The treatment of hepatitis C has dramatically improved over the past decade. Unlike any other chronic viral infection, a significant proportion of patients with chronic hepatitis C can be cured. However, the current standard therapy—pegylated interferon alpha and ribavirin—has its limitations. Limited efficacy in patients with hepatitis C virus (HCV) genotype 1 and the side effect profile will necessitate the development of new therapeutic approaches. This review describes the efficacy and optimisation of the current standard therapy of hepatitis C and its problems in special patient populations. New treatment directions beyond interferon alpha based therapies are on the horizon.
Management of acute hepatitis C
Early identification of patients with acute HCV infection is important for their optimal management. The rate of chronic evolution is 50–90%, and the natural course of chronic hepatitis C can be associated with severe complications. Patients with chronic hepatitis C have the potential risk of developing liver cirrhosis and hepatocellular carcinoma.1 The social burden of HCV infection is high, including for health care workers. Extrahepatic manifestations of HCV are often troublesome and may not be reversible with viral eradication.2 These are good reasons for the design of a prophylactic vaccine but as this has yet to be accomplished, early treatment of acute HCV infection with interferon alpha (IFN) is the only option to prevent chronicity.
Immediate treatment of patients with symptomatic acute hepatitis C with recombinant IFN or pegylated IFN (PEG‐IFN) monotherapy for 24 weeks can prevent the development of chronic hepatitis C in approximately 90% of cases.3,4,5 Combination with ribavirin is not necessary.6 However, symptomatic patients also have a good chance to clear HCV spontaneously.7,8 This usually occurs in the first 12 weeks after the onset of symptoms. A wait and see strategy (that is, treatment of only those patients who remain HCV‐RNA positive 12 weeks after the onset of symptoms) resulted in an overall sustained virological response (self limited and treatment induced) in 91% of patients.8 A study coordinated by the German competence network for viral hepatitis (Hep‐Net)9 is underway to test if a wait and see strategy may be as effective as immediate treatment (www.kompetenznetz‐hepatitis.de/study_house/hcv_III_studie.htm). Asymptomatic patients however should be treated immediately as they have a higher risk for chronic evolution. Post exposure prophylaxis (for example, short duration IFN administration after a needlestick injury to prevent HCV infection) is not necessary.10 The future may bring highly effective antiviral drugs which allow short term treatment for all acutely infected patients.
Standard therapy of chronic hepatitis C
The importance of an effective treatment against hepatitis C is reflected by the 170 million people that are chronically infected with HCV. Despite implementation of blood donor screening in the early 1990s, an increase in HCV related cirrhosis, hepatic decompensation, and hepatocellular carcinoma over the next 10–20 years is still anticipated.11
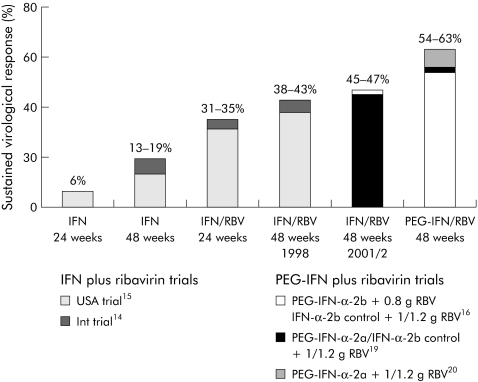
Before identification of HCV as the infectious agent for non‐A, non‐B hepatitis,12 it was found that IFN may lead to normalisation of transaminases and improvement in liver histology.13 After identification of HCV, it became possible to measure the success of therapy as long lasting disappearance of HCV‐RNA from serum, a so‐called sustained virological response (SVR). Since that time, the SVR rate has increased from 5% to 20% with IFN monotherapy and from 40% to 50% with the combination of IFN and ribavirin (fig 1). 14,15,16,17 The development of PEG‐IFN was a further milestone in the treatment of chronic hepatitis C.18 Two PEG‐IFNs are available: PEG‐IFN alpha‐2b (PEG‐Intron; Schering‐Plough, Kenilworth, New Jersey, USA) and PEG‐IFN alpha‐2a (PEGASYS; Roche). Pegylation of IFN allows once weekly administration due to an improved pharmacokinetic profile. PEG‐IFN/ribavirin combination therapy improved the overall SVR to 54–63% (fig 1).16,19,20 There seems to be no difference between both PEG‐IFNs in combination with ribavirin in terms of SVR.21 However, both PEG‐IFNs have different pharmacokinetic profiles due to their different polyethylene glycol moieties. PEG‐IFN alpha‐2b is bound to a single linear 12 kDa polyethylene glycol molecule whereas PEG‐IFN alpha‐2a is covalently attached to a 40 kDa branched chain polyethylene glycol moiety. The distinct sizes of the PEG‐IFNs influence the volume of distribution. PEG‐IFN alpha‐2b is given adjusted for body weight (1.5 μg/kg once weekly) while the larger PEG‐IFN alpha‐2a is given in a fixed dose of 180 μg once weekly (reviewed by Cornberg and colleagues18 and Pedder22) (table 1).
Figure 1 Development of therapy for chronic hepatitis C is a story of success. Sustained virological response rates have been improved from approximately 5% with interferon (IFN) monotherapy in the early 1990s to >60% with the optimised standard therapy of pegylated IFN (PEG‐IFN) and ribavirin.
Table 1 Current treatment recommendations for patients with chronic hepatitis C.
| HCV genotype | Duration (weeks) | PEG‐IFN dose (1×/week sc) | Ribavirin dose (daily orally) |
|---|---|---|---|
| Genotype 1 | 48 | 180 μg PEG‐IFN alpha‐2a | 1000 mg (<75 kg) |
| Genotypes 4–6* | 1200 mg (⩾75 kg) | ||
| 1.5 μg/kg PEG‐IFN alpha‐2b | 800 mg (<65 kg) | ||
| 1000 mg (65–85 kg) | |||
| 1200 mg (>85 kg) | |||
| Genotypes 2/3 | 24 | 180 μg PEG‐IFN alpha‐2a | 800 mg (all) |
| 1.5 μg/kg PEG‐IFN alpha‐2b | 800 mg (<65 kg) | ||
| 1000 mg (65–85 kg) | |||
| 1200 mg (>85 kg) |
*Hepatitis C virus (HCV) genotypes 4–6 may respond better to pegylated IFN (PEG‐IFN)/ribavirin than HCV genotype 1 patients. For HCV genotype 4, 36 weeks may be sufficient.109 Data are limited for HCV genotypes 5/6.
sc, subcutaneously.
Ribavirin should be administered according to the body weight of the patient. A retrospective analysis of a large PEG‐IFN alpha‐2b/ribavirin pivotal trial revealed that the optimal ribavirin dose is at least 10.6 mg/kg (table 2).16 Therefore, ribavirin (Rebetol; Schering‐Plough) is recommended at a concentration of approximately 11 mg/kg body weight in combination with PEG‐IFN alpha‐2b (table 1). When combined with PEG‐IFN alpha‐2a, a ribavirin (Copegus; Roche, Basel, Switzerland) dose of 1000 mg if <75 kg or 1200 mg if ⩾75 kg is recommended for HCV genotype 1 patients, while 800 mg ribavirin are suggested for patients with HCV genotypes 2 and 3 (tables 1, 2). The benefit of higher ribavirin doses has not been observed for genotype 2/3 patients in combination with PEG‐IFN alpha‐2a.20 The Hadziyannis study20 also confirmed the 24 week schedule for HCV genotype 2/3 patients whereas patients with HCV genotype 1 require 48 weeks of therapy (table 2). The 24 week regimen for patients with HCV genotypes 2 and 3 has also been confirmed for the combination of PEG‐IFN alpha‐2b and ribavirin (table 2).23,24
Table 2 Efficacy of hepatitis C treatment with pegylated interferon (PEG‐IFN) plus ribavirin.
| Study | Treatment | HCV genotype | Duration (weeks) | SVR (%) |
|---|---|---|---|---|
| Manns16 | 1.5 μg/kg PEG‐IFN alpha‐2b | HCV‐1 | 48 | 42 |
| 800 mg ribavirin | HCV‐2/3 | 48 | 82 | |
| 1.5 μg/kg PEG‐IFN alpha‐2b | HCV‐1 | 48 | 48 (retrospective) | |
| >10.6 mg/kg ribavirin | HCV‐2/3 | 48 | 88 (retrospective) | |
| Fried19 | 180 μg PEG‐IFN alpha‐2a | HCV‐1 | 48 | 46 |
| 1000/1200 mg ribavirin | HCV‐2/3 | 48 | 76 | |
| Hadziyannis20 | 180 μg PEG‐IFN alpha‐2a | HCV‐1 | 24 | 29 |
| 800 mg ribavirin | 48 | 40 | ||
| HCV‐2/3 | 24 | 78 | ||
| 48 | 73 | |||
| 180 μg PEG‐IFN alpha‐2a | HCV‐1 | 24 | 41 | |
| 1000/1200 mg ribavirin | 48 | 51 | ||
| HCV‐2/3 | 24 | 78 | ||
| 48 | 77 | |||
| Zeuzem24 | 1.5 μg/kg PEG‐IFN alpha‐2b | HCV‐2 | 24 | 93 |
| 800–1400 mg ribavirin | HCV‐3 | 79 | ||
| Kamal109 | 1.5 μg/kg PEG‐IFN alpha‐2b | HCV‐4 | 24 | 29 |
| 1000/1200 mg ribavirin | 36 | 66 | ||
| 48 | 69 | |||
| von Wagner27 | 180 μg PEG‐IFN alpha‐2a | HCV‐2/3 | 24 | 36 if TW4 HCV‐RNA ⩾600 IU/ml |
| 800–1200 mg ribavirin | 24 | 80 if TW4 HCV‐RNA <600 IU/ml | ||
| 16 | 82 if TW4 HCV‐RNA <600 IU/ml | |||
| Zeuzem32 | 1.5 μg/kg PEG‐IFN alpha‐2b | HCV‐1 LVL | 24 | 50 |
| 800–1400 mg ribavirin | (<600 000 IU/ml) | 89 if TW4 HCV‐RNA negative (<29 IU/ml) |
Sustained virological response rates (SVR) depend on hepatitis C virus (HCV) genotype, dose, and duration of treatment.
TW4, four weeks of therapy.
Early HCV‐RNA kinetics predict the outcome and success of treatment. Patients with HCV genotype 1, who do not show a HCV‐RNA decline of more than 2 log10 or have serum concentrations of more than 30 000 IU/ml HCV‐RNA after 12 weeks of therapy (TW12), have no chance of achieving an SVR.25,26 Thus therapy should be discontinued in these patients.
The main challenge for the future is to improve the success rates for the difficult to treat and non‐responsive HCV genotype 1 patients. While patients with HCV genotypes 2 and 3 can be cured in more than 75% of cases, the 40–50% SVR for patients with HCV genotype 1 is still unsatisfactory.
Individualisation and optimisation of the current standard therapy
Adherence to therapy
Adherence to therapy is one of the most important factors associated with the success of therapy.17 The definition of adherence used here is the 80/80/80 rule, as patients who received more than 80% of IFN, more than 80% of ribavirin, and were treated for more than 80% of the planned duration of treatment are considered adherent. One of the first studies investigating the effect of adherence demonstrated that patients who fulfilled the 80/80/80 rule had a 63% sustained response compared with 52% of those with less than 80% adherence.17 This was statistically significant for HCV genotype 1 patients. Therefore, it is important to reduce side effects and motivate patients to adhere to treatment in order to optimise treatment responses, especially in difficult to treat genotype 1 patients.
Optimal treatment duration
Optimal treatment duration may also improve the management of chronic hepatitis C. There are two different concepts to optimise treatment duration. While some patients with HCV genotype 1 may need longer treatment to improve the response, patients with HCV genotypes 2 and 3 may be treated for a shorter period of time to reduce costs and side effects.
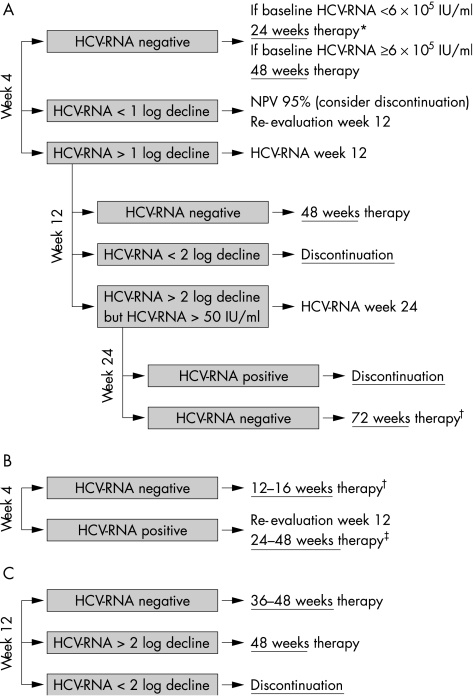
Many studies are investigating reductions in treatment duration for HCV genotypes 2 and 3 to 16, 14, or even 12 weeks. The first reported results are very promising but we have to consider individual factors when treating patients for less than 24 weeks. The early virological response (EVR) after four weeks of therapy (HCV‐RNA negative in serum at TW4) is one of the critical factors associated with the success of shorter therapy. Only patients who showed an EVR at week 4 had high SVR rates after 16 weeks27 (table 2), 14 weeks,28 or even after 12 weeks of therapy,29 whereas those without an EVR had low response rates, even with the 24 week schedule. However, 12 weeks seems to be the limit for some patients as relapse rates after 12 weeks were higher compared with the standard 24 week schedule.29 In addition to EVR, other factors are associated with response in patients with HCV genotypes 2 and 3. These are HCV genotype and baseline viral load. Patients with HCV genotypes 2 and 3 should be analysed separately because those with HCV genotype 2 respond better to PEG‐IFN and ribavirin therapy than those infected with HCV genotype 3 (table 2).24,29 Furthermore, the shorter treatment schedules revealed that HCV genotype 3 patients with low baseline viraemia (HCV‐RNA <600 000–800 000 IU/ml) had a much better chance of responding than those with a high viral load (HCV‐RNA >600 000–800 000 IU/ml).27,28 In conclusion, patients with HCV genotype 2 and those with HCV genotype 3 and low viral load who have an EVR after four weeks of therapy may be treated for less than 24 weeks, and patients without an EVR (especially HCV genotype 3 and high viral load) may be treated for more than 24 weeks (fig 2). The promising results obtained in these pilot studies need to be confirmed in large multicentre studies for both PEG‐IFNs. Tailoring treatment individually for patients with HCV genotype 2 and 3 will reduce costs, side effects, and further optimise response rates.
Figure 2 Optimisation of treatment duration for patients with hepatitis C virus (HCV) genotype 1 (A), 2/3 (B), and 4 (C). Sensitive HCV RNA assays at weeks 4, 12, and 24 may determine treatment duration. *Approved for pegylated interferon (PEG‐IFN) alpha‐2b only; †not yet approved but supported by preliminary study results.27,28,29,30,31; ‡longer treatment may be necessary for some patients who were HCV RNA positive at week 4.
We face the opposite problem in patients with HCV genotype 1. Extending treatment duration beyond 48 weeks is one strategy that may improve response rates in some of these difficult to treat patients. The rationale is to extend the time of HCV‐RNA negativity, especially in patients with a slow viral decline (first time HCV‐RNA negative between TW12 and TW24) to reduce relapse rates in these so called “late responders” (fig 2). Several studies investigated the efficacy and safety of 48 weeks versus 72 weeks of treatment with PEG‐IFN plus ribavirin in patients with chronic hepatitis C. Sanchez‐Tapias et al reported the benefit of extended therapy in patients who were HCV‐RNA positive at treatment week 4. Relapse rate after 72 weeks of therapy was significantly reduced in these patients.30 However, treatment duration beyond one year may lead to higher dropout rates which results in lower intent to treat responses.30,31 Multivariate analyses of these studies will hopefully reveal factors such as viral kinetics that will help to identify patients who will benefit from extended therapy. In conclusion, extension of therapy to 72 weeks may improve response rates for patients with a slow viral response (>2 log10 decline but >50 IU/ml at TW12 (fig 2)) but high motivation and compliance of the patient is mandatory.
On the other hand, it is possible to reduce treatment duration to 24 weeks in patients with HCV genotype 1 who have a low viral load at baseline and an EVR after four weeks of therapy (table 2, fig 2).32
Amantadine
Another strategy to enhance the success of therapy in patients with HCV genotype 1 may be the additional use of amantadine. In 1997, JP Smith reported that amantadine treatment could improve both biochemical and virological markers in patients with hepatitis C who had previously not responded to treatment with IFN.33 The effect of amantadine monotherapy could not be confirmed in other studies. However, these data led to numerous studies analysing the efficacy of amantadine in combination with IFN or IFN/ribavirin. Brillanti et al were among the first who demonstrated promising SVR with the triple therapy (IFN/ribavirin/amantadine) in prior IFN non‐responders.34 The dilemma of all of these small studies was that the results varied from study to study. While some studies confirmed the results, others demonstrated no additional benefit of amantadine in combination with IFN or IFN/ribavirin. A large German placebo controlled multicentre study treated 400 naïve patients with IFN/ribavirin/placebo or with IFN/ribavirin/amantadine. Triple therapy increased SVR by 8% in HCV genotype 1 patients but this was not statistically significant.35 A placebo controlled study with more than 700 patients in cooperation with Hep‐Net, testing the addition of amantadine to PEG‐IFN/ribavirin therapy in treatment naïve patients will hopefully provide the final answer. Perhaps addition of amantadine can reduce IFN side effects such as fatigue and depression36 and thus help patients to adhere to treatment. Amantadine is inexpensive. However, amantadine may cause QT prolongation in the electrocardiogram and thus is contraindicated in patients with prolonged QT times.
Other interferons
There are other type 1 interferons in development. Albuferon‐alpha (Human Genome Sciences, Rockville, Maryland, USA), which is an 85.7 kDa protein consisting of interferon alpha‐2b genetically fused to human serum albumin, further extends the half life of the IFN to approximately 148 hours (table 3). The pharmacokinetic profile of albuferon allows dosing at intervals of 2–4 weeks compared with one week with PEG‐IFNs. Results of a phase II trial testing multiple doses of albuferon in HCV genotype 1 patients demonstrated high antiviral efficacy.37 These data led to the initiation of phase III clinical trials evaluating the efficacy of albuferon in combination with ribavirin.
Table 3 Future drugs for the treatment of hepatitis C.
| Future therapies | Characteristics |
|---|---|
| Albuferon‐alpha (Human Genome Science) phase III | Longer half life than PEG‐IFN due to fusion of albumin to IFN (85.7 kDa). |
| Viramidine (Valeant) phase III | Prodrug of ribavirin with less anaemia. |
| HCV protease inhibitors, HCV polymerase inhibitors phase I/II (see table 5) | Direct antiviral action with fewer side effects than IFN. Direct inhibition of the HCV protease/HCV polymerase which are crucial for viral replication. |
| Other small molecules (eg ribozymes, siRNA, antisense molecules) phase I/II | Direct antiviral effect with potentially fewer side effects than IFN. Interference with the HCV genome or blocking translation leading to inhibition of viral replication. |
| Caspase inhibitors (eg IDN‐6556, Pfizer) phase I/II | Antifibrogenic effect. Reduction of hepatocyte apoptosis and reduction of hepatic stellate cell activation. |
| Toll‐like receptor agonists (CpG 10101‐TLR‐9 agonist, Coley; ANA‐975 TLR‐7 agonist; Anadys) phase I | Therapeutic stimulation of Toll‐like receptor pathways to modulate (Th1 shift) immune responses and to stimulate innate immunity. |
| Therapeutic vaccination (E1y—protein vaccine, Innogenetics; IC‐41—peptide vaccine, Intercell) phase I/II | Stimulation of the impaired HCV specific immune response in order to control viral replication or alter the natural course of the disease (regression of fibrosis). |
HCV, hepatitis C virus; PEG‐IFN, pegylated interferon.
Consensus interferon (CIFN) or interferon alphacon‐1 (Infergen; Valeant, Costa Mesa, California, USA) is another type 1 interferon that is already in use for the treatment of chronic hepatitis C. The “consensus” molecule, composed of conserved amino acids of type 1 interferons, shows greater biological activity than other type 1 interferons in vitro.38,39 Despite this in vitro advantage, a head to head study comparing CIFN and standard IFN monotherapy revealed only minor differences in efficacy. The results suggested that patients with HCV genotype 1 may have a small advantage with CIFN.40 A recent study reported better SVR in naïve patients with chronic hepatitis C when treated with CIFN in combination with ribavirin compared with standard IFN plus ribavirin.41 Some studies investigating the effect of high and daily dosing of CIFN, in combination with ribavirin in naïve as well as in non‐responders, demonstrated promising SVR.42,43 However, daily dosing requires high levels of motivation and compliance as adherence to therapy is an important factor influencing treatment outcome.
Side effects and complications
Severe side effects may reduce adherence to therapy and may result in dose modifications which will result in less response. Both IFN and ribavirin induce side effects that have to be considered in the management of patients with chronic hepatitis C (table 4). IFN related side effects can be divided into IFN induced bone marrow depression, flu‐like symptoms, neuropsychiatric disorders, and autoimmune syndromes. The main problem with ribavirin is haemolytic anaemia. Overall, side effects result in 10–20% premature withdrawals from therapy and an additional 20–30% of patients require dose modifications. These numbers are lower in recent than in earlier studies, suggesting improved understanding and management of adverse events,44 potentially also leading to higher SVR (fig 1). However, these percentages were recorded from registration trials using careful selection of patients. This may differ in general clinical practice where patients with, for example, a history of depression, low platelets, or thyroid disease are being treated.
Table 4 Common side effects (>20% of patients) recorded in major pegylated interferon (PEG‐IFN)/ribavirin trials16,19,20.
| Side effect | Incidence with PEG‐IFN alpha and ribavirin16,19,20 (%) |
|---|---|
| Headache | 47–62 |
| Pyrexia | 40–46 |
| Myalgia | 37–56 |
| Rigors | 24–48 |
| Arthralgia | 24–34 |
| Nausea | 35–43 |
| Loss of appetite | 21 |
| Weight loss | 29 |
| Diarrhoea | 22 |
| Alopecia | 21–36 |
| Rash/dermatitis | 20–24 |
| Injection site inflammation | 25 |
| Pruritus | 25–29 |
| Dyspnoea | 26 |
| Fatigue | 48–64 |
| Insomnia | 33–40 |
| Irritability | 24–35 |
| Depression | 22–31 |
The incidence of side effects between different studies is difficult to compare as the studies had significant differences in genetic and socioeconomic backgrounds. Furthermore, there were methodological differences in assessing the side effects. Patients were selected on the basis of well defined inclusion and exclusion criteria. Normal thyroid stimulating hormone levels pretreatment were a prerequisite.
IFN side effects
The effect of IFN on bone marrow results in decreased granulocytes and thrombocytes during treatment. These are usually moderate if normal counts are present initially. However, dose modifications are necessary, especially in patients with initially low counts. This limits the use of IFN in patients with advanced liver cirrhosis who often have low platelets and are also more vulnerable to infections. Neutropenia is one of the most common reasons for dose modifications. This granulocyte macrophage‐colony‐stimulating factor could potentially be used to stabilise neutrophil counts during IFN therapy.45,46 Cost benefit analyses and further trials are required to recommend routine use of these agents. However, our own experience and other reports suggest that IFN induced neutropenia is generally not associated with an increased risk of bacterial infections.47
Flu‐like symptoms usually occur during the first weeks of treatment and severity declines in the later treatment period. These side effects include fever, chills, headaches, arthralgia, and myalgia (table 4). Antipyretic drugs such as paracetamol can help to prevent or reduce these side effects.
Neuropsychiatric side effects such as irritability, severe fatigue, and apathy are frequent (table 4) and a great problem for many patients, which also affect family members. Severe depression can occur and even suicide has been reported.48 Psychiatric care and the use of antidepressants, especially serotonin uptake inhibitors, may help to reduce IFN induced depression49 and consequently improve adherence to therapy and response rates.50 Prospective placebo controlled trials are underway to confirm these preliminary findings.
IFN has immunomodulatory properties, and treatment can induce autoimmune phenomena.51 The most frequent problem is the development of autoimmune thyroiditis. In most cases thyroiditis starts with hyperthyroidism that later becomes hypothyroidism. Autoimmune thyroiditis has been reported in up to 20% of patients under or after IFN based therapies. This may not be reversible after stopping therapy.52 Predisposed patients with pre‐existing thyroid antibodies have a higher risk and it is possible that hepatitis C itself may be a cause of autoimmune thyroiditis.53
Other autoimmune diseases can also be aggravated by IFN therapy (for example, diabetes or autoimmune hepatitis). Patients with documented hepatitis C infection may deteriorate during IFN treatment if an underlying autoimmune hepatitis is present. This has been observed particularly in LKM antibody positive individuals. These patients require careful monitoring if IFN is considered as firstline treatment. However, IFN therapy seems to be safe in most HCV/anti‐LKM‐1 positive patients.54,55
Ribavirin side effects
The main side effect of ribavirin is haemolytic anaemia as this complication may frequently result in ribavirin dose reduction or even discontinuation, which may significantly affect the overall SVR, especially in patients with HCV genotype 1.16
Treatment with erythropoietin can effectively reverse ribavirin associated anaemia and allow full adherence to ribavirin therapy.56 This will improve response rates but the treatment is expensive and not reimbursed in many countries. This problem emphasises the need for alternative ribavirin‐like drugs with less toxicity and/or higher antiviral efficacy. Unfortunately, the mechanism by which ribavirin enhances the efficacy of IFN treatment and prevents relapse remains largely unknown. Proposed mechanisms are immunmodulatory effects, inhibition of inosine monophosphate dehydrogenase (IMPDH) activity, and induction of RNA mutagenesis.57,58 More potent IMPDH inhibitors such as mycophenolate mofetil (MMF, Cell Cept; Roche) or VX‐497 have been studied59 but with limited effects.60 Another approach is the development of a ribavirin prodrug. Viramidine is the amidine version of ribavirin and is converted by the enzyme adenosine deaminase to ribavirin, mainly in hepatocytes (table 3). Therefore, there is less uptake of ribavirin into red blood cells after administration of viramidine and consequently less haemolytic anaemia.61 First results of a phase II study demonstrated that viramidine in combination with PEG‐IFN alpha‐2a led to significantly less anaemia compared with ribavirin plus PEG‐IFN.62 Phase III studies with both PEG‐IFNs in combination with viramidine have been carried out (VISER 1 and 2). Preliminary data (VISER 1) confirmed the superior safety profile of viramidine plus PEG‐IFN alpha‐2b but viramidine did not meet the non‐inferiority to ribavirin efficacy endpoint on an intent‐to‐treat basis (SVR: 38% v 52%).[63a] Further subanalyses, the VISER 2 trial, and possibly weight based dosing of viramidine, may be awaited to draw final conclusions.
Meanwhile, drug monitoring of ribavirin could be an option to optimise the ribavirin dose without losing efficacy.64 The pharmacokinetic properties of ribavirin suggest that not only body weight but also renal function (glomerular filtration rate) should be considered when selecting the ribavirin dose.65
Treatment of hepatitis C in special populations
Patients with normal aminotransferase levels
Approximately 30% of patients with chronic hepatitis C maintain persistently normal alanine aminotransferase (ALT) levels despite having detectable HCV‐RNA in serum. Treatment indications for these patients are questionable. Firstly, these patients have generally mild liver disease and show a slow progression to cirrhosis. Secondly, treatment with IFN has been shown to be associated with ALT flares in the past (reviewed by Tassopoulos66). Thirdly, the efficacy of therapy may be lower as patients with elevated transaminases seem to respond better.67 However, up to one third of patients with normal ALT can present with significant liver fibrosis which necessitates an effective treatment.68,69 Zeuzem et al demonstrated that 48 weeks of PEG‐IFN alpha‐2a and ribavirin combination led to SVR rates of 52% in patients with chronic hepatitis C and persistently normal ALT levels. Treatment related flares in ALT activity were not observed.68 The efficacy and tolerability of PEG‐IFN/ribavirin combination therapy in patients with persistently normal ALT levels seem to be comparable with patients with elevated ALT levels. The decision to treat or not to treat patients with chronic hepatitis C and persistently normal ALT levels should be made on an individual basis, independent of ALT levels.
HIV/HCV coinfection
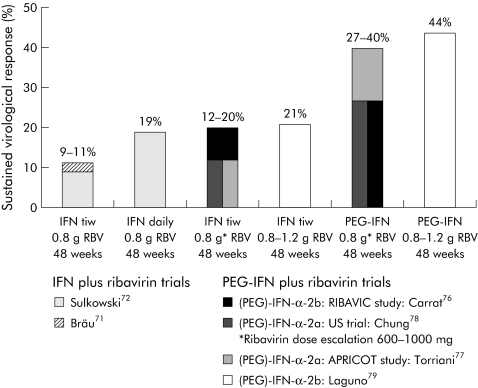
A significant portion of individuals infected with the human immune deficiency virus (HIV) are coinfected with HCV.70 The first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV coinfected patients was held in February 2005.71 HCV induced liver disease is now a major cause of morbidity for these patients following the introduction of highly active antiretroviral therapy (HAART). Hepatitis C liver disease may progress much more rapidly in HIV infected patients than in immunocompetent patients. Effective anti‐HCV treatment is therefore needed for HIV patients. The first studies using conventional IFN plus ribavirin showed disappointing SVR (fig 3). Side effects leading to frequent early discontinuation may have contributed to these results.70,72,73 In addition, ribavirin induced haemolysis is a particular problem for HIV patients and thus the ribavirin dose was often not adequate in these studies. Another problem of ribavirin is exacerbation of mitochondrial toxicity caused by anti‐HIV drugs, such as didanosine.74 This may explain the reported cases of pancreatitis in patients treated with didanosine containing antiretroviral regimens in some studies (reviewed by Manns and Wedemeyer75). Didanosine treatment was also associated with an increased risk of hepatic decompensation in patients with cirrhosis who were treated with PEG‐IFN and ribavirin.76 However, the overall risk for hepatic decompensation in HIV/HCV coinfected patients without cirrhosis was rather low. Therefore, patients with cirrhosis need careful monitoring and didanosine should be avoided. With added experience and the development of PEG‐IFN, SVR increased to more than 40% (fig 3) and early discontinuation due to adverse events declined to 12–17%.77,78,79,80 The efficacy of PEG‐IFN and ribavirin in HCV/HIV coinfected patients is comparable with HCV mono infected patients if the adequate ribavirin dose is used. In particular, for these patients, approaches to improve the adherence to therapy would further improve the success of treatment in coinfected patients. A dilemma of antiviral therapy with IFN is that GBV‐C (hepatitis G) may also be cleared. However, GBV‐C coinfection is associated with an improved natural course of HIV infection,81,82,83 even with HAART. A recent study showed that patients who lost GBV‐C had the poorest prognosis.84 GBV‐C status should be considered and careful follow up monitoring after anti‐HCV therapy may reveal the impact of HCV/HIV/GBV‐C coinfections.
Figure 3 Development of therapy for chronic hepatitis C in human immune deficiency virus (HIV) coinfected patients. Sustained virological response rates were improved from approximately 10% with interferon (IFN) plus ribavirin (RBV) to >40% with the optimised standard therapy of pegylated IFN (PEG‐IFN) and ribavirin. Sustained virological response rates for hepatitis C virus (HCV) genotype 1 patients were 14–38% in these PEG‐IFN/RBV trials.76,77,78,79
HCV and liver transplantation
HCV reinfection occurs in almost all patients after liver transplantation. While the course of hepatitis C in liver transplant recipients was believed to be rather benign in the late 1980s and early 1990s,85 HCV has led to a more rapid course post transplant in recent years86 with progression to cirrhosis within the first 5–10 years in 20–30% of patients. Thus HCV takes a more rapid course post‐transplant than in immunocompetent individuals, and treatment needs are obvious.
Antiviral therapy for HCV may be administered before transplantation to prevent reinfection of the graft. If this approach is successful, reinfection can be prevented in two thirds of patients.87 However, treatment with IFN and ribavirin is only poorly tolerated in decompensated cirrhosis and thus this approach will be feasible in only a minority of patients.88 Pre‐emptive treatment within the first 4–6 weeks post transplantation has been disappointing, with SVR between 0% and 33% for different regimens, including IFN monotherapy and IFN plus ribavirin combination therapy.89,90 There is more experience on the treatment of established recurrent hepatitis C. The most recent studies using PEG‐IFN in combination with ribavirin showed an initial virological response rate of up to 55%.91 Treatment duration should be at least similar to non‐transplanted patients considering early viral kinetics and HCV genotype. However, bone marrow toxicity, depression, and rejection are limiting factors that require aggressive management (for example, growth factors).92,93 The ribavirin dose may have to be adjusted as several patients have some degree of renal insufficiency. Interestingly, the risk for IFN induced graft rejection seems to be higher if ribavirin is not used.
Overall, several issues in the sometimes rather complicated management of post transplant hepatitis C have yet to be resolved. Patients with established graft hepatitis should be treated with PEG‐IFN and ribavirin. Whether reinfection can be prevented in future either by the new direct antivirals inhibiting HCV replication or by a combination with anti‐HCV antibodies with neutralising properties will have to be addressed in studies performed in the near future.
Dialysis patients
Treatment needs for dialysis patients with hepatitis C are obvious, especially if patients are considered for kidney transplantation. The outcome of HCV post kidney transplantation is worse than for HCV negative patients after renal transplantation. However, IFN based therapies are contraindicated post transplantation as they may induce rejection. Thus, if possible, HCV should be eliminated before transplantation. There have been several smaller reports on the treatment of HCV with IFN monotherapy in patients with end stage renal disease.94 Surprisingly, the results for IFN monotherapy under dialysis were better than in patients not undergoing dialysis, with SVR of 21–64%. Data on combination therapies with ribavirin are limited as ribavirin has traditionally been considered to be contraindicated in this setting. However, ribavirin can be given at lower doses in dialysis patients, usually between 200 and 400 mg daily.95 Several trials on the use of PEG‐IFNs plus ribavirin in dialysis patients are ongoing and final data are not available yet. However, it has to be considered that there might be significant differences between the two pegylated interferons in the setting of dialysis as PEG‐IFN alpha‐2a is eliminated mainly by the liver while PEG‐IFN alpha‐2b is cleared via the kidney.18 Future studies need to evaluate the potential of viramidine in particular for this special patient population.
Treatment of the future and drugs in the pipeline
Future aims should be to develop a treatment beyond IFN with less side effects and higher efficacy. Knowledge of the molecular structure of the hepatitis C proteins has allowed the design of new drugs that directly target the sites of HCV encoded enzymes that are important for the replication of the virus. The HCV protease and HCV polymerase are the main targets for these enzyme inhibitors (tables 3, 5). The first drug that has been tested in patients and demonstrated the proof of concept in humans for a HCV protease inhibitor was BILN‐2061 (Boehringer‐Ingelheim, Biberach, Germany). BILN‐2061 given twice daily as monotherapy for two days reduced HCV‐RNA by 2–3 log10 in most patients infected with HCV genotype 1.96 Unfortunately, further clinical trials are on hold due to preclinical cardiac toxicity issues. Other promising HCV protease inhibitors97,98,99 and HCV polymerase inhibitors100,101 are under investigation (table 5). Drug resistance may become a problem with these new compounds102 and combination therapies may be unavoidable. Phase II trials investigating these new drugs in combination with PEG‐IFN are ongoing. The majority of these new compounds were developed using an in vitro replicon system103 which was derived from patients with HCV genotype 1. This probably explains why BILN‐2061, for example, was less effective in patients with HCV genotypes 2 and 3.104 Thus other in vitro replicon systems derived from patients with non‐genotype 1 disease need to be developed. Recently, a new in vitro culture generated HCV clone derived from a patient infected with HCV genotype 2a was established and for the first time this was shown to be infectious in a chimpanzee.105 The HCV polymerase inhibitor valopicitabine (NM‐283; Idenix Pharmaceuticals, Cambridge, Massachusetts, USA) was developed using a bovine diarrhoea virus in vitro model. Therefore, this drug may by HCV genotype independent.
Table 5 Current status of hepatitis C virus (HCV) enzyme inhibitor development (April/2006).
| Stage of development | |
|---|---|
| HCV protease inhibitors | |
| BILN‐2061 (Boehringer Ingelheim) | Phase I (2–3 log10 decline in HCV‐RNA after 2 days). More effective in HCV genotype 1 than 3 patients.96,104Program halted |
| VX‐950 (Vertex) | Phase I monotherapy (4.4 log10 decline in HCV‐RNA after 14 days),97 in combination with PEG‐IFN. 5.5 log decline in HCV‐RNA after 14 days.111 Phase IIb studies have been initiated (prove 1 and 2). |
| SCH503034 (Schering‐Plough) | Phase I monotherapy (2.06 log10 decline of HCV‐RNA after 14 days), stronger antiviral effect in combination with PEG‐IFN.98,99 Phase IIb trial started |
| HCV polymerase inhibitors | |
| NM‐283, Valopicitabine (Idenix) | Phase I (∼1 log decline after 14 days), phase II (NM‐283 plus PEG‐IFN stronger effect compared with PEG‐IFN plus ribavirin after 12 weeks therapy).100,101,112 However, 800 mg dose group was stopped due to gastrointestinal symptoms. Studies are ongoing with 200–400 mg NM‐283.112 |
| R1626 (Roche) | Phase I (1.2 log decline in HCV‐RNA after 14 days with 1500 mg bid).113 |
Large phase II trials in combination with pegylated interferon (PEG‐IFN) with or without ribavirin have started. The combination of PEG‐IFN may be necessary to prevent drug resistance. More HCV enzyme inhibitors are in development (for example, GS‐9132, Gilead, Achillion; ITMN‐191, Intermune; JTK‐002/3, Japan Tobacco; HCV‐796, ViroPharma, Wyeth; BILB‐1941, Boehringer Ingelheim).
Key points
Sustained clearance of HCV can be achieved in 50–60% of patients with chronic hepatitis C.
Patients with HCV genotypes 2 and 3 are more sensitive to treatment than patients with HCV genotypes 1 and 4.
Treatment with pegylated interferon alpha and ribavirin can be associated with a wide variety of side effects.
Optimal management of side effects and optimisation of standard therapy can enhance response rates.
Another approach to treatment of HCV infection is induction of HCV specific immune responses (table 3). Spontaneous recovery after acute HCV infection is associated with a strong and broad immune response while development of chronic hepatitis C is associated with an impaired immune response.106,107 The aim of therapeutic vaccination is to stimulate the hepatitis C specific immune responses to control viral replication. The first therapeutic vaccines are currently being tested in phase I/II studies.108,109
Other advances include the development of small molecules such as ribozymes, antisense oligonucleotides, and small interfering RNAs that have been designed to control viral gene expression. There are many more approaches to fight hepatitis C and its complications. Tables 3 and 5 give an overview of the drugs in the pipeline.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Acknowledgements
Supported by the German Competence Network for Viral Hepatitis (Hep‐Net), funded by the German Ministry of Education and Research (BMBF‐O1 KI 0401).
Footnotes
Conflict of interest: None declared.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Hoofnagle J H. Course and outcome of hepatitis C. Hepatology 200236(5 suppl 1)S21–S29. [DOI] [PubMed] [Google Scholar]
- 2.Manns M P, Rambusch E G. Autoimmunity and extrahepatic manifestations in hepatitis C virus infection. J Hepatol 199931(suppl 1)39–42. [DOI] [PubMed] [Google Scholar]
- 3.Jaeckel E, Cornberg M, Wedemeyer H.et al Treatment of acute hepatitis C with interferon alfa‐2b. N Engl J Med 20013451452–1457. [DOI] [PubMed] [Google Scholar]
- 4.Santantonio T, Fasano M, Sinisi E.et al Efficacy of a 24‐week course of PEG‐interferon alpha‐2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol 200542329–333. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand J, Buggisch P, Boecher W.et al Early monotherapy with pegylated interferon alfa‐2b for acute hepatitis C infection: The HEP‐NET Acute‐HCV‐II Study. Hepatology 200643250–256. [DOI] [PubMed] [Google Scholar]
- 6.Kamal S M, Ismail A, Graham C S.et al Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus‐specific T cell response kinetics. Hepatology 2004391721–1731. [DOI] [PubMed] [Google Scholar]
- 7.Hofer H, Watkins‐Riedel T, Janata O.et al Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology 20033760–64. [DOI] [PubMed] [Google Scholar]
- 8.Gerlach J T, Diepolder H M, Zachoval R.et al Acute hepatitis C: high rate of both spontaneous and treatment‐induced viral clearance. Gastroenterology 200312580–88. [DOI] [PubMed] [Google Scholar]
- 9.Manns M P, Meyer S, Wedemeyer H. The German network of excellence for viral hepatitis (Hep‐Net). Hepatology 200338543–544. [DOI] [PubMed] [Google Scholar]
- 10.Chung H, Kudo M, Kumada T.et al Risk of HCV transmission after needlestick injury, and the efficacy of short‐duration interferon administration to prevent HCV transmission to medical personnel. J Gastroenterol 200338877–879. [DOI] [PubMed] [Google Scholar]
- 11.Davis G L, Albright J E, Cook S F.et al Projecting future complications of chronic hepatitis C in the United States. Liver Transpl 20039331–338. [DOI] [PubMed] [Google Scholar]
- 12.Choo Q L, Kuo G, Weiner A J.et al Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989244359–362. [DOI] [PubMed] [Google Scholar]
- 13.Hoofnagle J H, Mullen K D, Jones D B.et al Treatment of chronic non‐A, non‐B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med 19863151575–1578. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Marcellin P, Lee S S.et al Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 19983521426–1432. [DOI] [PubMed] [Google Scholar]
- 15.McHutchison J G, Gordon S C, Schiff E R.et al Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 19983391485–1492. [DOI] [PubMed] [Google Scholar]
- 16.Manns M P, McHutchison J G, Gordon S C.et al Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001358958–965. [DOI] [PubMed] [Google Scholar]
- 17.McHutchison J G, Manns M, Patel K.et al Adherence to combination therapy enhances sustained response in genotype‐1‐infected patients with chronic hepatitis C. Gastroenterology 20021231061–1069. [DOI] [PubMed] [Google Scholar]
- 18.Cornberg M, Wedemeyer H, Manns M P. Treatment of chronic hepatitis C with PEGylated interferon and ribavirin. Curr Gastroenterol Rep 2002423–30. [DOI] [PubMed] [Google Scholar]
- 19.Fried M W, Shiffman M L, Reddy K R.et al Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002347975–982. [DOI] [PubMed] [Google Scholar]
- 20.Hadziyannis S J, Sette H, Jr, Morgan T R.et al Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004140346–355. [DOI] [PubMed] [Google Scholar]
- 21.Mauss S, Berger F, Felten G.et al Peginterferon alfa‐2a versus peginterferon alfa‐2b in the treatment of chronic hepatitis C. J Hepatol 200542213 [Google Scholar]
- 22.Pedder S C. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis 200323(suppl 1)19–22. [DOI] [PubMed] [Google Scholar]
- 23.Cornberg M, Huppe D, Wiegand J.et al Treatment of chronic hepatitis C with PEG‐interferon alpha‐2b and ribavirin: 24 weeks of therapy are sufficient for HCV genotype 2 and 3. Z Gastroenterol 200341517–522. [DOI] [PubMed] [Google Scholar]
- 24.Zeuzem S, Hultcrantz R, Bourliere M.et al Peginterferon alfa‐2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol 200440993–999. [DOI] [PubMed] [Google Scholar]
- 25.Davis G L, Wong J B, McHutchison J G.et al Early virologic response to treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C. Hepatology 200338645–652. [DOI] [PubMed] [Google Scholar]
- 26.Berg T, Sarrazin C, Herrmann E.et al Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology 200337600–609. [DOI] [PubMed] [Google Scholar]
- 27.von Wagner M, Huber M, Berg T.et al Peginterferon‐alpha‐2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology 2005129522–527. [DOI] [PubMed] [Google Scholar]
- 28.Dalgard O, Bjoro K, Hellum K B.et al Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology 2004401260–1265. [DOI] [PubMed] [Google Scholar]
- 29.Mangia A, Santoro R, Minerva N.et al Peginterferon alfa‐2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med 20053522609–2617. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez‐Tapias J M, Diago M, Escartin P.et al Longer treatment duration with peginterferon alfa‐2A (40kd) (Pegasys (R)) and ribavirin (Copegus (R)) in naive patients with chronic hepatitis C and detectable HCV RNA by week 4 of therapy: Final results of the randomized, multicenter TeraViC‐4 study. Hepatology 200440218A [Google Scholar]
- 31.Berg T, von Wagner M, Nasser S.et al Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 20061301086–1097. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Buti M, Ferenci P.et al Efficacy of 24 weeks treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol 20064497–103. [DOI] [PubMed] [Google Scholar]
- 33.Smith J P. Treatment of chronic hepatitis C with amantadine. Dig Dis Sci 1997421681–1687. [DOI] [PubMed] [Google Scholar]
- 34.Brillanti S, Levantesi F, Masi L.et al Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology 200032630–634. [DOI] [PubMed] [Google Scholar]
- 35.Berg T, Kronenberger B, Hinrichsen H.et al Triple therapy with amantadine in treatment‐naive patients with chronic hepatitis C: a placebo‐controlled trial. Hepatology 2003371359–1367. [DOI] [PubMed] [Google Scholar]
- 36.Teuber G, Berg T, Naumann U.et al Randomized, placebo‐controlled, double‐blind trial with interferon‐alpha with and without amantadine sulphate in primary interferon‐alpha nonresponders with chronic hepatitis C. J Viral Hepat 20018276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bain V, Kaita K, Yoshida E.et al A phase 2 study to assess antiviral response, safety, and pharmacokinetics of albuferon in IFN‐alfa naive subjects with genotype 1 chronic hepatitis c. J Hepatol 2005429 [Google Scholar]
- 38.Blatt L M, Davis J M, Klein S B.et al The biologic activity and molecular characterization of a novel synthetic interferon‐alpha species, consensus interferon. J Interferon Cytokine Res 199616489–499. [DOI] [PubMed] [Google Scholar]
- 39.Ozes O N, Reiter Z, Klein S.et al A comparison of interferon‐Con1 with natural recombinant interferons‐alpha: antiviral, antiproliferative, and natural killer‐inducing activities. J Interferon Res 19921255–59. [DOI] [PubMed] [Google Scholar]
- 40.Tong M J, Reddy K R, Lee W M.et al Treatment of chronic hepatitis C with consensus interferon: a multicenter, randomized, controlled trial. Consensus Interferon Study Group. Hepatology 199726747–754. [DOI] [PubMed] [Google Scholar]
- 41.Sjogren M H, Sjogren R, Holtzmuller K.et al Interferon alfacon‐1 and ribavirin versus interferon alpha‐2b and ribavirin in the treatment of chronic hepatitis C. Dig Dis Sci 200550727–732. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser S, Hass H G, Gregor M. Successful retreatment of interferon/ribavirin nonresponders with daily dosing of consensus interferon. J Hepatol 200542(suppl 2)207–208. [Google Scholar]
- 43.Cornberg M, Hadem J, Herrmann E.et al Treatment with daily consensus interferon (CIFN) plus ribavirin in non‐responder patients with chronic hepatitis C: A randomized open‐label pilot study. J Hepatol 200644291–301. [DOI] [PubMed] [Google Scholar]
- 44.Fried M W. Side effects of therapy of hepatitis C and their management. Hepatology 200236(5 suppl 1)S237–S244. [DOI] [PubMed] [Google Scholar]
- 45.Shiffman M L, Hofmann C M, Luketic V A.et al Use of granulocyte macrophage colony stimulating factor alone or in combination with interferon‐alpha‐2b for treatment of chronic hepatitis C. J Hepatol 199828382–389. [DOI] [PubMed] [Google Scholar]
- 46.Van Thiel D H, Faruki H, Friedlander L.et al Combination treatment of advanced HCV associated liver disease with interferon and G‐CSF. Hepatogastroenterology 199542907–912. [PubMed] [Google Scholar]
- 47.Soza A, Everhart J E, Ghany M G.et al Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology 2002361273–1279. [DOI] [PubMed] [Google Scholar]
- 48.Janssen H L, Brouwer J T, van der Mast R C.et al Suicide associated with alfa‐interferon therapy for chronic viral hepatitis. J Hepatol 199421241–243. [DOI] [PubMed] [Google Scholar]
- 49.Musselman D L, Lawson D H, Gumnick J F.et al Paroxetine for the prevention of depression induced by high‐dose interferon alfa. N Engl J Med 2001344961–966. [DOI] [PubMed] [Google Scholar]
- 50.Schaefer M, Schwaiger M, Garkisch A S.et al Prevention of interferon‐alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol 200542793–798. [DOI] [PubMed] [Google Scholar]
- 51.Wesche B, Jaeckel E, Trautwein C.et al Induction of autoantibodies to the adrenal cortex and pancreatic islet cells by interferon alpha therapy for chronic hepatitis C. Gut 200148378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lisker‐Melman M, Di Bisceglie A M, Usala S J.et al Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology 19921022155–2160. [DOI] [PubMed] [Google Scholar]
- 53.Marazuela M, Garcia‐Buey L, Gonzalez‐Fernandez B.et al Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon‐alpha therapy. Clin Endocrinol (Oxf) 199644635–642. [DOI] [PubMed] [Google Scholar]
- 54.Dalekos G N, Wedemeyer H, Obermayer‐Straub P.et al Epitope mapping of cytochrome P4502D6 autoantigen in patients with chronic hepatitis C during alpha‐interferon treatment. J Hepatol 199930366–375. [DOI] [PubMed] [Google Scholar]
- 55.Todros L, Saracco G, Durazzo M.et al Efficacy and safety of interferon alfa therapy in chronic hepatitis C with autoantibodies to liver‐kidney microsomes. Hepatology 1995221374–1378. [PubMed] [Google Scholar]
- 56.Afdhal N H, Dieterich D T, Pockros P J.et al Epoetin alfa maintains ribavirin dose in HCV‐infected patients: a prospective, double‐blind, randomized controlled study. Gastroenterology 20041261302–1311. [DOI] [PubMed] [Google Scholar]
- 57.Lau J Y, Tam R C, Liang T J.et al Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 2002351002–1009. [DOI] [PubMed] [Google Scholar]
- 58.Perelson A S, Ribeiro R M. Mutagenic effects of ribavirin in vivo. J Hepatol 200543553–555. [DOI] [PubMed] [Google Scholar]
- 59.Sintchak M D, Nimmesgern E. The structure of inosine 5′‐monophosphate dehydrogenase and the design of novel inhibitors. Immunopharmacology 200047163–184. [DOI] [PubMed] [Google Scholar]
- 60.Cornberg M, Hinrichsen H, Teuber G.et al Mycophenolate mofetil in combination with recombinant interferon alfa‐2a in interferon‐nonresponder patients with chronic hepatitis C. J Hepatol 200237843–847. [DOI] [PubMed] [Google Scholar]
- 61.Watson J. Prospects for hepatitis C virus therapeutics: levovirin and viramidine as improved derivatives of ribavirin. Curr Opin Investig Drugs 20023680–683. [PubMed] [Google Scholar]
- 62.Benhamou Y, Pockros P, Rodriguez‐Torres M.et al The safety and efficacy of viramidine plus pegylated interferon alpha‐2b versus ribavirin plus pegylated interferon alpha‐2b in therapy‐naive patients infected with HCV: phase 3 results. J Hepatol 200644S273 [Google Scholar]
- 63.Gish R G, Nelson D, Arora S.et al Virological response and safety outcomes in therapy‐naive patients treated for chronic hepatitis C with viramidine in combination with pegylated interferon alfa‐2a. J Hepatol 20054239. [DOI] [PubMed] [Google Scholar]
- 64.Svensson J O, Bruchfeld A, Schvarcz R.et al Determination of ribavirin in serum using highly selective solid‐phase extraction and high‐performance liquid chromatography. Ther Drug Monit 200022215–218. [DOI] [PubMed] [Google Scholar]
- 65.Bruchfeld A, Lindahl K, Schvarcz R.et al Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit 200224701–708. [DOI] [PubMed] [Google Scholar]
- 66.Tassopoulos N C. Treatment of patients with chronic hepatitis C and normal ALT levels. J Hepatol 199931(suppl 1)193–196. [DOI] [PubMed] [Google Scholar]
- 67.Zeuzem S, Feinman S V, Rasenack J.et al Peginterferon alfa‐2a in patients with chronic hepatitis C. N Engl J Med 20003431666–1672. [DOI] [PubMed] [Google Scholar]
- 68.Zeuzem S, Diago M, Gane E.et al Peginterferon alfa‐2a (40 kilodaltons) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterology 20041271724–1732. [DOI] [PubMed] [Google Scholar]
- 69.Bacon B R. Treatment of patients with hepatitis C and normal serum aminotransferase levels. Hepatology 200236(5 suppl 1)S179–S184. [DOI] [PubMed] [Google Scholar]
- 70.Brau N. Treatment of chronic hepatitis C in human immunodeficiency virus/hepatitis C virus‐coinfected patients in the era of pegylated interferon and ribavirin. Semin Liver Dis 20052533–51. [DOI] [PubMed] [Google Scholar]
- 71.Alberti A, Clumeck N, Collins S.et al Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co‐infected patients. J Hepatol 200542615–624. [DOI] [PubMed] [Google Scholar]
- 72.Brau N, Rodriguez‐Torres M, Prokupek D.et al Treatment of chronic hepatitis C in HIV/HCV‐coinfection with interferon alpha‐2b+ full‐course vs. 16‐week delayed ribavirin. Hepatology 200439989–998. [DOI] [PubMed] [Google Scholar]
- 73.Sulkowski M S, Felizarta F, Smith C.et al Daily versus thrice‐weekly interferon alfa‐2b plus ribavirin for the treatment of chronic hepatitis C in HIV‐infected persons: a multicenter randomized controlled trial. J Acquir Immune Defic Syndr 200435464–472. [DOI] [PubMed] [Google Scholar]
- 74.Bani‐Sadr F, Carrat F, Pol S.et al Risk factors for symptomatic mitochondrial toxicity in HIV/hepatitis C virus‐coinfected patients during interferon plus ribavirin‐based therapy. J Acquir Immune Defic Syndr 20054047–52. [DOI] [PubMed] [Google Scholar]
- 75.Manns M P, Wedemeyer H. Treatment of hepatitis C in HIV‐infected patients: significant progress but not the final step. JAMA 20042922909–2913. [DOI] [PubMed] [Google Scholar]
- 76.Mauss S, Valenti W, DePamphilis J.et al Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon‐based therapy. AIDS 200418F21–F25. [DOI] [PubMed] [Google Scholar]
- 77.Carrat F, Bani‐Sadr F, Pol S.et al Pegylated interferon alfa‐2b vs standard interferon alfa‐2b, plus ribavirin, for chronic hepatitis C in HIV‐infected patients: a randomized controlled trial. JAMA 20042922839–2848. [DOI] [PubMed] [Google Scholar]
- 78.Torriani F J, Rodriguez‐Torres M, Rockstroh J K.et al Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection in HIV‐infected patients. N Engl J Med 2004351438–450. [DOI] [PubMed] [Google Scholar]
- 79.Chung R T, Andersen J, Volberding P.et al Peginterferon alfa‐2a plus ribavirin versus interferon alfa‐2a plus ribavirin for chronic hepatitis C in HIV‐coinfected persons. N Engl J Med 2004351451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laguno M, Murillas J, Blanco J L.et al Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for treatment of HIV/HCV co‐infected patients. AIDS 200418F27–F36. [DOI] [PubMed] [Google Scholar]
- 81.Heringlake S, Ockenga J, Tillmann H L.et al GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus‐infected patients? J Infect Dis 19981771723–1726. [DOI] [PubMed] [Google Scholar]
- 82.Tillmann H L, Heiken H, Knapik‐Botor A.et al Infection with GB virus C and reduced mortality among HIV‐infected patients. N Engl J Med 2001345715–724. [DOI] [PubMed] [Google Scholar]
- 83.Xiang J, Wunschmann S, Diekema D J.et al Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med 2001345707–714. [DOI] [PubMed] [Google Scholar]
- 84.Williams C F, Klinzman D, Yamashita T E.et al Persistent GB virus C infection and survival in HIV‐infected men. N Engl J Med 2004350981–990. [DOI] [PubMed] [Google Scholar]
- 85.Boker K H, Dalley G, Bahr M J.et al Long‐term outcome of hepatitis C virus infection after liver transplantation. Hepatology 199725203–210. [DOI] [PubMed] [Google Scholar]
- 86.Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol 200542448–456. [DOI] [PubMed] [Google Scholar]
- 87.Forns X, Garcia‐Retortillo M, Serrano T.et al Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol 200339389–396. [DOI] [PubMed] [Google Scholar]
- 88.Everson G T. Treatment of chronic hepatitis C in patients with decompensated cirrhosis. Rev Gastroenterol Disord 20044(suppl 1)S31–S38. [PubMed] [Google Scholar]
- 89.Terrault N A. Prophylactic and preemptive therapies for hepatitis C virus‐infected patients undergoing liver transplantation. Liver Transpl 20039S95–100. [DOI] [PubMed] [Google Scholar]
- 90.Chalasani N, Manzarbeitia C, Ferenci P.et al Peginterferon alfa‐2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology 200541289–298. [DOI] [PubMed] [Google Scholar]
- 91.Dumortier J, Scoazec J Y, Chevallier P.et al Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa‐2b and ribavirin combination. J Hepatol 200440669–674. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez‐Luna H, Khatib A, Sharma P.et al Treatment of recurrent hepatitis C infection after liver transplantation with combination of pegylated interferon alpha2b and ribavirin: an open‐label series. Transplantation 200477190–194. [DOI] [PubMed] [Google Scholar]
- 93.Neff G W, Montalbano M, O'Brien C B.et al Treatment of established recurrent hepatitis C in liver‐transplant recipients with pegylated interferon‐alfa‐2b and ribavirin therapy. Transplantation 2004781303–1307. [DOI] [PubMed] [Google Scholar]
- 94.Fabrizi F, Poordad F F, Martin P. Hepatitis C infection and the patient with end‐stage renal disease. Hepatology 2002363–10. [DOI] [PubMed] [Google Scholar]
- 95.Bruchfeld A, Stahle L, Andersson J.et al Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection—a pilot study. J Viral Hepat 20018287–292. [DOI] [PubMed] [Google Scholar]
- 96.Hinrichsen H, Benhamou Y, Wedemeyer H.et al Short‐term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology 20041271347–1355. [DOI] [PubMed] [Google Scholar]
- 97.Reesink H W, Zeuzem S, van Vliet A.et al Initial results of a phase 1B, multiple‐dose study of VX‐950, a hepatitis C virus protease inhibitor. Gastroenterology 2005128(suppl 2)A697 [Google Scholar]
- 98.Zeuzem S, Sarrazin C, Rouzier R.et al Anti‐viral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype‐1 (HCV‐1) patients refractory to pegylated interferon (PEG‐IFN‐alpha). Hepatology 200542233–4A. [Google Scholar]
- 99.Zeuzem S, Sarrazin C, Wagner F.et al Combination therapy with the HCV protease inhibitor, SCH 503034, plus peg‐intron in hepatitis C genotype‐1 peg‐intron non‐responders: Phase IB results. Hepatology 200542276–7A. [Google Scholar]
- 100.Afdhal N, Rodrriguez‐Torres M, Lawitz E.et al Enhanced antiviral efficacy for valopicitabine (NM283) plus PEG‐Interferon in hepatitis C patients with HCV genotype‐1 infection: Results of a phase IIa multicenter trial. J Hepatol 20054239–40. [Google Scholar]
- 101.O'Brien C, Godofsky E, Rodriguez‐Torres M.et al Randomized trial of valopicitabine (NM283), alone or with peg‐interferon, vs. retreatment with peg‐interferon plus ribavirin (pegifn/RBV) in hepatitis C patients with previous non‐response to pegifn/RBV: First interim results, Hepatology 200542234A [Google Scholar]
- 102.Sarrazin C, Kieffer T, Bartels D.et al Characterization of viral variants in the HCV NS3 protease domain of genotype 1 patients that are selected during 14 days of dosing with VX‐950. Hepatology 200542751A [Google Scholar]
- 103.Lohmann V, Korner F, Koch J.et al Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 1999285110–113. [DOI] [PubMed] [Google Scholar]
- 104.Reiser M, Hinrichsen H, Benhamou Y.et al Antiviral efficacy of NS3‐serine protease inhibitor BILN‐2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology 200541832–835. [DOI] [PubMed] [Google Scholar]
- 105.Wakita T, Pietschmann T, Kato T.et al Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 200511791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 20055215–229. [DOI] [PubMed] [Google Scholar]
- 107.Wedemeyer H, He X S, Nascimbeni M.et al Impaired effector function of hepatitis C virus‐specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol 20021693447–3458. [DOI] [PubMed] [Google Scholar]
- 108.Nevens F, Roskams T, Van Vlierberghe H.et al A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology 2003381289–1296. [DOI] [PubMed] [Google Scholar]
- 109.Manns M P, Berg T, Wedemeyer H.et al Immunization with the therapeutic hepatitis C virus (HCV) peptide vaccine IC41 in 66 chronic hepatitis C non‐responder patients. Hepatology 200440251A [Google Scholar]
- 110.Kamal S M, El Tawil A A, Nakano T.et al Peginterferon (alpha)‐2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut 200554858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reesink H W, Forestier N, Weegink C J.et al Initial results of a 14‐day study of the hepatitis C virus inhibitor protease VX‐950, in combination with peginterferon alpha‐2a. J Hepatol 200644S272 [Google Scholar]
- 112.Dieterich D, Lawitz E, Nguyen T.et al Early clearance of HCV RNA with valopicitabine (NM283) plus Peg‐interferon in treatment‐naive patients with HCV‐1 infection: First results from a phase IIb trial. J Hepatol 200644S271 [Google Scholar]
- 113.Roberts S, Cooksley G, Shaw D.et al Interim results of a multiple ascending dose study of R1626, a novel nucleoside analog targeting HCV polymerase in chronic HCV patients. J Hepatol 200644S269 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.