Abstract
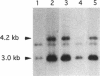
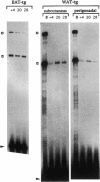
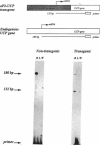
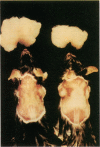
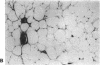
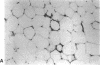
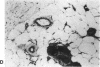
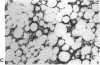
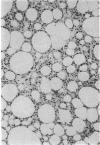
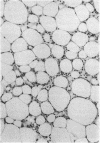
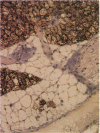
The brown fat-specific mitochondrial uncoupling protein (UCP) provides a mechanism for generating heat by uncoupling respiration and oxidative phosphorylation. It has been suggested that this system of thermogenesis can provide a defense against obesity. To test this idea, we created a transgenic mouse in which the fat-specific aP2 gene promoter directed Ucp expression in white fat and provided for the constitutive expression of Ucp in brown fat. Transgenic mice showed both Ucp mRNA and immunoreactive UCP in white fat at 2-10% the level normally measured in brown fat. A reduction in subcutaneous fat of aP2-Ucp C57BL/6J mice was observed at 3 mo of age. When the transgene was expressed in Avy genetically obese mice reductions in total body weight and subcutaneous fat stores were observed. Female transgenic Avy mice at 13 mo of age weighed 35 grams, a weight indistinguishable from nontransgenic C57BL/6J mice. Gonadal fat showed an increase in a novel adipocyte derivative that did not accumulate lipids and that constituted approximately 80% of the mass of the tissue in Avy transgenic. A major effect of aP2-Ucp in brown fat was to reduce endogenous gene expression by as much as 95%. The results suggest that UCP synthesized from the aP2 gene promoter is thermogenically active and capable of reducing fat stores.
Full text
PDF
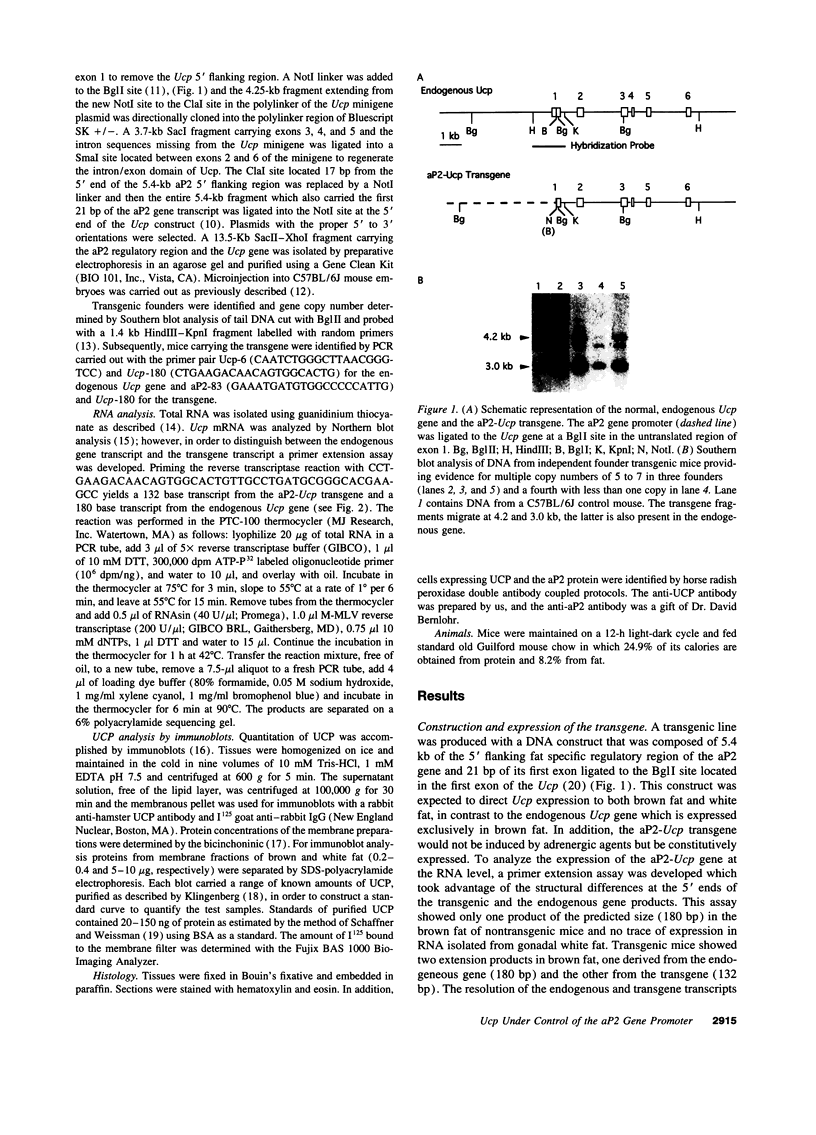




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer B. B., Kozak L. P. The mitochondrial uncoupling protein gene in brown fat: correlation between DNase I hypersensitivity and expression in transgenic mice. Mol Cell Biol. 1991 Aug;11(8):4147–4156. doi: 10.1128/mcb.11.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Casteilla L., Blondel O., Klaus S., Raimbault S., Diolez P., Moreau F., Bouillaud F., Ricquier D. Stable expression of functional mitochondrial uncoupling protein in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5124–5128. doi: 10.1073/pnas.87.13.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Brown adipose tissue thermogenesis and obesity. Prog Lipid Res. 1989;28(2):67–115. doi: 10.1016/0163-7827(89)90009-x. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Brown adipose tissue thermogenesis, energy balance, and obesity. Can J Biochem Cell Biol. 1984 Jul;62(7):610–617. doi: 10.1139/o84-081. [DOI] [PubMed] [Google Scholar]
- Houstek J., Kopecký J., Drahota Z. Specific properties of brown adipose tissue mitochondrial membrane. Comp Biochem Physiol B. 1978;60(3):209–214. doi: 10.1016/0305-0491(78)90088-3. [DOI] [PubMed] [Google Scholar]
- Houstek J., Kopecký J., Rychter Z., Soukup T. Uncoupling protein in embryonic brown adipose tissue--existence of nonthermogenic and thermogenic mitochondria. Biochim Biophys Acta. 1988 Aug 17;935(1):19–25. doi: 10.1016/0005-2728(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Nucleotide binding to uncoupling protein. Mechanism of control by protonation. Biochemistry. 1988 Jan 26;27(2):781–791. doi: 10.1021/bi00402a044. [DOI] [PubMed] [Google Scholar]
- Kozak L. P., Britton J. H., Kozak U. C., Wells J. M. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988 Sep 5;263(25):12274–12277. [PubMed] [Google Scholar]
- Kozak U. C., Kozak L. P. Norepinephrine-dependent selection of brown adipocyte cell lines. Endocrinology. 1994 Feb;134(2):906–913. doi: 10.1210/endo.134.2.7905411. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993 Dec 23;366(6457):740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Murdza-Inglis D. L., Patel H. V., Freeman K. B., Jezek P., Orosz D. E., Garlid K. D. Functional reconstitution of rat uncoupling protein following its high level expression in yeast. J Biol Chem. 1991 Jun 25;266(18):11871–11875. [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Porter R. K., Brand M. D. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature. 1993 Apr 15;362(6421):628–630. doi: 10.1038/362628a0. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Graves R. A., Greenstein A., Platt K. A., Shyu H. L., Mellovitz B., Spiegelman B. M. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Salans L. B., Cushman S. W., Weismann R. E. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973 Apr;52(4):929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Surwit R. S., Kuhn C. M., Cochrane C., McCubbin J. A., Feinglos M. N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988 Sep;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Wagner T. E., Hoppe P. C., Jollick J. D., Scholl D. R., Hodinka R. L., Gault J. B. Microinjection of a rabbit beta-globin gene into zygotes and its subsequent expression in adult mice and their offspring. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6376–6380. doi: 10.1073/pnas.78.10.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]