Abstract
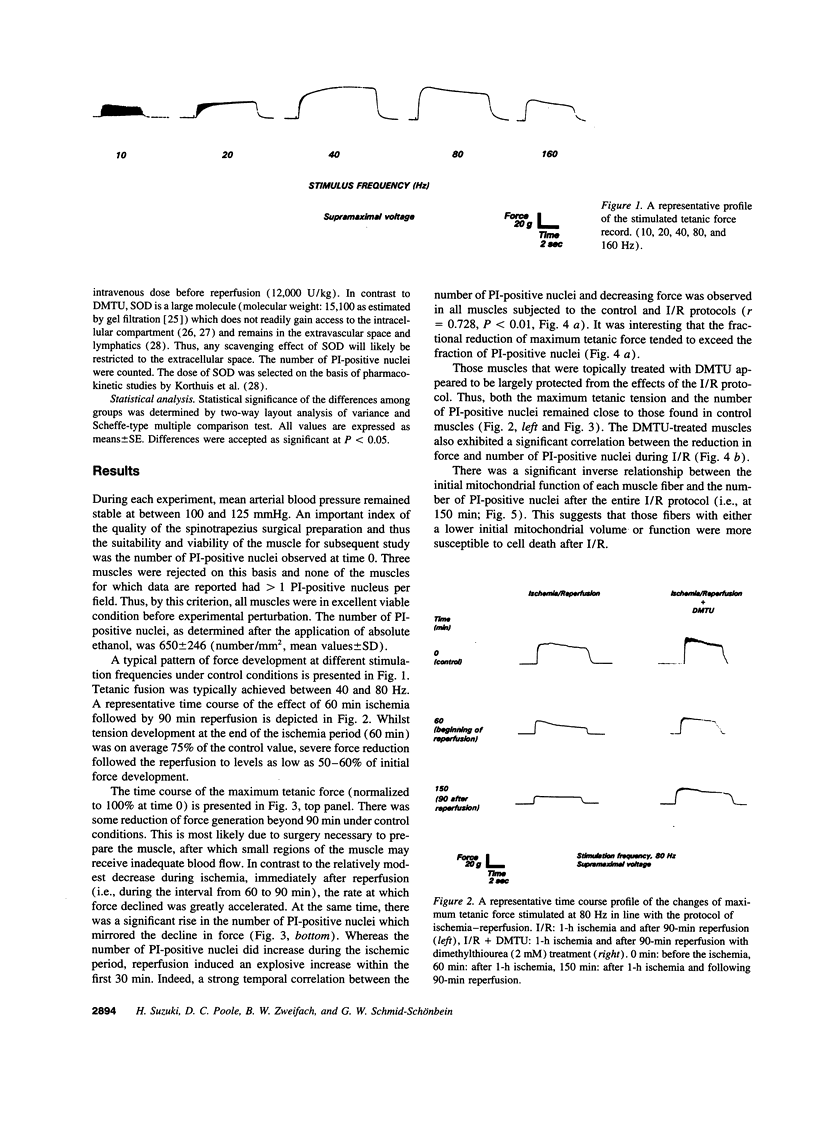
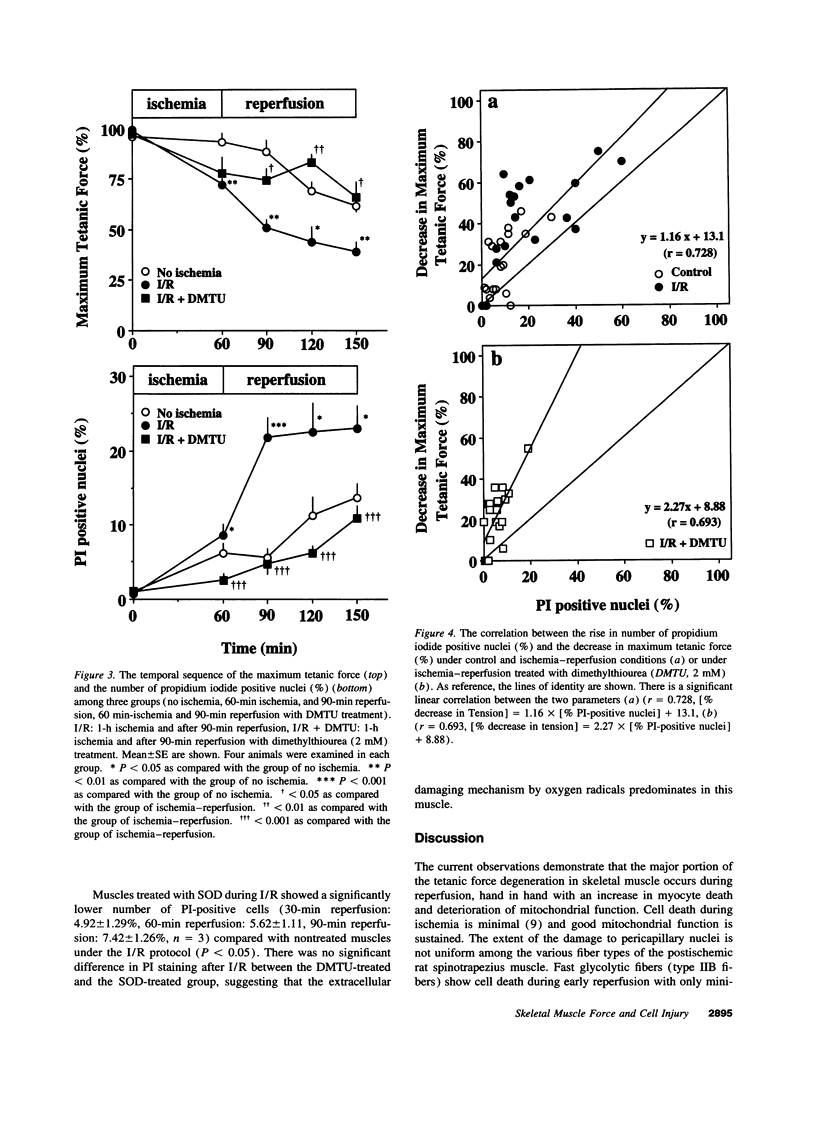
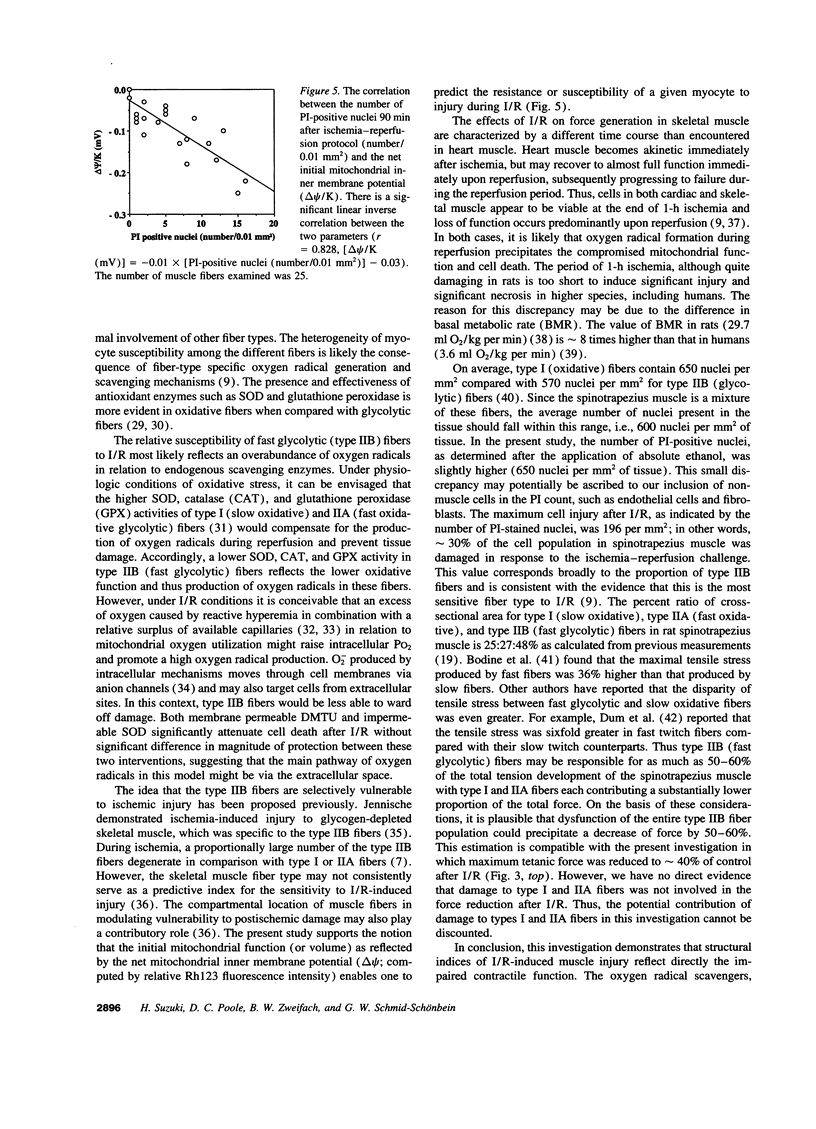
To gain insight into the mechanisms responsible for muscle dysfunction after ischemia-reperfusion, a rat spinotrapezius muscle preparation was developed which enabled sequential measurements of in vivo maximum tetanic force production and cell death assessed using digital microfluorographic determination of propidium iodide (PI) staining. After 60 min of no-flow ischemia, maximum tetanic force fell significantly during 90 min of reperfusion compared with control, nonischemic muscles. The most striking fall was evident within 30 min of reperfusion and occurred concomitant with an explosive increase in PI-positive myocyte nuclei. Treatment with the oxygen radical scavenger, dimethylthiourea, attenuated both the fall in force and increased PI staining. Indeed, the rise in PI-positive nuclei correlated closely (r= 0.728) with the reduction of maximum tetanic force developed following ischemia and reperfusion under all conditions. Superoxide dismutase also attenuated the rise in PI-positive nuclei. Assessment of mitochondrial inner membrane potential (deltapsi) using Rhodamine 123 fluorescence revealed that myocytes with the lowest initial mitochondrial membrane potential were subject to the greatest injury after 90 min of reperfusion (r= 0.828). These results support the hypothesis that myocyte injury, as visualized by PI-staining, reflects an impaired contractile function in fibers with a low oxidative potential which is likely mediated by oxygen radicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adembri C., Domenici L. L., Formigli L., Brunelleschi S., Ferrari E., Novelli G. P. Ischemia-reperfusion of human skeletal muscle during aortoiliac surgery: effects of acetylcarnitine. Histol Histopathol. 1994 Oct;9(4):683–690. [PubMed] [Google Scholar]
- Akimitsu T., Gute D. C., Korthuis R. J. Leukocyte adhesion induced by inhibition of nitric oxide production in skeletal muscle. J Appl Physiol (1985) 1995 May;78(5):1725–1732. doi: 10.1152/jappl.1995.78.5.1725. [DOI] [PubMed] [Google Scholar]
- Apple F. S., Hyde J. E., Ingersoll-Stroubos A. M., Theologides A. Geographic distribution of xanthine oxidase, free radical scavengers, creatine kinase, and lactate dehydrogenase enzyme systems in rat heart and skeletal muscle. Am J Anat. 1991 Nov;192(3):319–323. doi: 10.1002/aja.1001920311. [DOI] [PubMed] [Google Scholar]
- Atherton G. W., James N. T. Stereological analysis of the number of nuclei in skeletal muscle fibres. Acta Anat (Basel) 1980;107(2):236–240. doi: 10.1159/000145248. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Eldred E., Edgerton V. R. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J Neurophysiol. 1987 Jun;57(6):1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991 Mar;5 (Suppl 2):249–268. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- Brooks G. A., White T. P. Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Dec;45(6):1009–1015. doi: 10.1152/jappl.1978.45.6.1009. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Burke R. E., O'Donovan M. J., Toop J., Hodgson J. A. Motor-unit organization in flexor digitorum longus muscle of the cat. J Neurophysiol. 1982 Jun;47(6):1108–1125. doi: 10.1152/jn.1982.47.6.1108. [DOI] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner V. O., Caiozzo V. J., Long S. T., Stoffel J., McMaster W. C., Prietto C. A. Contractile properties of slow and fast muscle following tourniquet ischemia. Am J Sports Med. 1984 Nov-Dec;12(6):417–423. doi: 10.1177/036354658401200602. [DOI] [PubMed] [Google Scholar]
- Gray S. D. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res. 1973 May;5(3):395–400. doi: 10.1016/0026-2862(73)90055-1. [DOI] [PubMed] [Google Scholar]
- Gray S. D., Renkin E. M. Microvascular supply in relation to fiber metabolic type in mixed skeletal muscles on rabbits. Microvasc Res. 1978 Nov;16(3):406–425. doi: 10.1016/0026-2862(78)90073-0. [DOI] [PubMed] [Google Scholar]
- Harris K., Walker P. M., Mickle D. A., Harding R., Gatley R., Wilson G. J., Kuzon B., McKee N., Romaschin A. D. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 1986 Feb;250(2 Pt 2):H213–H220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- Hudlicka O., Hoppeler H., Uhlmann E. Relationship between the size of the capillary bed and oxidative capacity in various cat skeletal muscles. Pflugers Arch. 1987 Nov;410(4-5):369–375. doi: 10.1007/BF00586513. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., White C. W., Parker N. B., Ryan J. W., Repine J. E. Dimethylthiourea consumption reflects H2O2 concentrations and severity of acute lung injury. J Appl Physiol (1985) 1985 Dec;59(6):1995–1998. doi: 10.1152/jappl.1985.59.6.1995. [DOI] [PubMed] [Google Scholar]
- Jennische E. Ischaemia-induced injury in glycogen-depleted skeletal muscle. Selective vulnerability of FG-fibres. Acta Physiol Scand. 1985 Dec;125(4):727–734. doi: 10.1111/j.1748-1716.1985.tb07776.x. [DOI] [PubMed] [Google Scholar]
- Kaufman R. P., Jr, Klausner J. M., Anner H., Feingold H., Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Inhibition of thromboxane (Tx) synthesis by free radical scavengers. J Trauma. 1988 Apr;28(4):458–464. doi: 10.1097/00005373-198804000-00007. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Granger D. N., Townsley M. I., Taylor A. E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985 Oct;57(4):599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Kubes P., Tso P., Perry M., Granger D. N. Transport kinetics for superoxide dismutase and catalase between plasma and interstitial fluid in the rat small intestine. Free Radic Biol Med. 1991;11(3):293–298. doi: 10.1016/0891-5849(91)90126-n. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Smith J. K., Carden D. L. Hypoxic reperfusion attenuates postischemic microvascular injury. Am J Physiol. 1989 Jan;256(1 Pt 2):H315–H319. doi: 10.1152/ajpheart.1989.256.1.H315. [DOI] [PubMed] [Google Scholar]
- Laughlin M. H., Simpson T., Sexton W. L., Brown O. R., Smith J. K., Korthuis R. J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol (1985) 1990 Jun;68(6):2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Pedowitz R. A., Fridén J., Gershuni D. H. Decreased muscle speed, strength and fatigability following two hours of tourniquet-induced ischaemia. Scand J Plast Reconstr Surg Hand Surg. 1992;26(2):127–132. doi: 10.3109/02844319209016002. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Mazzoni M. C., Skalak T. C., Schmid-Schönbein G. W. Effects of skeletal muscle fiber deformation on lymphatic volumes. Am J Physiol. 1990 Dec;259(6 Pt 2):H1860–H1868. doi: 10.1152/ajpheart.1990.259.6.H1860. [DOI] [PubMed] [Google Scholar]
- Mäkitie J. Microvasculature of rat striated muscle after temporary ischemia. Acta Neuropathol. 1977 Mar 31;37(3):247–253. doi: 10.1007/BF00686886. [DOI] [PubMed] [Google Scholar]
- Mäkitie J., Teräväinen H. Ultrastructure of striated muscle of the rat after temporary ischemia. Acta Neuropathol. 1977 Mar 31;37(3):237–245. doi: 10.1007/BF00686885. [DOI] [PubMed] [Google Scholar]
- Paky A., Michael J. R., Burke-Wolin T. M., Wolin M. S., Gurtner G. H. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol (1985) 1993 Jun;74(6):2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- Pedowitz R. A., Fridén J., Thornell L. E. Skeletal muscle injury induced by a pneumatic tourniquet: an enzyme- and immunohistochemical study in rabbits. J Surg Res. 1992 Mar;52(3):243–250. doi: 10.1016/0022-4804(92)90081-a. [DOI] [PubMed] [Google Scholar]
- Sexton W. L., Korthuis R. J., Laughlin M. H. Microvascular injury after ischemia and reperfusion in skeletal muscle of exercise-trained rats. J Appl Physiol (1985) 1990 Jun;68(6):2329–2336. doi: 10.1152/jappl.1990.68.6.2329. [DOI] [PubMed] [Google Scholar]
- Sternbergh W. C., 3rd, Adelman B. Skeletal muscle fiber type does not predict sensitivity to postischemic damage. J Surg Res. 1992 Nov;53(5):535–541. doi: 10.1016/0022-4804(92)90103-7. [DOI] [PubMed] [Google Scholar]
- Sternbergh W. C., 3rd, Adelman B. The temporal relationship between endothelial cell dysfunction and skeletal muscle damage after ischemia and reperfusion. J Vasc Surg. 1992 Jul;16(1):30–39. [PubMed] [Google Scholar]
- Suematsu M., DeLano F. A., Poole D., Engler R. L., Miyasaka M., Zweifach B. W., Schmid-Schönbein G. W. Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab Invest. 1994 May;70(5):684–695. [PubMed] [Google Scholar]
- Suematsu M., Schmid-Schönbein G. W., Chavez-Chavez R. H., Yee T. T., Tamatani T., Miyasaka M., Delano F. A., Zweifach B. W. In vivo visualization of oxidative changes in microvessels during neutrophil activation. Am J Physiol. 1993 Mar;264(3 Pt 2):H881–H891. doi: 10.1152/ajpheart.1993.264.3.H881. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Suzuki H., Ishii H., Kato S., Hamamatsu H., Miura S., Tsuchiya M. Topographic dissociation between mitochondrial dysfunction and cell death during low-flow hypoxia in perfused rat liver. Lab Invest. 1992 Oct;67(4):434–442. [PubMed] [Google Scholar]
- Suzuki H., Suematsu M., Ishii H., Kato S., Miki H., Mori M., Ishimura Y., Nishino T., Tsuchiya M. Prostaglandin E1 abrogates early reductive stress and zone-specific paradoxical oxidative injury in hypoperfused rat liver. J Clin Invest. 1994 Jan;93(1):155–164. doi: 10.1172/JCI116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Swei A., Zweifach B. W., Schmid-Schönbein G. W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995 May;25(5):1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- Taylor K., Calvey T. N. Histochemical characteristics and contractile properties of the spinotrapezius muscle in the rat and the mouse. J Anat. 1977 Feb;123(Pt 1):67–76. [PMC free article] [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]


