Summary
The peroxisome proliferator activated receptor γ (PPARγ) is a nuclear receptor highly expressed in the colon and playing a key role in bacterial induced inflammation. Regulation of colon inflammation by this receptor has been well demonstrated in many experimental models of colitis but also in patients with ulcerative colitis, characterised by impaired expression of PPARγ confined to their colon epithelial cells. Recent data showing that PPARγ was the major functional receptor mediating the common aminosalicylate activities in inflammatory bowel diseases (IBD) have also reinforced the roles of this receptor in the control of intestinal inflammation. The aims of this review are to discuss the potential roles of PPARγ in the physiopathology of IBD, as well as the emerging therapeutic strategies targeting this receptor.
Introduction
Current evidence suggests that Crohn's disease (CD) and ulcerative colitis (UC) result from a complex interplay between genetic and environmental factors, leading to an abnormal innate and adaptive immune response of the gut directed against luminal constituents in genetically determined patients. Identification of cytoplasmic receptors of bacterial peptidoglycan, namely nucleotide oligomerisation domain (NOD)2/caspase recruitment domain (CARD)15 and NOD1/CARD4, as CD susceptibility genes reinforced the pivotal role of the interactions between enteric microbes and the intestinal immune system in the physiopathology of IBD.1,2,3 Furthermore, recent advances in our laboratory and others also indicate the involvement of another key receptor, PPARγ, which regulates colon inflammation. This represents a new target in the development of therapeutic molecules in IBD.
PPARγ is a nuclear receptor discovered in mammals in 1993 as an orphan receptor.4 Until recently, PPARγ was known as a receptor mainly expressed by adipose tissue and involved in the regulation of insulin resistance. PPARγ is activated by antidiabetic thiazolidinedione drugs.5 In 1998, the first studies were published reporting a potential link between this receptor and intestinal diseases, originally described in colon cancer6,7,8 and one year later during intestinal inflammation.9 There is now emerging interest in the roles of this receptor in the regulation of gut homeostasis. Using a computerised medical literature search of all English language articles selected from the “PubMed” online database with the keywords “peroxisome proliferator‐activated receptor gamma”, “inflammatory bowel disease”, “Crohn's disease”, “ulcerative colitis”, “colitis”, “ileitis”, and “intestinal diseases”, more than 100 articles were found that reported a role for PPARγ, mainly in colon cancer and intestinal inflammation.
After a brief presentation of PPARγ and its ligands, the aims of this review are to outline the potential roles of PPARγ in the physiopathology of IBD and highlight areas for future therapeutic strategies targeting this receptor.
PPARγ structure, expression, and regulation
PPARγ structure and function
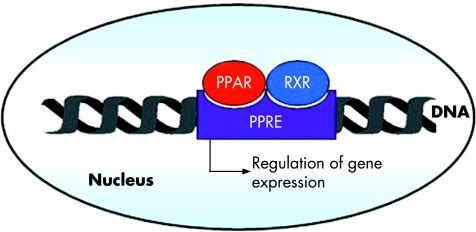
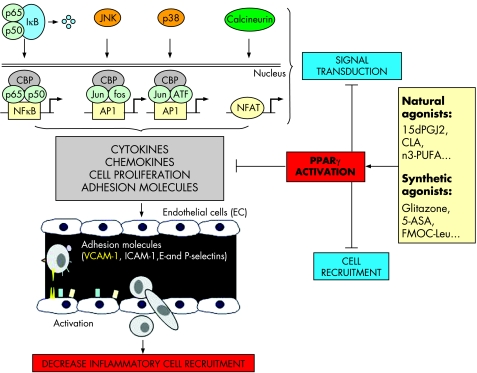
PPARγ belongs to the nuclear receptor family consisting of a group of approximately 50 transcription factors implicated in many different biological processes and considered as important targets in the development of new drugs.10 PPARγ is an essential nuclear receptor controlling the expression of a large number of regulatory genes in lipid metabolism and insulin sensitisation, as well as in inflammation and cell proliferation.11,12 Its activation requires heterodimerisation in the nucleus of the cells with another nuclear receptor, known as the retinoid X receptor α (RXR α) (fig 1), leading to binding of this heterodimer to specific DNA sequence elements termed peroxisome proliferator response elements (PPRE).13 It has been demonstrated that these two nuclear factors play a central role in the regulation of inflammatory signalling pathways by acting on kinases and transcription factors, such as nuclear factor κB (NFκB), c‐Jun, c‐Fos, and nuclear factor of activated T cell (NFAT)9,14,15 (fig 2) and inhibiting mucosal production of inflammatory cytokines (interleukin (IL)‐1β and tumour necrosis factor α (TNF‐α))14 and chemokines,16 proliferation of inflammatory cells,17 and expression of some adhesion molecules (fig 2).18
Figure 1 Peroxisome proliferator activated receptor γ (PPARγ) is a nuclear receptor which forms a heterodimer with retinoid X receptor (RXR). PPARγ may be activated by different natural and synthetic ligands allowing its heterodimerisation with RXR and binding, in the nucleus of the cell, on the peroxisome proliferator response element (PPRE). This binding regulates gene expression involved in the control of many biological processes, particularly inflammation.
Figure 2 Interferences of peroxisome proliferator activated receptor γ (PPARγ) with inflammatory signalling pathways. PPARγ inhibits nuclear factor κB (NFκB) signalling pathway through interactions with NFκB, the inhibitory protein called IκB, and CBP, a coactivator of p65. The MAPK pathway is also regulated by PPARγ, which reduces JNK and p38 activation and inhibits the transcription factors c‐jun, c‐fos, and nuclear factor of activated T cell (NFAT). Regulation of these main signalling pathways results in inhibition of cytokine and chemokine production, cell proliferation, and adhesion molecule expression (mainly VCAM‐1), which decrease inflammatory cell recruitment in inflamed tissues. 15dPGJ2, 15‐deoxy‐Δ12,14‐prostaglandin J2; PUFAs, polyunsaturated fatty acids; 5‐ASA, 5‐aminosalicylic acid.
PPARγ is highly expressed in the colon
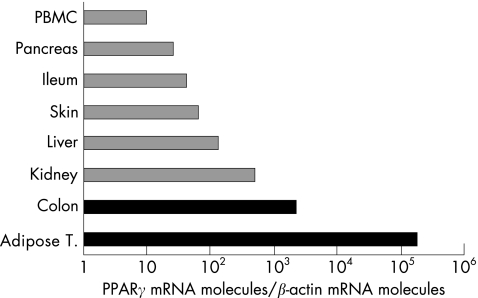
High levels of PPARγ expression have been reported in both colonic and adipose tissues. Originally described as a receptor expressed by adipose tissue where it plays a role in adipocyte differentiation and in the regulation of insulin responses, other tissues and cells are now known to express PPARγ (fig 3).19 Among them, the colon is a major tissue expressing PPARγ in epithelial cells and to a lesser degree macrophages and lymphocytes.20,21,22,23,24
Figure 3 Peroxisome proliferator activated receptor γ (PPARγ) mRNA expression in different tissues. PPARγ mRNA was quantified by reverse transcription‐competitive polymerase chain reaction in different human organs and tissues. The main sources of PPARγ are adipose tissue and the colon. PBMC, peripheral blood mononuclear cells.
Microorganisms regulate PPARγ expression in the colon
PPARγ is a modestly inducible receptor. Regulation of its expression remains poorly investigated although some reports suggest that it might be dependent at least in part on the cellular environment. In vivo, PPARγ mRNA and protein levels are negatively regulated by long term hypocaloric diet,25 fasting, and insulin deficient diabetes,26 and positively by obesity and a diet rich in fatty acids.25,26 More precisely, two classical pathways acting on PPARγ expression have been commonly observed using adipocyte cell lines. Firstly, specific natural or synthetic ligands of PPARγ can induce a mean 2–3‐fold expression of this receptor in a positive feedback loop.27 Secondly, different studies have demonstrated in vitro a synergistic effect of insulin and corticosteroids in inducing in vitro human PPARγ expression by cultured adipocytes.25,28 The NFκB and stress kinase pathways seem to be essential in post translational modifications of this nuclear receptor, but their regulatory effects on PPARγ expression remain uncertain. Other factors involving growth hormone,29,30 signal transducer and activator of transcription 5,31,32 and insulin growth factor 133 have also been proposed in the regulation of PPARγ expression, but these results need confirmation.29,30,34
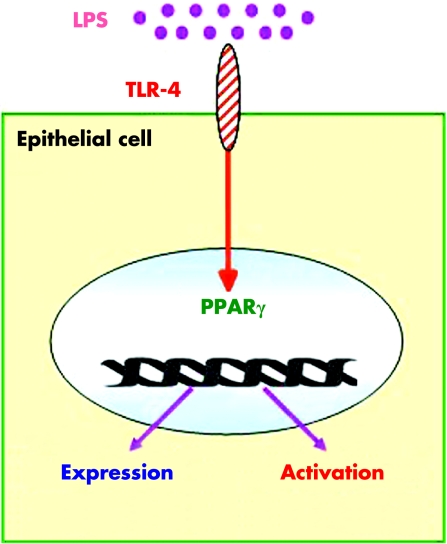
Recent research also indicates close links between intestinal‐microbial interactions and regulation of PPARγ expression by epithelial cells of the colon. To clarify the involvement of bacteria in the regulation of PPARγ expression in vivo, we showed over expression of PPARγ in the colon of mice with conventional or humanised flora compared with germ free animals.22 Similarly, in vitro studies using HT‐29 and/or Caco‐2 colon epithelial cells or KatoIII gastric cells have demonstrated the ability of lipopolysaccharide (LPS),22,35Saccharomyces boulardii,36 and Helicobacter pylori37 to increase by up to 2–4‐fold PPARγ mRNA and protein expression. Enhancement of PPARγ expression by microorganisms is probably multifactorial and involves at least in part the LPS recognition Toll‐like receptor (TLR)‐4, expressed by activated epithelial cells. This was demonstrated in vivo by very weak expression of PPARγ in the colon of mice with non‐functional TLR4 due to a naturally occurring mutation within the third exon of the TLR4 gene (C3H/HeJ Lpsd/Lpsdmice) compared with wild‐type animals.22 These results were confirmed in vitro after transfection of Caco‐2 cells with the constitutively active form of TLR4 leading to a fourfold induction of PPARγ expression (fig 4).22 An alternative way to regulate PPARγ expressed by epithelial cells through bacteria might be production of the volatile fatty acid butyrate produced by commensal intestinal flora. In contrast with other short chain fatty acids such as propionate or valerate, butyrate 2 mM caused a two‐ and sevenfold increase in PPARγ protein expression, respectively, after three and seven days of incubation of Caco‐2 epithelial cells.38
Figure 4 Modulation of peroxisome proliferator activated receptor γ (PPARγ) by Toll‐like receptor‐4 (TLR4). Activation of TLR4 by lipopolysaccharide (LPS) induces PPARγ expression and activation in transfected Caco‐2 cells.
Taken together, these results indicate the pivotal role of bacteria in the regulation of PPARγ expression by epithelial cells, which might account for the characteristic and important PPARγ pattern expression in the colon compared with other parts of the digestive tract. Although all microorganisms probably do not have the same ability to induce PPARγ expression, it seems that LPS of Gram negative bacteria are critical in colonic steady state PPARγ expression through TLR4. Studies are now in progress to evaluate the capacity of commensal bacteria to induce PPARγ expression and activation and to use this property as a criterion for probiotic selection.
Natural and synthetic ligands of PPARγ
Natural ligands
Many natural endogenous lipophilic species such as the polyunsaturated fatty acids (PUFAs)39 and eicosanoids40 are classically proposed as natural PPARγ ligands (table 1). However, their intrinsically low binding affinities and weak in vivo concentrations in intestinal cells do not support physiological functions of many of these compounds.
Table 1 Affinity and physiological roles of natural peroxisome proliferator activated receptor γ (PPARγ) modulators.
| Modulators | EC50/Ki | Plasma concentration | Sources | Effects in colon through PPARγ |
|---|---|---|---|---|
| PUFAs41 | ||||
| Omega 3 | ||||
| α‐linolenic acid | ND | 0.27 mM | High fat fish, marine mammals, milk | ND |
| γ‐linolenic acid | ND | High fat fish, marine mammals, milk | ND | |
| Eicosapentanoic acid | ND | 1.01 mM | High fat fish, marine mammals, milk | ND |
| Docohexanoic acid | ND | 3.54 mM | High fat fish, marine mammals, milk | ND |
| Omega 6 | ||||
| Linoleic acid | −/∼10 µM | 21.45 mM | Meats, eggs, milk, vegetable oils | ND |
| Dihomo‐γ‐linolenic acid | ND | 3.06 mM | Meats, eggs, milk, vegetable oils | ND |
| Arachidonic acid | ND | 11.36 mM | Meats, eggs, milk, vegetable oils | ND |
| Omega 9 | ||||
| Palmitoleic acid | ND | Rapeseed | ND | |
| Oleic acid | ND | Olive oil | ND | |
| Derivatives | ND | |||
| Conjugated linoleic acid42 | ND | – | Milk, meat, seeds | Yes |
| Nitrolinoleic acid43 | −/133 nM | 500 nM | Endogenous | ND |
| Nitrooleic acid44 | −/100 nM | 619 nM | Endogenous | ND |
| Eicosanoids:41 | ||||
| 8S‐hydroxyeicosapentaenoic acid | ND | − | Endogenous | ND |
| 12‐hydroxyeicosatetraenoic acid | ND | − | Endogenous | ND |
| 15‐hydroxyeicosatetraenoic acid | ND | − | Endogenous | ND |
| 9‐hydroxyoctodecadienoic acid | ND | − | Endogenous | ND |
| 13‐hydroxyoctodecadienoic acid | ND | − | Endogenous | ND |
| 15dPGJ245 | 1 µM/− | − | Endogenous | Yes |
| Miscellaneous | ||||
| Swietenia mahagony extract46 | 50 µg/l | − | S mahagony | ND |
| Lysophosphatidic acid47 | 2 µM | 5–25 µM | Platelets | ND |
| 9‐tetrahydrocannabinol48 | ND | − | Cannabis sativa | ND |
| Soy isoflavavone49 | ND | − | Soy | ND |
15dPGJ2, 15‐deoxy‐Δ12,14‐prostaglandin J2; ND, not determined; PUFAs, polyunsaturated fatty acids.
Although many PUFA activate PPARγ in micromolar amounts and are recorded as functional in human plasma at these concentrations,39 their in vivo intestinal effects through PPARγ activation remain hypothetical as concentrations of these fatty acids within colonic cells are unknown. Recently, two studies performed by the group of Bassaganya‐Riera et al demonstrated that, in contrast with a mixture of eicosapentaenoic and docohexaenoic acids, food supplemented with conjugated linoleic acid (CLA) efficiently prevents the development of colitis in pigs and mice.50,51 Moreover, they confirmed the direct involvement of PPARγ in the mechanism of action of CLA using colonic PPARγ null mice obtained by a Cre‐lox recombination system.51 Chemically, CLA is a mixture of four isomers (cis‐9, cis‐10, trans‐11, and trans‐12) of linoleic acid with both distinct biological properties. As CLA is mainly found in milk and meat products and may also be generated from linoleic acid by human gut microflora,52 these studies are important, identifying for the first time that PPARγ natural ligands present in food or synthesised by commensal flora may improve colon inflammation.
The eicosanoid 15‐deoxy‐prostaglandin J2 (15d‐PGJ2) is also proposed as a natural ligand of PPARγ.40 Preventive intravenous administration of high doses of 15d‐PGJ2 (0.3 mg/kg) reduces ileal injury and mortality induced by intestinal ischaemia and reperfusion in rats.53 However, the physiological role of 15d‐PGJ2 in PPARγ activation in the colon is still open for debate as minimal concentrations of 15d‐PGJ2 required to activate PPARγ are approximately 10–150‐fold higher that those found in human intestinal epithelial cells.54
Recently, the unsaturated fatty acid derivative nitrolinoleic acid (LNO2), generated via nitric oxide dependent oxidative inflammatory reactions, has been identified as a new PPARγ agonist.43 Present in the vascular cell wall as the most abundant bioactive oxide of nitrogen and in the blood of healthy individuals at concentrations of approximately 500 nM, LNO2 is considered at present to be one of the most potent physiological endogenous natural ligand of PPARγ. It works at nanomolar concentrations and displays 10‐fold more efficacy than other known natural ligands such as lysophosphatidic acid, isomers of CLA, and 15d‐PGJ2.43 If LNO2 seems interesting in vascular diseases, future studies are needed to determine its intestinal effects in the maintenance of gut homeostasis and during inflammatory disorders.
Synthetic ligands
PPARγ has a large ligand binding pocket that accommodates lipophilic ligands, belonging to several different groups of chemical compounds such as thiazolidinediones, also known as glitazones which bind selectively PPARγ, and glitazars which bind both PPARα and PPARγ. Troglitazone was the first glitazone developed for therapeutic use in patients with diabetes and withdrawn from the market due to severe hepatic toxic effects. After demonstration that liver injury of troglitazone was idiosyncratic and independent of PPARγ stimulation, numerous additional glitazone molecules have been developed and two are already approved in the treatment of type 2 diabetes (rosiglitazone‐avandia and pioglitazone‐actos) (table 2). Glitazar is a novel family of dual acting PPARα/γ agonists developed as an oral treatment for insulin resistance related glucose and lipid abnormalities associated with type 2 diabetes and the metabolic syndrome.55 Four glitazar molecules have been developed and are awaiting FDA approval (table 2). Non‐steroidal anti‐inflammatory drugs are also reported in vitro as PPARγ ligands but in vivo their binding affinities of 0.1 mM are 1000‐fold higher than the mean concentrations found in patients conventionally treated with these drugs (table 2).56
Table 2 Affinity and intestinal functions of synthetic peroxisome proliferator activated receptor γ (PPARγ) modulators.
| Modulators | EC50/Ki | Effects in colon through PPARγ |
|---|---|---|
| Glitazones | ||
| Rosiglitazone45 | 89 nM/8 nM | Yes |
| Ciglitazone57 | 3 µM/− | ND |
| Troglitazone45 | 0.54 µM/474 nM | Yes |
| Pioglitazone45 | 0.59 µM/364 nM | Yes |
| Netoglitazone (MCC‐555)58 | 8 µM/− | ND |
| Glitazars | ||
| Muraglitazar59 | 110 nM/− | ND |
| Tesaglitazar60 | 0.25 µM/18 nM | ND |
| Farglitazar61 | 0.0034 µM/− | ND |
| Ragaglitazar62 | 2.1 µM/− | ND |
| NSAIDs56 | ||
| Indomethacin | 40 µM/− | ND |
| Flufenamic acid | ND | ND |
| Fenoprofen | ND | ND |
| Ibuprofen | ND | ND |
| L‐Tyrosine derived compounds | ||
| FMOC‐L‐Leu63 | −/15 µM | Yes |
| Miscellaneous | ||
| 5‐ASA64 | −/28.7 mM | Yes |
| CDDO65 | −/310 nM | Yes |
| COOH66 | ND | ND |
| Triphenyltin67 | 95 nM/− | ND |
| BADGE68 | ND | ND |
5‐ASA, 5‐aminosalycilic acid; BADGE, bisphenol A diglycidyl ether; CDDO, 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid; COOH, 2‐(2‐(4‐phenoxy‐2‐propylphenoxy) ethyl)indole‐5‐acetic acid; FMOC‐L‐Leu, fluorenylmethyloxycarbonyl‐L‐leucine; ND, not determined.
To date, only one open label pilot trial has evaluated the efficacy of the PPARγ ligand rosiglitazone (4 mg orally twice daily) in 15 patients with active UC, refractory to conventional treatment with either corticosteroids or immunomodulators and 5‐aminosalicylic acid.69 After 12 weeks of treatment with rosiglitazone, a substantial decrease in disease activity index score was reported, with clinical and endoscopic remission (27% and 20%, respectively) or part response (27%) in eight patients.69 Due to their systemic effects, the most well known adverse events of thiazolidinediones observed in patients with diabetes are weight gain and infrequent hepatotoxicity. This study in IBD patients led to new clinical trials in IBD with these chemical compounds, and may lead to the development of safer PPARγ agonist with topical effects and targeting selectively the colon.
5‐Aminosalicylic acid (5‐ASA): a prototype of a new class of PPARγ agonists
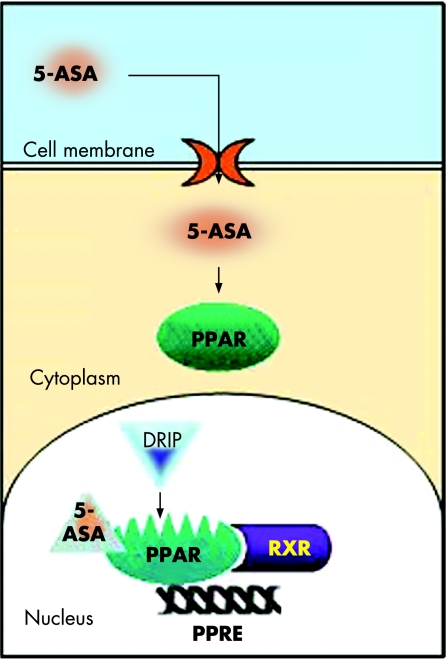
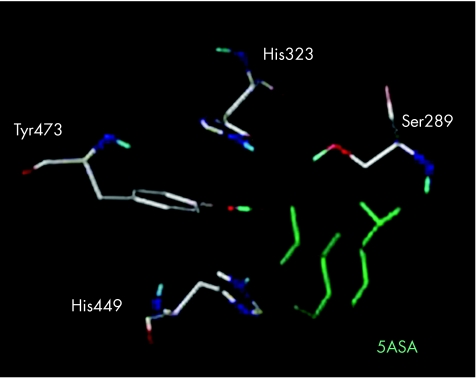
Recently, we published studies showing functional, biological, pharmacological, and chemical evidence that aminosalicylates are a new functional synthetic ligand for PPARγ in colonic epithelial cells.64 5‐ASA is one of the oldest anti‐inflammatory agents in use for the treatment of IBD, but the mechanism underlying its intestinal effects remains unknown. We showed that chemically induced colitis in mice heterozygous at the PPARγ locus (PPARγ +/−) was refractory to 5‐ASA therapy, arguing for a major role of PPARγ in mediating in vivo the anti‐inflammatory effect of 5‐ASA in the gut. Using the HT‐29 colon epithelial cell line, we found that 5‐ASA induced PPARγ expression. 5‐ASA was also able to bind PPARγ, to induce its translocation from the cytosol of epithelial cells to the nucleus, to promote a PPARγ conformational change, and to recruit a coactivator named DRIP (fig 5). Docking simulations showed a binding mode of 5‐ASA very similar to the crystal orientation of the thiazolidinedione head group of rosiglitazone. 5‐ASA fitted tightly with the PPARγ ligand binding domain interacting via hydrogen bonding with His‐323, His‐449, Tyr‐473, and Ser‐289, considered as key determinants required for molecular recognition and PPARγ activation (fig 6).64 Taken together, these data show that PPARγ is an essential receptor mediating the common 5‐ASA activities in IBD.
Figure 5 Molecular mechanisms of peroxisome proliferator activated receptor γ (PPARγ) activation by 5‐aminosalicylic acid (5‐ASA). After oral administration, 5‐ASA crosses the cell membrane of the epithelial cell through a transporter and binds to PPARγ in the cytoplasm. 5‐ASA then induces its nuclear translocation, promotes a PPARγ conformational change, and recruits the coactivator DRIP, leading to formation of a heterodimer between PPARγ and retinoid X receptor (RXR) and activation of the PPARγ response elements (PPRE).
Figure 6 Structural aspects of 5‐aminosalicylic acid (5‐ASA) binding to peroxisome proliferator activated receptor γ (PPARγ) ligand binding domain. 5‐ASA, in green, located in the PPARγ ligand binding domain, interacts via hydrogen bonding with His‐323, His‐449, Tyr‐473, and Ser‐289, coloured by atom type, and considered as key determinants required for molecular recognition and PPARγ activation.
PPARγ in IBD
PPARγ and experimental models of colitis
The first evidence of the involvement of PPARγ in the regulation of intestinal inflammation came from the use of the PPARγ synthetic agonist thiazolidinedione in mice with colitis induced by oral administration of dextran sodium sulfate (DSS).9 In this study, the two thiazolinediones troglitazone and rosiglitazone dramatically reduced disease severity in mice with colitis from 47% to 70%, seven days after DSS administration compared with the placebo treated group. These results were confirmed and extended several months later in another model of experimental colitis induced in mice by intrarectal administration of 2,4,6‐trinitrobenzene sulfonic acid (TNBS). Thiazolidinediones given preventively or in treatment mode have a therapeutic effect, reducing mortality, intensity of macroscopic and histological lesions, and levels of biological markers of colon inflammation, including the NFκB and stress kinase pathways involved in transduction of inflammation.14 In addition, genetic involvement of PPARγ in the protection against colon inflammation was shown by the increased susceptibility of PPARγ heterozygous mice (PPARγ+/−) to TNBS induced inflammation compared with their wild‐type littermates.14 At the present time, more than 20 published studies have reported similar prophylactic and therapeutic effects of PPARγ in different strains of mice, rats, or pigs with acute colitis induced by chemical compounds,9,14 bacteria,50 ischaemia‐reperfusion,70 and also in chronic colitis occurring after the transfer of immunocompetent T cells in SCID mice51 or spontaneously in IL‐10 deficient mice71 and SAMP1/YitFc animals (table 3).72
Table 3 Anti‐inflammatory properties of peroxisome proliferator activated receptor γ (PPARγ) in experimental models of inflammatory bowel diseases.
| Model | Modulators | Reference |
|---|---|---|
| Acute colitis | ||
| DSS | Troglitazone | Su9 |
| Rosiglitazone | Saubermann74 | |
| Pioglitazone | Takagi,75 Schaefer76 | |
| CLA | Bassaganya‐Riera51 | |
| TNBS | Troglitazone | Desreumaux14 |
| Rosiglitazone | ||
| Pioglitazone | Schaefer76 | |
| FMOC‐L‐leu | Rocchi63 | |
| 5‐ASA | Rousseaux64 | |
| Ischaemia/reperfusion | Rosiglitazone | Nakajima70 |
| 15‐d‐PGJ2 | Cuzzocrea53 | |
| NS‐398 | Sato77 | |
| Glutamine | Sato78 | |
| Bacteria induced colitis | CLA | Hontecillas50 |
| Chronic colitis | ||
| DSS | Troglitazone | Tanaka79 |
| TNBS | Rosiglitazone | Sanchez‐Hidalgo80 |
| CD4+CD45RBhigh | CLA | Bassaganya‐Riera51 |
| IL‐10 KO | Rosiglitazone | Lytle71 |
| SAMP1/YitFc | Rosiglitazone | Sugawara72 |
| Genetic evidence | ||
| PPARγ+/− | Desreumaux,14 Nakajima,70 Saubermann74 | |
| AdPPARγ | Katayama73 | |
| SAMP1/YitFc | Sugawara72 | |
| PPARγfl/fl Cre+ | Bassaganya‐Riera51 |
5‐ASA, 5‐aminosalycilic acid; 15dPGJ2, 15‐deoxy‐Δ12,14‐prostaglandin J2; CLA, conjugated linoleic acid; DSS, dextran sodium sulphate; FMOC‐L‐Leu, fluorenylmethyloxycarbonyl‐L‐leucine; IL‐10 KO, interleukin 10 knockout mice; PPARγfl/fl Cre+, PPARγ conditional knockout mice; TNBS, 2,4,6‐trinitrobenzene sulfonic acid.
Lessons from these animal studies are numerous. Firstly, natural and synthetic ligands of PPARγ are both effective in the treatment of acute and chronic colitis, with a similar beneficial effect of CLA and thiazolidinediones. Secondly, even if these treatments are efficacious when they are administered preventively or in treatment mode, a prophylactic effect is always more pronounced suggesting that PPARγ agonists may have higher efficacy in maintenance than in induction treatment in IBD patients. Thirdly, the therapeutic effect of PPARγ is mainly dependent on its abundance in target tissues. This notion is supported by the different susceptibility to colitis of animals in which the PPARγ gene has been disrupted14,51 or enhanced through gene transfer using adenoviruses,73 and also by analysis of SAMP1/YitFc animals where specific impaired expression and activation of PPARγ in the crypts of the small intestine is associated with ileitis.72 As PPARγ is expressed in the colon by epithelial cells and lamina propria mononuclear cells such as macrophages, and T and B cells, additional investigations in animals with cell type specific expression of PPARγ are required to determine the main cellular source responsible for the therapeutic effect of PPARγ.
PPARγ in patients with ulcerative colitis and Crohn's disease
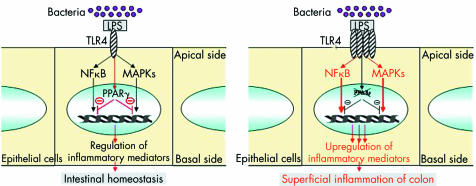
Despite in vitro and in vivo evidence of the anti‐inflammatory functions of the PPARγ/RXR heterodimer in the colon, very few studies have assessed the role of PPARγ in UC and CD.22,69,72 As PPARγ is mainly expressed in the colon by epithelial cells, prior expectation might be that decreased expression of this receptor may be found in an inflammatory disorder confined to superficial layers of the intestine and limited to the colon, such as UC rather than CD. Using quantitative polymerase chain reaction, ribonuclease protection assay, western blot, and imuunohistochemical methods, 60% decreased expression of PPARγ was observed at the mRNA and protein levels in the colon of UC patients compared with patients with CD and controls.22 This impaired expression was found in both healthy and inflamed colon and was limited to epithelial cells, suggesting that perturbed levels of PPARγ in UC are not secondary to the inflammatory process. The aetiology underlying impaired PPARγ expression in colonic epithelial cells of UC patients remains unknown. Comparable levels of PPARγ in peripheral mononuclear cells of IBD patients and controls and absence of specific mutations of the PPARγ gene or its promoter in UC patients suggest that epigenetic events may account for impaired PPARγ expression in UC patients.22 Another attractive possibility may be that TLR4 signalling to PPARγ is impaired in UC and an imbalance between elevated levels of TLR422 and impaired expression of PPARγ in epithelial cells of UC patients may alter mucosal tolerance to luminal LPS, resulting in superficial colonic inflammation (fig 7). More generally, we can hypothesise that impaired expression of PPARγ in UC may be secondary to non‐functional regulation of PPARγ expression in epithelial cells due to abnormal signalling pathways and/or lack of luminal stimuli induced by natural ligands or microorganisms. Further study is required to investigate more precisely the complex regulation of PPARγ expression by epithelial cells in UC patients.
Figure 7 Physiopathological model integrating impairment of peroxisome proliferator activated receptor γ (PPARγ) regulation by Toll‐like receptor 4 (TLR4) in patients with ulcerative colitis.19 Induction of PPARγ expression in intestinal epithelial cells by lipopolysaccharide (LPS) activated TLR4 leads to regulation of nuclear factor κB (NFκB) and MAPK pathways and control of the inflammatory response. Upregulation of TLR4 expression together with impaired expression of PPARγ in epithelial cells may lead to superficial colonic inflammation in patients with ulcerative colitis.
More recent data suggest that the role of PPARγ in the physiopathology of IBD will not be limited solely to UC but may also involve CD. Based on SAMP1/YitFc animal findings developing spontaneous ileitis due to a defect in expression of PPARγ in ileal crypts, secondary to inheritance of AKR alleles in the region of PPARγ, Sugawara et al tested the relationship between PPARγ alleles and CD in humans. They demonstrated that two intronic polymorphisms SNP1 (p<10−5) and SNP2 (p⩽10−3) exhibited lower allele frequencies in 134 CD patients compared with 125 controls.72 Replication of these results in independent cohorts of patients, family based analyses, and genotype/phenotype correlation studies will be necessary to conclude more definitely that PPARγ is a susceptibility gene in CD.
Conclusion and perspectives
PPARγ is highly expressed in the colon and a key receptor in the regulation of intestinal inflammation induced by bacteria. Other studies also indicate a role of PPARγ in tumour suppression, particularly in colon cancer.6,7,8 Therefore, greater knowledge of PPARγ expression and function in intestinal homeostasis and during inflammation will fuel speculations about its potential therapeutic effects in IBD to prevent inflammation and colorectal cancer.81 The discovery that 5‐ASA is a new topical ligand for this receptor expressed by colonic epithelial cells paves the way for the development of new molecules specifically targeting intestinal PPARγ. Because 5‐ASA was originally developed without any prior knowledge of its molecular target, there is hope that the research described above will lead to rationale optimisation or development of better PPARγ ligands. To date, 20 new molecules have been developed, optimised by docking analysis to activate PPARγ in intestinal epithelial cells. Among them, two families of compounds have been selected having 30–50‐fold more efficacy than 5‐ASA in activating PPARγ (personal communication). Optimisation of these new molecules is now in progress. Improvements in efficacy and safety may reside not solely in new compounds with higher affinity but also in a combination of agents with additive or synergic effects on PPARγ/RXR heterodimer. In this way, studies showing the synergistic effects of PPARγ and RXR agonists must be considered.14 Furthermore, of considerable interest is the recent discovery that some commensal bacteria and natural ligands present in food may induce PPARγ expression and activation in the colon. These data suggest the potential of associating a natural regulator and a synthetic ligand of PPARγ as drug therapy for IBD patients.
Acknowledgements
The authors would like to thank the Association François Aupetit, IRMAD, UCB Pharma, and Giuliani Spa for their support. We thank Dr LJ Egan for critical review of the manuscript and improvement of language style.
Footnotes
Conflict of interest: None declared.
References
- 1.Hugot J P, Chamaillard M, Zouali H.et al Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature 2001411599–603. [DOI] [PubMed] [Google Scholar]
- 2.Ogura Y, Bonen D K, Inohara N.et al A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001411603–606. [DOI] [PubMed] [Google Scholar]
- 3.McGovern D P, Hysi P, Ahmad T.et al Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet 2005141245–1250. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Alvares K, Huang Q.et al Cloning of a new member of the peroxisome proliferator‐activated receptor gene family from mouse liver. J Biol Chem 199326826817–26820. [PubMed] [Google Scholar]
- 5.Lehmann J M, Moore L B, Smith‐Oliver T A.et al An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator‐activated receptor gamma (PPAR gamma). J Biol Chem 199527012953–12956. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre A M, Chen I, Desreumaux P.et al Activation of the peroxisome proliferator‐activated receptor gamma promotes the development of colon tumors in C57BL/6J‐APCMin/+ mice. Nat Med 199841053–1057. [DOI] [PubMed] [Google Scholar]
- 7.Saez E, Tontonoz P, Nelson M C.et al Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med 199841058–1061. [DOI] [PubMed] [Google Scholar]
- 8.Sarraf P, Mueller E, Jones D.et al Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med 199841046–1052. [DOI] [PubMed] [Google Scholar]
- 9.Su C G, Wen X, Bailey S T.et al A novel therapy for colitis utilizing PPAR‐gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest 1999104383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. A unified nomenclature system for the nuclear receptor superfamily. Cell 199997161–163. [DOI] [PubMed] [Google Scholar]
- 11.Debril M B, Renaud J P, Fajas L.et al The pleiotropic functions of peroxisome proliferator‐activated receptor gamma. J Mol Med 20017930–47. [DOI] [PubMed] [Google Scholar]
- 12.Fajas L, Debril M B, Auwerx J. Peroxisome proliferator‐activated receptor‐gamma: from adipogenesis to carcinogenesis. J Mol Endocrinol 2001271–9. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer S A, Umesono K, Noonan D J.et al Convergence of 9‐cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 1992358771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desreumaux P, Dubuquoy L, Nutten S.et al Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator‐activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 2001193827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X Y, Wang L H, Chen T.et al Activation of human T lymphocytes is inhibited by peroxisome proliferator‐activated receptor gamma (PPARgamma) agonists. PPARgamma co‐association with transcription factor NFAT. J Biol Chem 20002754541–4544. [DOI] [PubMed] [Google Scholar]
- 16.Marx N, Mach F, Sauty A.et al Peroxisome proliferator‐activated receptor‐gamma activators inhibit IFN‐gamma‐induced expression of the T cell‐active CXC chemokines IP‐10, Mig, and I‐TAC in human endothelial cells. J Immunol 20001646503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris S G, Phipps R P. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur J Immunol 2001311098–1105. [DOI] [PubMed] [Google Scholar]
- 18.Jackson S M, Parhami F, Xi X P.et al Peroxisome proliferator‐activated receptor activators target human endothelial cells to inhibit leukocyte‐endothelial cell interaction. Arterioscler Thromb Vasc Biol 1999192094–2104. [DOI] [PubMed] [Google Scholar]
- 19.Dubuquoy L, Dharancy S, Nutten S.et al Role of peroxisome proliferator‐activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet 20023601410–1418. [DOI] [PubMed] [Google Scholar]
- 20.Fajas L, Auboeuf D, Raspe E.et al The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 199727218779–18789. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre M, Paulweber B, Fajas L.et al Peroxisome proliferator‐activated receptor gamma is induced during differentiation of colon epithelium cells. J Endocrinol 1999162331–340. [DOI] [PubMed] [Google Scholar]
- 22.Dubuquoy L, Jansson E A, Deeb S.et al Impaired expression of peroxisome proliferator‐activated receptor gamma in ulcerative colitis. Gastroenterology 20031241265–1276. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelman B M. PPARgamma in monocytes: less pain, any gain? Cell 199893153–155. [DOI] [PubMed] [Google Scholar]
- 24.Tontonoz P, Nagy L, Alvarez J G.et al PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 199893241–252. [DOI] [PubMed] [Google Scholar]
- 25.Vidal‐Puig A J, Considine R V, Jimenez‐Linan M.et al Peroxisome proliferator‐activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 1997992416–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal‐Puig A, Jimenez‐Linan M, Lowell B B.et al Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 1996972553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamura T, Nohara E, Nagai Y.et al Stage‐specific effects of a thiazolidinedione on proliferation, differentiation and PPARgamma mRNA expression in 3T3‐L1 adipocytes. Eur J Pharmacol 200142223–29. [DOI] [PubMed] [Google Scholar]
- 28.Rieusset J, Andreelli F, Auboeuf D.et al Insulin acutely regulates the expression of the peroxisome proliferator‐activated receptor‐gamma in human adipocytes. Diabetes 199948699–705. [DOI] [PubMed] [Google Scholar]
- 29.Rieusset J, Seydoux J, Anghel S I.et al Altered growth in male peroxisome proliferator‐activated receptor gamma (PPARgamma) heterozygous mice: involvement of PPARgamma in a negative feedback regulation of growth hormone action. Mol Endocrinol 2004182363–2377. [DOI] [PubMed] [Google Scholar]
- 30.Tominaga S, Morikawa M, Osumi T. Growth hormone has dual stage‐specific effects on the differentiation of 3T3‐L1 preadipocytes. J Biochem (Tokyo) 2002132881–889. [DOI] [PubMed] [Google Scholar]
- 31.Meirhaeghe A, Fajas L, Gouilleux F.et al A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler Thromb Vasc Biol 200323289–294. [DOI] [PubMed] [Google Scholar]
- 32.Shipley J M, Waxman D J. Simultaneous, bidirectional inhibitory crosstalk between PPAR and STAT5b. Toxicol Appl Pharmacol 2004199275–284. [DOI] [PubMed] [Google Scholar]
- 33.Bogazzi F, Ultimieri F, Raggi F.et al Peroxisome proliferator activated receptor gamma expression is reduced in the colonic mucosa of acromegalic patients. J Clin Endocrinol Metab 2002872403–2406. [DOI] [PubMed] [Google Scholar]
- 34.Khalfallah Y, Sassolas G, Borson‐Chazot F.et al Expression of insulin target genes in skeletal muscle and adipose tissue in adult patients with growth hormone deficiency: effect of one year recombinant human growth hormone therapy. J Endocrinol 2001171285–292. [DOI] [PubMed] [Google Scholar]
- 35.Eun C S, Han D S, Lee S H.et al Attenuation of colonic inflammation by PPAR‐gamma in intestinal epithelial cells: Effect on TLR pathways. Gastroenterology 2005128T1526 [Google Scholar]
- 36.Lee S K, Kim H J, Chi S G.et al Saccharomyces boulardii activates expression of peroxisome proliferator‐activated receptor‐gamma in HT‐29 cells. Korean J Gastroenterol 200545328–334. [PubMed] [Google Scholar]
- 37.Konturek P C, Kania J, Kukharsky V.et al Implication of peroxisome proliferator‐activated receptor gamma and proinflammatory cytokines in gastric carcinogenesis: link to Helicobacter pylori‐infection. J Pharmacol Sci 200496134–143. [DOI] [PubMed] [Google Scholar]
- 38.Wachtershauser A, Loitsch S M, Stein J. PPAR‐gamma is selectively upregulated in Caco‐2 cells by butyrate. Biochem Biophys Res Commun 2000272380–385. [DOI] [PubMed] [Google Scholar]
- 39.Schoonjans K, Martin G, Staels B.et al Peroxisome proliferator‐activated receptors, orphans with ligands and functions. Curr Opin Lipidol 19978159–166. [DOI] [PubMed] [Google Scholar]
- 40.Forman B M, Tontonoz P, Chen J.et al 15‐Deoxy‐delta 12, 14‐prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 199583803–812. [DOI] [PubMed] [Google Scholar]
- 41.Kliewer S A, Sundseth S S, Jones S A.et al Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator‐activated receptors alpha and gamma. Proc Natl Acad Sci U S A 1997944318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y, Correll P H, Vanden Heuvel J P. Conjugated linoleic acid decreases production of pro‐inflammatory products in macrophages: evidence for a PPAR gamma‐dependent mechanism. Biochim Biophys Acta 2002158189–99. [DOI] [PubMed] [Google Scholar]
- 43.Schopfer F J, Lin Y, Baker P R.et al Nitrolinoleic acid: an endogenous peroxisome proliferator‐activated receptor gamma ligand. Proc Natl Acad Sci U S A 20051022340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker P R, Lin Y, Schopfer F J.et al Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator‐activated receptor ligands. J Biol Chem 200528042464–42475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Chen J, Sun T.et al A yeast two‐hybrid technology‐based system for the discovery of PPARgamma agonist and antagonist. Anal Biochem 2004335253–259. [DOI] [PubMed] [Google Scholar]
- 46.Li D D, Chen J H, Chen Q.et al Swietenia mahagony extract shows agonistic activity to PPAR(gamma) and gives ameliorative effects on diabetic db/db mice. Acta Pharmacol Sin 200526220–222. [DOI] [PubMed] [Google Scholar]
- 47.McIntyre T M, Pontsler A V, Silva A R.et al Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A 2003100131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Sullivan S E, Tarling E J, Bennett A J.et al Novel time‐dependent vascular actions of delta9‐tetrahydrocannabinol mediated by peroxisome proliferator‐activated receptor gamma. Biochem Biophys Res Commun 2005337824–831. [DOI] [PubMed] [Google Scholar]
- 49.Mezei O, Banz W J, Steger R W.et al Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr 20031331238–1243. [DOI] [PubMed] [Google Scholar]
- 50.Hontecillas R, Wannemeulher M J, Zimmerman D R.et al Nutritional regulation of porcine bacterial‐induced colitis by conjugated linoleic acid. J Nutr 20021322019–2027. [DOI] [PubMed] [Google Scholar]
- 51.Bassaganya‐Riera J, Reynolds K, Martino‐Catt S.et al Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004127777–791. [DOI] [PubMed] [Google Scholar]
- 52.Alonso L, Cuesta E P, Gilliland S E. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. J Dairy Sci 2003861941–1946. [DOI] [PubMed] [Google Scholar]
- 53.Cuzzocrea S, Pisano B, Dugo L.et al Rosiglitazone and 15‐deoxy‐delta12,14‐prostaglandin J2, ligands of the peroxisome proliferator‐activated receptor‐gamma (PPAR‐gamma), reduce ischaemia/reperfusion injury of the gut. Br J Pharmacol 2003140366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald G A, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest 20011071335–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohray B B, Lohray V B, Bajji A C.et al (‐)3‐[4‐[2‐(Phenoxazin‐10‐yl)ethoxy]phenyl]‐2‐ethoxypropanoic acid [(‐)DRF 2725]: a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. J Med Chem 2001442675–2678. [DOI] [PubMed] [Google Scholar]
- 56.Lehmann J M, Lenhard J M, Oliver B B.et al Peroxisome proliferator‐activated receptors alpha and gamma are activated by indomethacin and other non‐steroidal anti‐inflammatory drugs. J Biol Chem 19972723406–3410. [DOI] [PubMed] [Google Scholar]
- 57.Willson T M, Cobb J E, Cowan D J.et al The structure‐activity relationship between peroxisome proliferator‐activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 199639665–668. [DOI] [PubMed] [Google Scholar]
- 58.Reginato M J, Bailey S T, Krakow S L.et al A potent antidiabetic thiazolidinedione with unique peroxisome proliferator‐activated receptor gamma‐activating properties. J Biol Chem 199827332679–32684. [DOI] [PubMed] [Google Scholar]
- 59.Devasthale P V, Chen S, Jeon Y.et al Design and synthesis of N‐[(4‐methoxyphenoxy)carbonyl]‐N‐[[4‐[2‐(5‐ methyl‐2‐phenyl‐4‐oxazolyl)ethoxy]phenyl] methyl]glycine [Muraglitazar/BMS‐298585], a novel peroxisome proliferator‐activated receptor alpha/gamma dual agonist with efficacious glucose and lipid‐lowering activities. J Med Chem 2005482248–2250. [DOI] [PubMed] [Google Scholar]
- 60.Ljung B, Bamberg K, Dahllof B.et al AZ 242, a novel PPARalpha/gamma agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats. J Lipid Res 2002431855–1863. [DOI] [PubMed] [Google Scholar]
- 61.Trifilieff A, Bench A, Hanley M.et al PPAR‐alpha and ‐gamma but not ‐delta agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF‐kappaB‐independent effect. Br J Pharmacol 2003139163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vikramadithyan R K, Hiriyan J, Suresh J.et al DRF 2655: a unique molecule that reduces body weight and ameliorates metabolic abnormalities. Obes Res 200311292–303. [DOI] [PubMed] [Google Scholar]
- 63.Rocchi S, Picard F, Vamecq J.et al A unique PPARgamma ligand with potent insulin‐sensitizing yet weak adipogenic activity. Mol Cell 20018737–747. [DOI] [PubMed] [Google Scholar]
- 64.Rousseaux C, Lefebvre B, Dubuquoy L.et al Intestinal antiinflammatory effect of 5‐aminosalicylic acid is dependent on peroxisome proliferator‐activated receptor‐gamma. J Exp Med 20052011205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Porter W W, Suh N.et al A synthetic triterpenoid, 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid (CDDO), is a ligand for the peroxisome proliferator‐activated receptor gamma. Mol Endocrinol 2000141550–1556. [DOI] [PubMed] [Google Scholar]
- 66.Berger J, Tanen M, Elbrecht A.et al Peroxisome proliferator‐activated receptor‐gamma ligands inhibit adipocyte 11beta ‐hydroxysteroid dehydrogenase type 1 expression and activity. J Biol Chem 200127612629–12635. [DOI] [PubMed] [Google Scholar]
- 67.Kanayama T, Kobayashi N, Mamiya S.et al Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator‐activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 200567766–774. [DOI] [PubMed] [Google Scholar]
- 68.Bishop‐Bailey D, Hla T, Warner T D. Bisphenol A diglycidyl ether (BADGE) is a PPARgamma agonist in an ECV304 cell line. Br J Pharmacol 2000131651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis J D, Lichtenstein G R, Stein R B.et al An open‐label trial of the PPAR‐gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol 2001963323–3328. [DOI] [PubMed] [Google Scholar]
- 70.Nakajima A, Wada K, Miki H.et al Endogenous PPAR gamma mediates anti‐inflammatory activity in murine ischemia‐reperfusion injury. Gastroenterology 2001120460–469. [DOI] [PubMed] [Google Scholar]
- 71.Lytle C, Tod T J, Vo K T.et al The peroxisome proliferator‐activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis 200511231–243. [DOI] [PubMed] [Google Scholar]
- 72.Sugawara K, Olson T S, Moskaluk C A.et al Linkage to peroxisome proliferator‐activated receptor‐gamma in SAMP1/YitFc mice and in human Crohn's disease. Gastroenterology 2005128351–360. [DOI] [PubMed] [Google Scholar]
- 73.Katayama K, Wada K, Nakajima A.et al A novel PPAR gamma gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 20031241315–1324. [DOI] [PubMed] [Google Scholar]
- 74.Saubermann L J, Nakajima A, Wada K.et al Peroxisome proliferator‐activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis 20028330–339. [DOI] [PubMed] [Google Scholar]
- 75.Takagi T, Naito Y, Tomatsuri N.et al Pioglitazone, a PPAR‐gamma ligand, provides protection from dextran sulfate sodium‐induced colitis in mice in association with inhibition of the NF‐kappaB‐cytokine cascade. Redox Rep 20027283–289. [DOI] [PubMed] [Google Scholar]
- 76.Schaefer K L, Denevich S, Ma C.et al Intestinal antiinflammatory effects of thiazolidenedione peroxisome proliferator‐activated receptor‐gamma ligands on T helper type 1 chemokine regulation include nontranscriptional control mechanisms. Inflamm Bowel Dis 200511244–252. [DOI] [PubMed] [Google Scholar]
- 77.Sato N, Kozar R A, Zou L.et al Peroxisome proliferator‐activated receptor gamma mediates protection against cyclooxygenase‐2‐induced gut dysfunction in a rodent model of mesenteric ischemia/reperfusion. Shock 200524462–469. [DOI] [PubMed] [Google Scholar]
- 78.Sato N, Moore F A, Kone B C.et al Differential induction of PPAR{gamma} by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am J Physiol Gastrointest Liver Physiol 2006290G616–G623. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka T, Kohno H, Yoshitani S.et al Ligands for peroxisome proliferator‐activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res 2001612424–2428. [PubMed] [Google Scholar]
- 80.Sanchez‐Hidalgo M, Martin A R, Villegas I.et al Rosiglitazone, an agonist of peroxisome proliferator‐activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol 2005691733–1744. [DOI] [PubMed] [Google Scholar]
- 81.Michalik L, Desvergne B, Wahli W. Peroxisome‐proliferator‐activated receptors and cancers: complex stories. Nat Rev Cancer 2004461–70. [DOI] [PubMed] [Google Scholar]