice (15), which lack BACE1, the major β-secretase (16). (TgAPP × TgBACE1-KO)F1 mice were intercrossed to produce TgAPP+ with two, one, or no copies of BACE1, as well as TgAPP− mice with two, one, or no copies of BACE1, in the same mixed strain background and were used for synaptic plasticity testing and biochemical examinations. (TgAPP × TgBACE1-KO)F1 mice were also backcrossed to mice lacking BACE1 to produce TgAPP+ with one or no copies of BACE1, as well as TgAPP− mice with one or no copies of BACE1. This second set of mice was used for behavioral testing and confirmation of the electrophysiological results from the intercrossed mice. The difference in background strain between the backcrossed mice (one and zero BACE1 copies) and the parent mice (two BACE1 copies) precludes direct comparisons between the parent and backcrossed mice. However, backcrossing still allows for comparisons within similar strain backgrounds while also generating sufficient numbers of mice with one and zero BACE1 copies for behavioral testing, which intercrossing failed to produce.
Consistent with previous reports (15, 17, 18), in the soluble extracellular-enriched brain fraction, Aβ levels were reduced by >90% (Fig. 5d) and sAPPα levels were increased by ≈120% (Fig. 5e) in BACE1-null TgAPP+ mice, but the levels of neither molecule changed significantly in TgAPP+ mice with one copy of BACE1. The levels of Aβ in the soluble intracellular-enriched and membrane-associated fractions were also examined and decreased significantly only in mice lacking both copies of BACE1 (data not shown). The levels of full-length APP remained unchanged regardless of BACE1 copy number (Fig. 5f). CTFβ levels decreased and CTFα levels increased in BACE1-null mice, in parallel with Aβ and sAPPα levels, respectively, but remained unchanged in mice with one copy of BACE1 (SI Fig. 7 a and b). Interestingly, AICD levels decreased dramatically (to below the detection limit) in both BACE1 heterozygous and null mice (Fig. 5c, lanes 10–12 vs. lanes 13–15 and 16–18). Although CTFα also generates AICD, its levels were unchanged in TgAPP mice with one copy of BACE1 and therefore could not contribute to AICD levels in a compensatory manner. Thus, the ablation of one BACE1 gene significantly reduced AICD without changing other APP cleavage product levels.
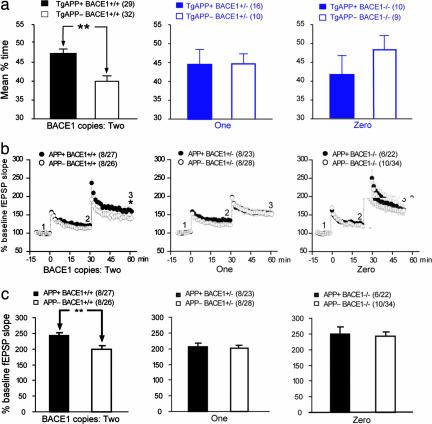
Fig. 5.
Dependence of enhanced memory and synaptic plasticity in TgAPP mice on BACE1 copy number. (a) The mean target quadrant occupancy of TgAPP+ and TgAPP− mice as a function of BACE1 copy number in backcrossed mice with one or zero copies of BACE1 (blue bars) and in parent mice with two copies of BACE1 (black bars). ∗∗, P < 0.01, t test. Numbers of mice tested are shown in parentheses. (b) P-LTP as a function of BACE1 copy number in intercrossed mice. ∗, P < 0.05, t test. (c) P-PTP as a function of BACE1 copy number in intercrossed mice. ∗∗, P < 0.01, t test. Bars are means; error bars are SEM. Number of mice/number of slices are shown in parentheses.
We tested TgAPP/BACE1-KO mice to examine how the changes in the levels of the various APP derivatives correspond to alterations in memory and synaptic plasticity. The elimination of one or both copies of BACE1 abolished the enhancement of spatial reference memory in TgAPP+ relative to TgAPP− mice. Target quadrant occupancies were not significantly different in TgAPP+ and TgAPP− mice lacking one or both copies of BACE1 in contrast to TgAPP+ and TgAPP− mice with intact BACE1 genes (Fig. 5a). The abolishment of enhanced memory was not caused by changes in navigational capability or extinction, because all groups of mice found the hidden platform equally quickly and showed similar levels of extinction in the probe trials (SI Fig. 8 a and b). Similarly, TgAPP+ mice with either one or no copies of BACE1 showed no enhancement of P-LTP relative to TgAPP− littermates. The loss of one or both copies of BACE1 did not prevent the establishment of LTP per se but only abolished the enhancement of P-LTP associated with the overexpression of APP (Fig. 5b). We observed a trend toward elevated P-LTP in both TgAPP+ and TgAPP− mice lacking both copies of BACE1. This observation could potentially be explained by increased levels of sAPPα (Fig. 4e). In addition, the enhancement in P-PTP was abolished when one or both copies of BACE1 were disrupted in TgAPP mice (Fig. 5c). Tests of backcrossed bigenic mice confirmed that the enhancement in P-LTP and P-PTP was absent in TgAPP mice lacking one or both copies of BACE1 (SI Fig. 9). Because the ablation of one BACE1 gene was sufficient to abolish the enhanced memory and synaptic plasticity in TgAPP mice and resulted in the reduction of levels of AICD but not of the other APP derivatives, we concluded that AICD correlated better than the other APP cleavage products with the enhanced memory and synaptic plasticity.
Discussion
Here we show that the limited overproduction of APP cleavage products, at levels that do not lead to the formation of Aβ plaques or Aβ*56, can improve the retention of spatial memory and enhance activity-dependent forms of long-lasting and short-term synaptic plasticity. The enhanced memory and synaptic plasticity depend on BACE1-mediated APP processing and correlate with elevated levels of AICD, but not with the other APP cleavage products. The data suggest that the regulated, activity-dependent cleavage of APP by BACE1 in neurons may facilitate aspects of normal brain function, in sharp contrast to previous studies in culture, which showed that synaptic function is inhibited by BACE1-mediated cleavage of APP (2). Thus, we propose that one of the normal physiological roles of BACE1-mediated cleavage of APP in the brain is to facilitate memory and synaptic plasticity, not to inhibit synaptic function.
The discrepancy between our work and that of Kamenetz et al. (2) may be reconciled by noting that they did not measure the aggregation state of the Aβ species generated by their culture preparations and were therefore unaware of whether oligomeric Aβ species, which impair memory and synaptic function (11, 19–22), were generated. In contrast, we were careful to study APP transgenic mice that do not generate oligomeric Aβ species, such as Aβ*56, which impair memory (11). We have studied APP transgenic mice, such as Tg2576, which generate oligomeric Aβ species (11), and have found impaired synaptic function (23, 24) and memory (10, 13).
Because TgAPP mice overexpress APP, the effects we observed may not necessarily reflect the physiological activity of APP. However, our results support, complement, and extend previous loss-of-function studies in mice lacking APP, BACE1, or presenilin-1 (6–9) by showing a gain-of-function memory and plasticity phenotype associated with APP and may therefore be relevant to normal physiology.
We dissociated the enhanced memory and synaptic plasticity in TgAPP mice from sAPPα, which has been reported to enhance memory and synaptic plasticity in rats (3, 4). Our findings are consistent with recent studies showing that sAPPα infused into the lateral ventricles of rats does not enhance LTP or cognitive function (21, 22). However, the negative results do not exclude other potential positive effects of sAPPα on brain function, which may be elicited only under specific testing conditions. We found no increase in fast-synaptic transmission in TgAPP mice relative to non-Tg mice, arguing against the synaptotrophic effects that were reported in mice overexpressing moderate levels of APP (25) as an explanation for the enhanced memory in TgAPP mice. We also failed to observe associations between enhanced memory or synaptic plasticity and the monomeric Aβ that was present in the three cellular compartments we examined (extracellular, cytosolic, and membrane), arguing against positive effects of monomeric Aβ on synaptic function that have been suggested by some in vitro studies (26, 27).
One possible mediator of the beneficial effects of APP on memory and synaptic plasticity is AICD, which was the only APP cleavage product that correlated with enhanced plasticity and memory in TgAPP mice under different BACE1 deletion backgrounds. AICD forms a complex that can activate the transcription of reporter genes (28), and has been implicated in the regulation of calcium signaling (29). The short time interval between priming and the enhancement of synaptic plasticity (30 min) in TgAPP mice places constraints on mechanisms involving transcriptional activation that might be involved in the synaptic effects we observed. The requirement for priming may also explain the controversy concerning the role of AICD in nuclear signaling (30). One scenario that would reconcile the original observations of Cao and Sudhof (28) identifying chimeric AICD/yeast DNA binding domain constructs as transcriptional regulators with recent work that suggests weak or no transcription regulatory activity of AICD in mammalian cell-based assays (30) is the possibility that the putative mammalian DNA-binding proteins that are regulated by AICD interact with AICD most efficiently under conditions that accompany synaptic activity. Thus, the optimal environment for observing AICD-mediated regulation of transcription may not be achieved in conventional mammalian cell-based assays that lack synaptic activity.
To address the effects of BACE1 on endogenous (murine) APP, we examined the effects of BACE1 deletions in TgAPP- mice. Although we were unable to compare memory in TgAPP- mice with different BACE1 deletions because of differences in strain backgrounds, we were able to study primed plasticity and saw no significant effect of BACE1 copy number on P-PTP or P-LTP (Fig. 5 b and c). Our failure to detect BACE1 effects in TgAPP- mice contrasts with previous reports by Laird et al. (9) of abnormal de-depression, an activity-dependent form of synaptic plasticity, in mice lacking both BACE1 copies. It is possible that our P-PTP and P-LTP protocols were insufficiently sensitive to detect differences under different BACE1 deletion backgrounds. Laird et al. reported spatial memory deficits in BACE1 KO mice that were rescued by human APP, which led them to suggest that the deficits in BACE1 KO mice were caused by a deficiency in AICD. However, they did not measure AICD and therefore could not provide direct evidence for the AICD hypothesis. Although our data provide direct biochemical support for this notion, firm proof will require specific application or targeting of AICD in assays of behavior or synaptic plasticity.
The curious disparity in TgAPP mice with one BACE1 gene between decreases in the levels of AICD and Aβ, which are usually generated in a one-to-one stoichiometric manner, is not unprecedented. Some presenilin-1 mutations and γ-secretase inhibitors alter Aβ and AICD levels differently (31–33). This finding may reflect differential effects of the mutations and drugs on TMP21, which negatively regulates presenilin complex activity at the γ- but not the ε-site (34). Conversely, our results suggest that the level, but not the enzymatic activity, of BACE1 may modulate ε-secretase activity. Potential differences in the kinetics of AICD and Aβ degradation may result in a more significant drop in AICD levels than in Aβ levels when one BACE1 gene is ablated.
A practical implication of this work involves safety considerations for experimental therapies of AD. To ensure that experimental therapies do not prevent BACE1-mediated facilitation of memory by APP, preclinical studies of experimental β-secretase inhibitors should be done not only in animal models of AD, but also in natural animals to evaluate their effects on normal cognitive function.
Methods
Mice.
The generation of TgAPP and TgBACE1-KO mice have been described in refs. 10 and 15. TgAPP and TgBACE1-KO mice were maintained in hybrid C57B6/SJL and 129SvJ/Black Swiss backgrounds, respectively.
Behavioral Testing.
Spatial reference memory was assessed by using a modified version of the Morris water maze (10). Mice received visible platform training for 3 days (eight trials per day), followed by hidden platform training for 9 days (four trials per day). Three 60-second probe trials were performed 20 h after 12, 24, and 36 hidden platform trials, and the average target quadrant occupancy for the three probe trials (MPS) was calculated. Quadrant occupancy at 5-second intervals was determined for each probe separately and then averaged for each interval. Performance-incompetent mice were excluded from analysis (outliers in the last block of visible platform training and mice failing to orient or follow an escape scoop). Trials were monitored by using a computerized video tracking system (HVS Image, Hampton, U.K.; or EthoVision 3.0; Noldus; Wageningen, The Netherlands) and further analyzed by using Wintrack software (35).
Slice Electrophysiology.
Hippocampal slices were prepared as described (36). Slices were superfused with a solution containing 119 mM NaCl, 2.5 mM KCl, 1.0 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, 1.3 mM MgCl2, and 11 mM glucose, saturated with 5% CO2/95% O2 at 30°C. Synaptic responses were evoked by stimulating Schaffer collaterals with 0.08-ms constant current pulses by using a tungsten bipolar electrode. Field excitatory postsynaptic potentials (fEPSPs) were recorded extracellularly in CA1 stratum radiatum by using a 5-MΩ tungsten monopolar electrode. The initial slope of the evoked fEPSPs, an indicator of synaptic strength, was measured as a function of the stimulation intensity (25–100 μA). Thereafter, a stimulation intensity, which elicited 30% of the maximum response, was used for the duration of the recording. Paired-pulse facilitation (PPF) was tested by applying pairs of pulses separated by interstimulus intervals of 25–500 ms. To induce LTP, stimulus trains of 10 pulses at 100 Hz were delivered; multiple trains (4×, 5×) were separated by 20 seconds. Data were sampled at 100 kHz and filtered at 10 Hz and 1 kHz. Data acquisition and analysis were done with pCLAMP (Molecular Devices, Sunnyvale, CA). Experimenters were blind to mouse genotype during slice preparation, recordings, and analysis of each slice until final grouping of all slice experiments. Experiments were ended if the 10-min baseline before LTP induction was unstable or rejected if the data were greater or <2 standard deviations from the mean group response.
Immunoblots.
Protein extraction, immunoprecipitation, and Western blot analysis were carried out as described (11). In brief, chunks of forebrain (remains after hippocampal dissection for electrophysiological recordings) were extracted with a four-step protocol to separate the extracellular-, intracellular-, membrane-enriched, and insoluble proteins (from intercrossed TgAPP/BACE1-KO mice, n = 3 for each group). The antibodies used were as follows : 6E10 (9320–10; Signet LaboratoryDedham, MA) for holo-APP, 6E10 for Aβ and sAPPα, APPC17-Cter (37) for holo-APP, CTFα, CTFβ and AICD. Protein levels in each of the four supernatant fractions were determined with Bradford tests before use for subsequent Western blots, in which equal amounts of proteins were loaded onto precast 10–20% SDS-polyacrylamide Tris-tricine gels (Bio-Rad, Hercules, CA) or 16% Tris-tricine gels. Gels were transferred to a PVDF membrane (Immobilon Psq membrane; Millipore, Billerica, MA) or 0.2 μm of nitrocellulose membrane (Bio-Rad). Blots were developed with an enhanced chemiluminescence (ECL) Western blotting detection system (Supersignal Pico Western system; Pierce Rockford, IL; or Western Lightning Chemiluminescence Reagent Plus; PerkinElmer, Boston, MA). Films were scanned and densitometry analyses were performed with OptiQuant software (Molecular Dynamics, Sunnyvale, CA).
Aβ Measurements.
ELISAs were used to measure Aβ40 and Aβ42 in hemibrains that were extracted with a two-step protocol as described in ref. 38.
Statistics.
Statistical significance was assessed with ANOVA and Student's t tests (with P < 0.05 accepted as significant).
Acknowledgments
We thank A. Mariash, D. Cooper-Blacketer, and A. Guimaraes for technical assistance; K. Sambamurti (Medical University of South Carolina, Charleston, SC) for providing the O443 antibody that was used in preliminary studies; K. SantaCruz (University of Minnesota) for photomicrographs of TgAPP and Tg2576 mice; and K. Zahs for critical discussions. This work was supported by the National Institutes of Health (K.H.A.) and the Minnesota Medical Foundation (L.M.B.).
Abbreviations
- Aβ
amyloid-β protein
- AD
Alzheimer's disease
- AICD
APP intracellular domain
- APP
amyloid precursor protein
- BACE1
β-site APP cleaving enzyme 1
- CTF
carboxyl-terminal fragment
- KO
knockout
- LTP
long-term potentiation
- non-Tg
nontransgenic
- P-LTP
primed LTP
- P-PTP
primed posttetanic potentiation
- sAPPα
secreted APPα.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609521104/DC1.
References
- 1.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 2.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 3.Ishida A, Furukawa K, Keller JN, Mattson MP. NeuroReport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 4.Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Proc Natl Acad Sci USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, et al. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, et al. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 8.Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 9.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, et al. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 12.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa K, Sopher B, Rydel R, Begley J, Pham D, Martin G, Fox M, Mattson M. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, et al. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 16.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 18.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, et al. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 19.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 22.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 23.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, et al. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 24.Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR. J Neurosci. 2001;21:4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucke L, Masliah E, Johnson WB, Ruppe MD, Alford M, Rockenstein EM, Forss-Petter S, Pietropaolo M, Mallory M, Abraham CR. Brain Res. 1994;666:151–167. doi: 10.1016/0006-8993(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Anwyl R, Rowan MJ. Eur J Pharmacol. 1995;284:R1–R3. doi: 10.1016/0014-2999(95)00539-w. [DOI] [PubMed] [Google Scholar]
- 27.Koudinov AR, Berezov TT. Acta Neurobiol Exp (Wars) 2004;64:71–79. doi: 10.55782/ane-2004-1492. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Sudhof TC. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 29.Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Muller U, Saftig P, De Strooper B, et al. Proc Natl Acad Sci USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hebert SS, Serneels L, Tolia A, Craessaerts K, Derks C, Filippov MA, Muller U, De Strooper B. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecimovic S, Wang J, Dolios G, Martinez M, Wang R, Goate AM. Neurobiol Dis. 2004;17:205–218. doi: 10.1016/j.nbd.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Chen F, Gu Y, Hasegawa H, Ruan X, Arawaka S, Fraser P, Westaway D, Mount H, St George-Hyslop P. J Biol Chem. 2002;277:36521–36526. doi: 10.1074/jbc.M205093200. [DOI] [PubMed] [Google Scholar]
- 33.Petit A, Bihel F, Alves da Costa C, Pourquie O, Checler F, Kraus JL. Nat Cell Biol. 2001;3:507–511. doi: 10.1038/35074581. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, et al. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 35.Wolfer DP, Madani R, Valenti P, Lipp HP. Physiol Behav. 2001;73:745–753. doi: 10.1016/s0031-9384(01)00531-5. [DOI] [PubMed] [Google Scholar]
- 36.Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. NeuroReport. 2003;14:219–223. doi: 10.1097/00001756-200302100-00012. [DOI] [PubMed] [Google Scholar]
- 37.Sergeant N, David JP, Champain D, Ghestem A, Wattez A, Delacourte A. J Neurochem. 2002;81:663–672. doi: 10.1046/j.1471-4159.2002.00901.x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]