Abstract
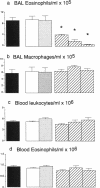
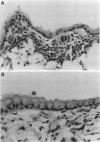
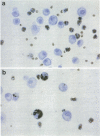
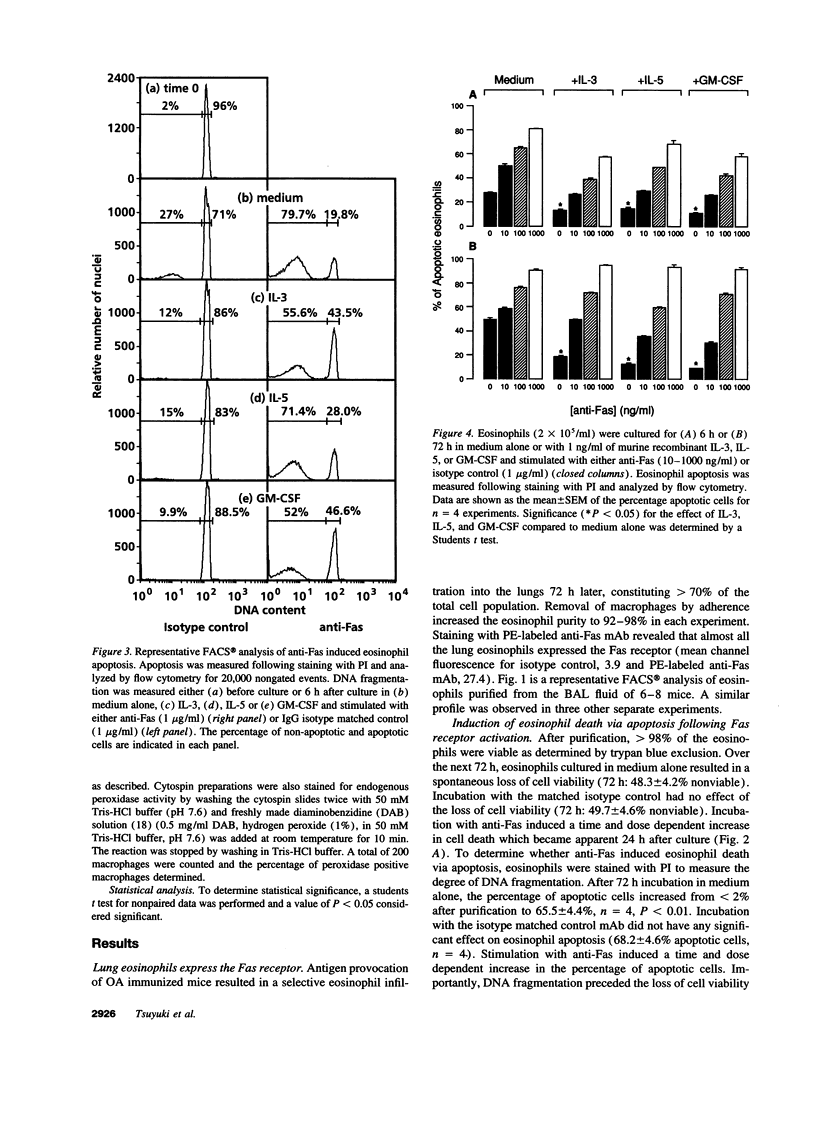
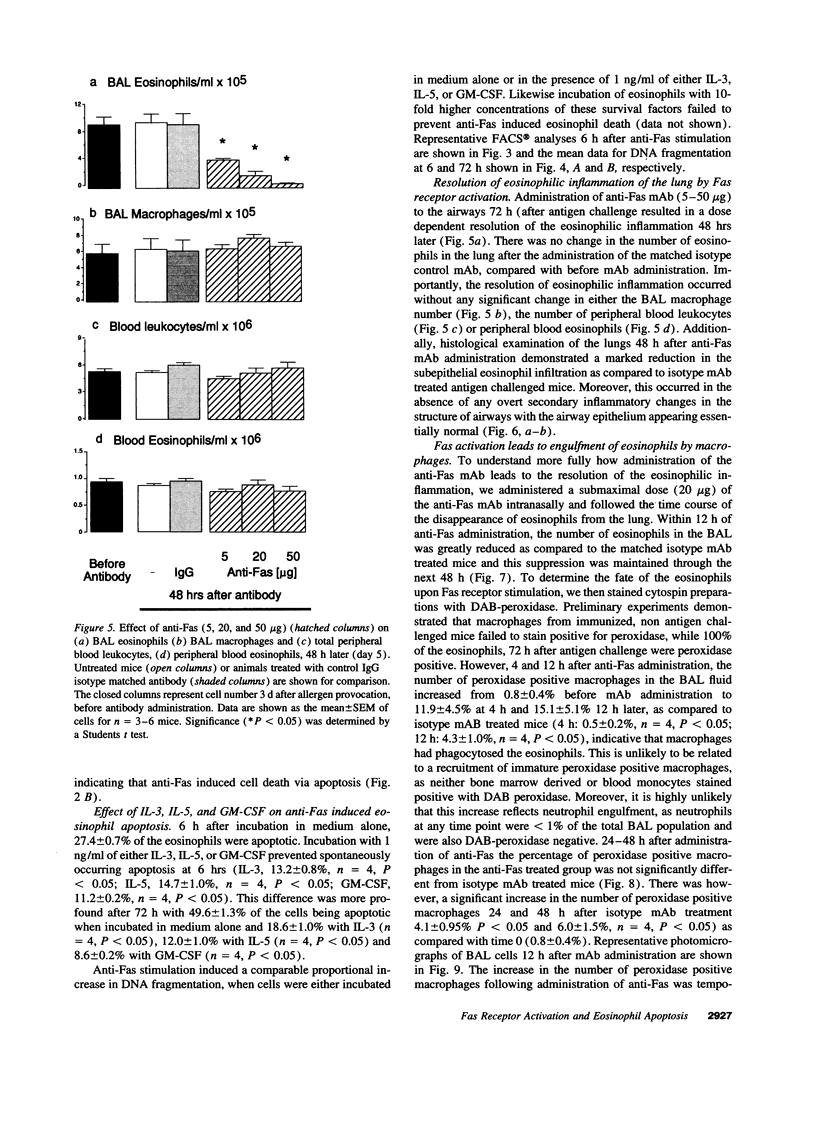
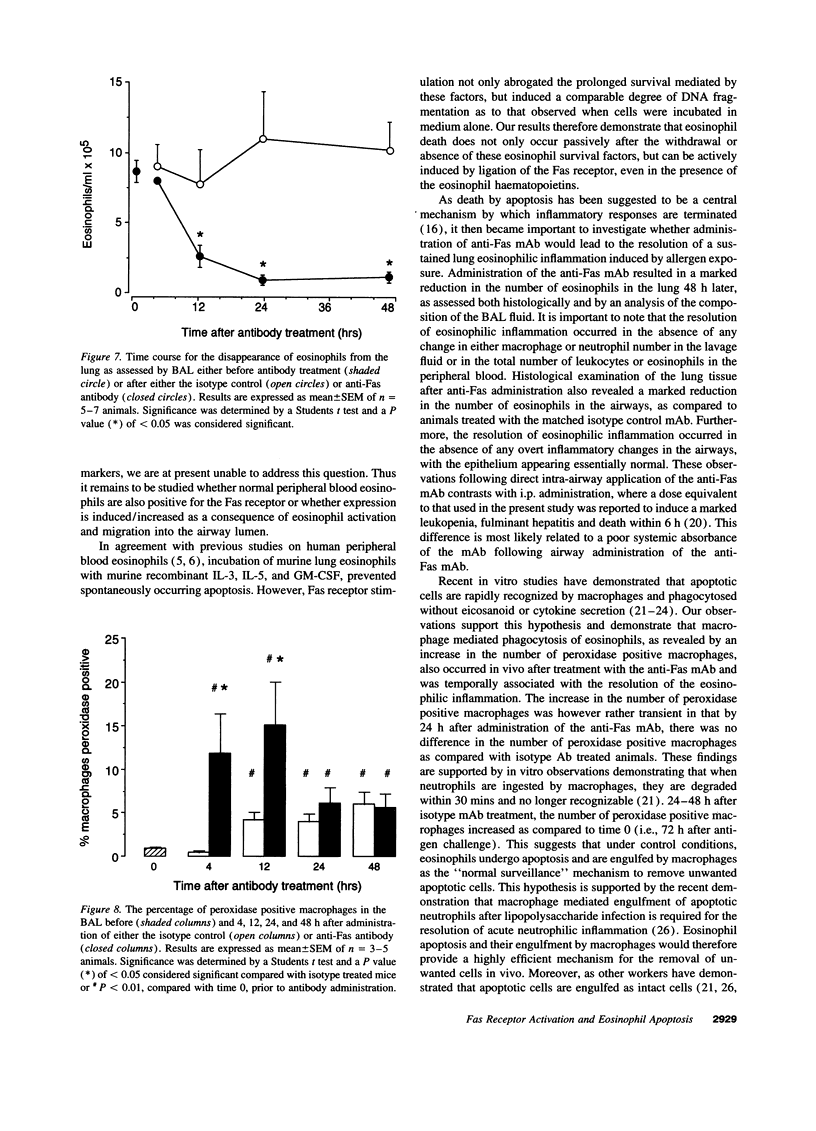
While considerable progress has been made in understanding the events by which eosinophils accumulate in various pathophysiological conditions, the mechanisms controlling the resolution of eosinophilic inflammation are poorly understood. In the present study, we demonstrate that lung eosinophils obtained by bronchoalveolar lavage (BAL) after aerosol allergen provocation of immunized mice expressed the Fas receptor. Stimulation of purified eosinophils in vitro with a monoclonal anti-Fas mAb (1 ng-1 microg/ml) induced a dose/time dependent loss of cell viability from 24-72 h. Measurement of DNA fragmentation with propidium iodide confirmed that anti-Fas induced eosinophil death by apoptosis. While incubation with IL-3, IL-5, or GM-CSF prevented spontaneous apoptosis, these factors failed to prevent anti-Fas induced apoptosis. Administration of anti-Fas mAb to the lungs after the induction of a lung eosinophilia increased the number of peroxidase positive macrophages in BAL fluid 4-12 h later which was followed by a marked reduction in the number of eosinophils in the airways. Importantly, Fas-mediated resolution of eosinophilic inflammation occurred in the absence of any overt secondary inflammatory changes in the lungs. We speculate that defects in this pathway may at least in part explain the chronic eosinophilic inflammation often observed in the lungs of asthmatic individuals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Savill J., Gombert W., Bofill M., Borthwick N. J., Whitelaw F., Grundy J., Janossy G., Salmon M. The specific recognition by macrophages of CD8+,CD45RO+ T cells undergoing apoptosis: a mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994 Nov 1;180(5):1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Cox G., Crossley J., Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995 Feb;12(2):232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- Coyle A. J., Ackerman S. J., Burch R., Proud D., Irvin C. G. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest. 1995 Apr;95(4):1735–1740. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A. J., Le Gros G., Bertrand C., Tsuyuki S., Heusser C. H., Kopf M., Anderson G. P. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995 Jul;13(1):54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Her E., Frazer J., Austen K. F., Owen W. F., Jr Eosinophil hematopoietins antagonize the programmed cell death of eosinophils. Cytokine and glucocorticoid effects on eosinophils maintained by endothelial cell-conditioned medium. J Clin Invest. 1991 Dec;88(6):1982–1987. doi: 10.1172/JCI115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron L. R., Eisenberg R. A., Roper E., Kakkanaiah V. N., Cohen P. L., Kotzin B. L. Selection of the T cell receptor repertoire in Lpr mice. J Immunol. 1993 Oct 1;151(7):3450–3459. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Cui H., Panka D. J., Ettinger R., Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., Panka D. J., Cui H., Ettinger R., el-Khatib M., Sherr D. H., Stanger B. Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb 2;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Tomoe S., Matsumura R., Tomioka H., Takatsu K., Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992 Aug;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
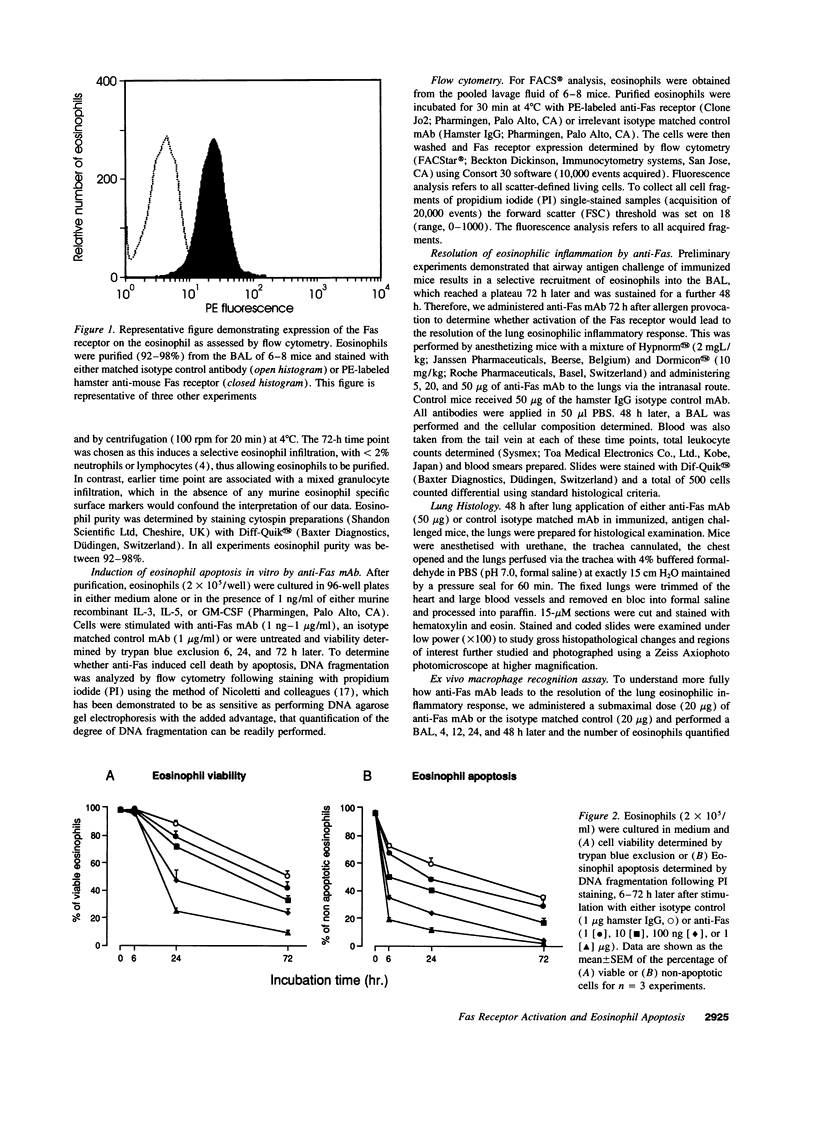
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Seaman M. S., Miller R. E., Picha K. S., Kennedy M. K., Lynch D. H. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994 Oct;6(10):1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Seaman M. S., Miller R. E., Tough T. W., Alderson M. R., Lynch D. H. gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol. 1994 Apr;24(4):928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Cooke M. P., Ho W. Y., Grein J., Townsend S. E., Davis M. M., Goodnow C. C. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995 Jul 13;376(6536):181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito J., Sniezek M., Warnock M. Acquisition of peroxidase activity by rat alveolar macrophages during pulmonary inflammation. Am J Pathol. 1987 Dec;129(3):567–577. [PMC free article] [PubMed] [Google Scholar]
- Stern M., Meagher L., Savill J., Haslett C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J Immunol. 1992 Jun 1;148(11):3543–3549. [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Ohta S., Tominaga K., Miura Y., Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991 Nov 15;78(10):2542–2547. [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G. The identification of eosinophil colonies in soft-agar cultures by differential staining for peroxidase. J Histochem Cytochem. 1976 Dec;24(12):1270–1272. doi: 10.1177/24.12.63511. [DOI] [PubMed] [Google Scholar]