Abstract
Background and aims
Portal hypertension in cirrhosis is mediated in part by increased intrahepatic resistance, reflecting an increased sensitivity of the hepatic microvasculature to vasoconstrictors. Activation of the RhoA/Rho‐kinase pathway is essential for contraction of vascular smooth muscle. The aim of this study was to investigate RhoA/Rho‐kinase mediated regulation of the intrahepatic vascular tone in cirrhotic rats.
Methods
Cirrhosis was induced by bile duct ligation (BDL). Hepatic RhoA and Rho‐kinase expressions were studied by real time reverse transcription polymerase chain reaction and western blot analysis. Hepatic Rho‐kinase activity in rat and human livers was assessed as phosphorylation of the Rho‐kinase substrate moesin. The effect of the Rho‐kinase inhibitor Y‐27632 on hepatic perfusion pressure was measured in livers perfused at constant flow. The in vivo effect of intravenous application of Y‐27632 was studied by haemodynamic measurements.
Results
Hepatic expressions of RhoA and Rho‐kinase were increased at mRNA and protein level in BDL rats. Intrahepatic moesin phosphorylation was increased in livers from cirrhotic rats and patients with alcohol induced cirrhosis. Y‐27632 reduced the basal perfusion pressure of in situ perfused livers in BDL rats but not in sham operated rats. Y‐27632 reduced the sensitivity to methoxamine in isolated perfused livers in sham operated rats more than in BDL rats. In vivo, Y‐27632 reduced portal pressure to a greater extent in BDL rats than in sham operated rats. Intrahepatic vascular resistance was decreased in response to bolus injection of Y‐27632 in BDL rats but not in sham operated rats.
Conclusions
Upregulation of RhoA and Rho‐kinase contributes to increased intrahepatic resistance in cirrhotic rats and to an increased sensitivity of cirrhotic livers to vasoconstrictors.
Keywords: cirrhosis, portal hypertension, intrahepatic vascular resistance, RhoA, Rho‐kinase
Increased resistance to portal blood flow is a primary factor in the pathophysiology of portal hypertension.1,2,3,4 Anatomical abnormalities—such as narrowing of intrahepatic microvessels because of fibrosis—are a major cause of the increased resistance to portal flow. However, a dynamic component caused by an abnormally active contraction of the hepatic microvasculature plays an additional role in the development of increased intrahepatic resistance.5,6 This part of the intrahepatic resistance to portal flow is regulated by intrahepatic portal venules and hepatic stellate cells (HSCs).7,8 Decreased formation and action of the vasodilator nitric oxide in the hepatic vascular bed supports the presence of portal hypertension.5,9,10,11,12,13,14,15,16 Furthermore, the intrahepatic resistance of cirrhotic livers shows hyperresponsiveness to vasoconstrictors such as noradrenaline (norepinephrine) and endothelin.5,6,17,18,19,20,21,22
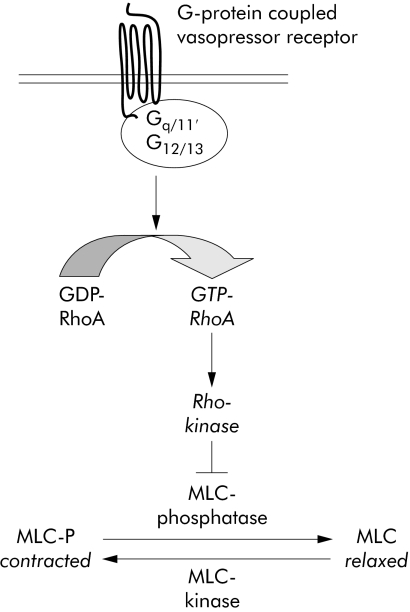
The RhoA/Rho‐kinase pathway is essentially involved in vasocontraction and the regulation of vascular tone.23,24,25,26,27,28,29,30 The pathway is activated by contractile agonists through G‐protein coupled vasopressor receptors (fig 1). These receptors activate the small monomeric GTPase, RhoA. Thereafter, RhoA activates Rho‐kinase, which subsequently inhibits myosin‐light‐chain‐phosphatase (MLC‐phosphatase). Inhibition of MLC‐phosphatase results in enhanced phosphorylation of MLCs and contraction.
Figure 1 RhoA/Rho‐kinase mediated vasocontraction. The RhoA/Rho‐kinase pathway is essentially involved in contraction of vascular smooth muscle and is linked to G‐protein coupled receptors for vasoconstrictors. The initial event is the activation of the small monomeric GTPtase RhoA by receptor associated heterotrimeric G‐proteins containing α subunits of the Gαq/11 and Gα12/13 family. RhoA activation is associated with an exchange of GDP to GDP at the protein. GTP‐RhoA subsequently activates Rho‐kinase. Rho‐kinase in turn phosphorylates and inhibits myosin‐light‐chain phosphatase. Inhibition of myosin‐light‐chain phosphatase results in enhanced phosphorylation of myosin‐light‐chains, which is the ultimate prerequisite for contraction of vascular smooth muscle. GDP, guanosine diphosphate; GTP, guanosine triphosphate.
Although it has been reported that RhoA is expressed in activated HSCs and that the RhoA/Rho‐kinase pathway plays an important role in HSC activation and hepatic fibrogenesis,31,32,33,34,35,36,37,38,39,40,41 the actions of this pathway in the regulation of intrahepatic resistance to portal blood flow remain unknown. We therefore studied the role of the RhoA/Rho‐kinase pathway in the intrahepatic vascular resistance of rats with secondary biliary cirrhosis and investigated whether it is involved in the pathogenesis of portal hypertension.
Methods
Animals
Male Sprague‐Dawley rats (180 to 200 g) were obtained from Charles River Laboratories (Sulzfeld, Germany) and maintained on standard chow on a 12 hour light/dark cycle. The rats were randomly divided into two groups. In one group, bile duct ligation was carried out as previously described.42 Briefly, rats were anaesthetised with ketamine hydrochloride (100 mg/kg); the common bile duct was exposed by an upper abdominal midline incision of 1.5 cm and was ligated twice with 5‐0 silk suture and resected between the ligatures; muscle and skin were sutured separately with 3‐0 silk. The rats of the other group were sham operated and served as controls. These animals therefore experienced the same procedures except that the bile duct was manipulated but not ligated and sectioned.
The study was approved by the local committee for animal studies (administrative authority, Cologne, Germany, 50.203‐Bn 15, 23/03).
Patients
Samples of liver tissue from patients with alcohol induced cirrhosis (n = 3) were obtained during liver transplantation. Non‐tumour‐bearing liver tissue obtained during resection of liver malignancies served as normal control (n = 3). The use of human liver specimens was approved by the local ethics committee.
Western blot analysis
Samples of shock frozen livers were homogenised in a buffer containing 25 mM Tris/HCl, 5 mM ethylenediamine tetra‐acetic acid, 10 μM phenylmethanesulphonyl fluoride, 1 mM benzamidine, and 10 μg/ml leupeptin. Protein determination of the homogenates was carried out with the Dc‐Assay kit (Biorad, Munich, Germany). Thereafter, homogenates were diluted with sample buffer. Samples (20 μg of protein per lane) were subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE) (15% gels for RhoA, 8% gels for Rho‐kinase), and proteins were blotted on nitrocellulose membranes. The membranes were blocked, incubated with primary antibodies (RhoA 119 and Rock‐2 H‐85, Santa Cruz Biotechnology, Santa Cruz, California, USA) and thereafter with corresponding secondary peroxidase coupled antibodies (Calbiochem, San Diego, California, USA). Blots were developed with enhanced chemiluminescence (ECL, Amersham, UK). Intensities of the resulting bands on each blot were compared densitometrically with a FLA‐3000 phosphoimager (Fuji‐Film, Düsseldorf, Germany).
Quantitative real time reverse transcription polymerase chain reaction
RNA was isolated from 30 mg shock frozen liver tissue using the RNeasy‐mini kit (Qiagen, Hilden, Germany) according to the manufacturer's guidelines. RNA concentrations were measured spectrophotometrically at 260 nm. For each sample, 1 μg of total RNA was used. Before reverse transcription, samples were DNA digested with RQ1 RNase‐free DNase (Promega, Madison, Wisconsin, USA). Reverse transcription was carried out using Moloney murine leukaemia virus (MMLV) reverse transcriptase (Invitrogen, Karlsruhe, Germany) and random primers (250 ng, Microsynth, Balgach, Switzerland). Control reactions did not contain reverse transcriptase. Primers and probes for real time reverse transcription polymerase chain reaction (RT‐PCR) were designed using the Primer Express Software (Applied Biosystems, Foster City, California, USA) and custom synthesised by Microsynth and Applied Biosystems, respectively. Sequences of the primers and probes are given in table 1. Primers and probes for the housekeeping gene (18SrRNA) were provided by Applied Biosystems as a ready‐to‐use mix. RT‐PCR was carried out using the ABI 7700 sequence detector (Applied Biosystems). The PCR reaction was done in a volume of 25 μl containing 12.5 μl 2× TaqMan PCR master mix (Roche Molecular Systems, Mannheim, Germany/Applied Biosystems) and 2 μl cDNA (equivalent to 67 ng total RNA). The final concentrations of the primers and probes are given in table 1. 18SrRNA served as the endogenous control. The final concentrations were 100 nM for primers and 200 nM for the probe. The results are expressed as the number of cycles (CT value) at which the fluorescence signal exceeded a defined threshold. The difference in CT value of the target cDNA and the endogenous control are expressed as negative ΔCT values (−ΔCT). Thus higher −ΔCT values denote higher mRNA levels. The ΔCT method was used for quantification of the results. For all target genes and 18SrRNA, validation experiments were carried out according to the manufacturer's guidelines. In these experiments, it was shown that the efficiencies of the RT‐PCR for the target gene and the endogenous control were approximately equal. Thus the ΔCT method is suitable for relative quantification.
Table 1 Primers and probes used for quantitative reverse transcription polymerase chain reaction for RhoA and Rho‐kinase.
| Gene | Primer/probe sequence 5′‐3′ (forward/reverse/probe) | Primer/probe concentration (nM) |
|---|---|---|
| RhoA | GGCAGAGATATGGCAAACAGG, | 300 |
| TCCGTCTTTGGTCTTTGCTGA, | 300 | |
| CACTCCATGTACCCAAAAGCGCCAAM | 100 | |
| Rho‐kinase | CCCGATCATCCCCTAGAACC, | 300 |
| TTGGAGCAAGCTGTCGACTG, | 300 | |
| ACAAAACCAGTCCATTCGGCGGC | 200 |
Assessment of Rho‐kinase activity
Rho‐kinase activity was assessed as phosphorylation of the endogenous Rho‐kinase substrate, moesin, at thr‐558.43,44,45,46,47,48 This was done by western blot analysis using a site specific and phosphospecific anti‐moesin antibody (Santa Cruz Biotechnology). In parallel, total moesin was analysed using a non‐phosphospecific antibody (Santa Cruz Biotechnology).
Immunohistochemistry
Immunohistochemical staining of liver sections was carried out using the indirect immunoperoxidase technique as previously described49 with the exception that the incubation with the primary antibody (site specific and phosphospecific anti‐moesin antibody) was prolonged (overnight), and a swine anti‐rabbit antibody (Dako, Carpinteria, California, USA) was used as secondary antibody.
In situ liver perfusion
In situ liver perfusion was carried out in a recirculating system according to a previously described technique.9 Briefly, rats were fasted overnight but allowed free access to water. Only cirrhotic rats with ascites were included in the study. After being anaesthetised with ketamine hydrochloride (60 mg/kg), the abdomen was opened and the bile duct was cannulated with a polyethyl tube to monitor bile flow. Loose ligatures were placed around portal vein, common hepatic artery, spleen vein, and posterior vena cava just cranially to the confluence of the right renal vein. A 500 U dose of heparin was injected into the posterior vena cava. The portal vein was cannulated with a 14‐gauge Teflon catheter, initiating liver exsanguinations by infusion (30 ml/min) of Krebs‐Henseleit solution containing heparin (2 U/ml) and oxygenated with carbogen (95% O2, 5% CO2). The posterior vena cava was immediately cut caudally to the loose ligature, allowing the perfusate to escape. Thereafter, the thorax was opened and the right atrium was cut. Another catheter was introduced in the right atrium and pushed forward to the inferior vena cava. Next, all ligatures were pulled tight. At a constant flow (30 ml/min), perfusion pressure was monitored continuously and recorded digitally on‐line. The preparation was allowed to stabilise for 20 minutes without any procedure.
Viability and stability of liver perfusion preparation
The criteria for liver viability included gross appearance of the liver, stable perfusion, bile production >0.4 μl/min*g, and stable buffer pH (7.4±0.1) during the initial 20 minute stabilisation period. If one of the viability criteria was not met, the experiment was discarded.
Effect of the α1 adrenoceptor agonist methoxamine on portal perfusion pressure
In one set of experiments, livers were initially perfused at a constant flow (30 ml/min) for a period of 20 minutes without any procedure in order to stabilise the entire system. Then cumulative concentration–response curves with the α1 adrenoceptor agonist methoxamine (0.1 µM to 100 µM) were obtained by addition of the agonist to the perfusate. Changes in perfusion pressure were expressed either as the absolute perfusion pressure after administration of methoxamine, or as change in perfusion pressure elicited by the given concentration of methoxamine in the perfusate (that is, perfusion pressure minus basal pressure).
Effect of the Rho‐kinase inhibitor Y‐27632 on methoxamine induced hepatic flow resistance
In another set of experiments, 10 minutes before addition of the first dose of methoxamine, Y‐27632 was added to the perfusate in different concentrations (1, 10, and 30 μM). Thereafter, cumulative concentration–response curves for methoxamine were constructed as described above.
Haemodynamic studies
Haemodynamic studies were carried out under ketamine anaesthesia (60 mg/kg intravenously). This condition has been shown to approximate most closely the conscious state in terms of cardiac output and regional blood flow and has been used extensively to investigate the haemodynamic effects of portal pressure lowering drugs in animal models of portal hypertension.2,42,50,51 The left femoral artery and vein were cannulated with PE‐50 catheters for measurement of arterial pressure and blood withdrawal, as well as for drug infusion. Median laparotomy was carried out and a PE‐50 catheter was introduced into a small ileocaecal vein and advanced to the confluence of the portal and splenic vein for the measurement of portal pressure. Through the right carotid artery another PE‐50 catheter was advanced into the left ventricle under pulse curve control. This catheter was used for microsphere application. The catheters in the femoral artery and the portal vein were connected to pressure transducers (Hugo Sachs Electronic, March‐Hugstetten, Germany) for blood pressure measurement. The zero point was 1 cm above the operating table.
Regional blood flows were measured using the coloured microsphere method, as previously described.42,52 A reference sample was obtained for one minute at a rate of 0.65 ml/min using a continuous withdrawal pump (Hugo Sachs Electronic). Then 300 000 yellow microspheres (15 μm diameter, Triton Technologies, San Diego, USA) were suspended in 0.3 ml saline containing 0.05% Tween and injected into the left ventricle 10 seconds after the withdrawal pump had been started. Upon completion of the haemodynamic measurements the animals were killed and the lungs, liver, kidneys, stomach, intestine, pancreas, and spleen were resected. The tissues were weighed, minced with scissors, and digested by addition of 14 ml/g tissue of 4 M KOH with 2% Tween, and subsequent boiling for one hour. The blood reference sample was digested by the addition of 3.8 ml 5.3 M KOH and 0.5 ml Tween and subsequent boiling for one hour. The digested tissues and blood samples were vortexed and filtered using Whatman Nucleopore filters (Whatman International, Maidstone, UK). The colour of the filtered microspheres was dissolved in 0.2 ml N,N‐dimethylformamide and the absorption was measured by spectrophotometry. Thereafter, the number of microspheres per organ and organ perfusion was calculated using software obtained from Triton Technologies.
Porto‐systemic shunting (PSS) was estimated as previously described, after the injection of 150 000 blue microspheres in 0.3 ml saline containing 0.05% Tween into an ileocaecal vein within 30 seconds.42,53 The tissue microsphere content was calculated as described for the measurement of organ blood flow. PSS was calculated as the number of microspheres in the lung×100 divided by the number of microspheres in lung and liver. Portal venous inflow (PVI) was calculated as the sum of the blood flows to stomach, spleen, intestines, pancreas, and mesentery. Collateral blood flow (ml/min×100 g) was estimated as PVI×PSS/100. Vascular resistances were calculated from the ratio between perfusion pressure and blood flow of each vascular territory.
Statistical analysis
Data are presented as means (SEM) with the indicated number (n) of experiments. Analysis of variance (ANOVA) followed by Bonferroni/Dunn or the Mann–Whitney U test was used for comparison between groups (StatView 5.0, SAS Institute, Cary, North Carolina, USA). Probability (p) values of <0.05 were considered statistically significant. For the analysis of the in situ liver perfusion studies with methoxamine, concentration–response curves were fitted by non‐linear regression, using the computer program Prism® (Graph Pad Software Inc, San Diego, California, USA). Emax (maximum contraction) and pEC50 values (negative logarithm of the concentration producing a half maximum effect) were calculated from the fitted curves.
Results
Hepatic expression of RhoA and Rho‐kinase
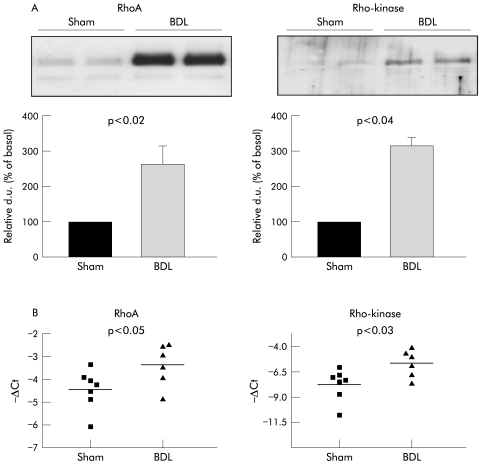
Western blot analysis of whole liver homogenates showed a strong upregulation of RhoA and Rho‐kinase protein levels in livers from BDL rats compared with sham operated rats (fig 2A). In parallel, as revealed by quantitative RT‐PCR with mRNA isolated from whole liver homogenates, both RhoA and Rho‐kinase mRNA were significantly raised in livers from sham operated and BDL rats (fig 2B).
Figure 2 Hepatic RhoA and Rho‐kinase expression in sham operated and bile duct ligated (BDL) rats. (A) Protein expression, western blot analysis. Shown are representative western blots of whole liver homogenates and densitometric quantification of all experiments. Data are means with SEM, n = 8–10 for each group. (B) mRNA expression, data from quantitative reverse transcription polymerase chain reaction. Shown are mRNA levels in whole liver homogenates from both groups (sham n = 7, BDL n = 6). d.u., densitometric units.
Hepatic moesin phosphorylation
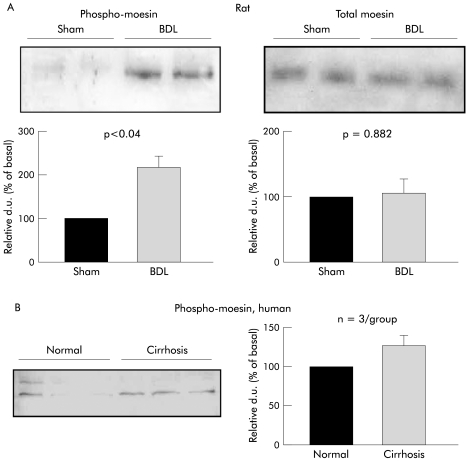
Phosphorylation of moesin—a marker for Rho‐kinase activity43,44,45,46,47,48—was greatly increased in livers of BDL rats (fig 3A). This difference was not associated with changes in total moesin, which was similar in both groups (fig 3A). As thr‐558 of moesin is preferentially phosphorylated by Rho‐kinase, these findings probably reflect an increased basal Rho‐kinase activity in livers of BDL rats.
Figure 3 Total and phospho‐moesin in whole liver homogenates in rat (A) and human (B) cirrhosis. Moesin is phosphorylated at threonine 558 by Rho‐kinase. (A) Phospho‐moesin and total moesin in livers from sham operated and bile duct ligated (BDL) rats (n = 5–6 for each group). (B) Phospho‐moesin in livers from cirrhotic and non‐cirrhotic patients (n = 3/group). Representative western blots of whole liver homogenates and densitometric quantification of all experiments are shown. Data are means with SEM; d.u., densitometric units.
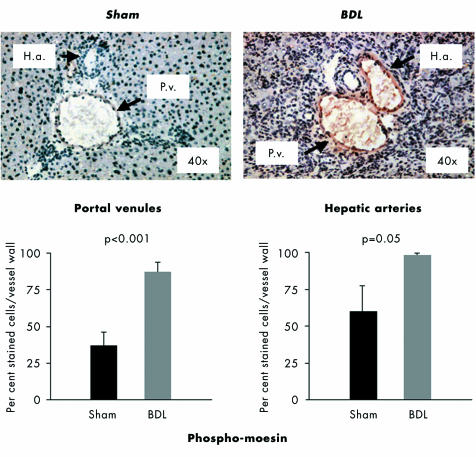
To localise the sites of intrahepatic moesin hyperphosphorylation in BDL rats, immunohistochemical investigations for phospho‐moesin were undertaken in liver sections from sham operated and BDL rats. Phosphorylated moesin was highly present within the walls of the intrahepatic branches of portal venules and hepatic arteries (fig 4). The staining for phospho‐moesin within these vessel walls was significantly stronger in BDL rats than in sham operated rats, and to some degree in the hepatic arteries (fig 4). In contrast, no phospho‐moesin was detected in the remaining intrahepatic sites (hepatocytes or extracellular space), and only weak staining in the perisinusoidal cells (fig 4).
Figure 4 Immunohistochemical staining of liver sections from sham operated (n = 7) and bile duct ligated (BDL) rats (n = 7) for phospho‐moesin. Representative experiments are shown, with quantification of the staining in the intrahepatic branches of the portal venules (P.v.) and hepatic arteries (H.a.). Data are means with SEM, n = 7 for each group (quantification was carried out in triplicate for each animal).
The phosphorylation state of moesin was also investigated by western blot analysis in human livers (whole liver homogenates from cirrhotic v non‐cirrhotic patients). These experiments showed a clear trend towards raised phospho‐moesin levels in livers from cirrhotic patients (alcohol induced cirrhosis) when compared with those from non‐cirrhotic patients (fig 3B).
Basal intrahepatic resistance and modulation by Rho‐kinase inhibition
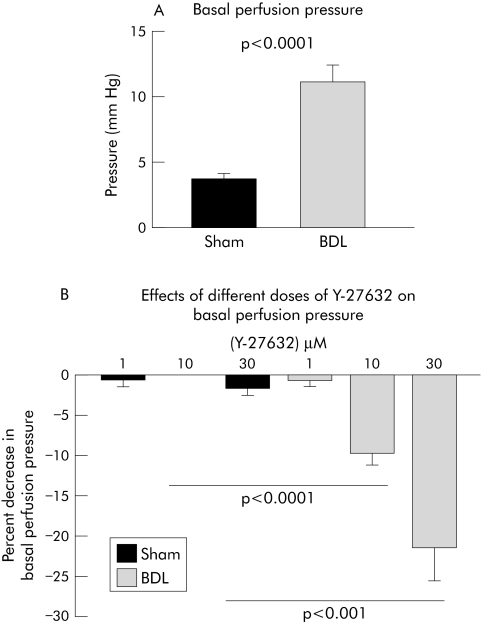
At a constant flow, changes in perfusion pressure reflect changes in intrahepatic resistance. In BDL rats, the perfusion pressure of in situ perfused livers was on average increased threefold (fig 5A). In order to assess whether increased hepatic expression and activity of Rho‐kinase is related to intrahepatic vascular resistance, we tested the effect of the Rho‐kinase inhibitor Y‐27632 on perfusion pressure. Y‐27632 had no significant effects at any concentration on perfusion pressure in sham operated rats (fig 5B). In contrast, in BDL rats intrahepatic perfusion pressure was significantly reduced by 10 μM and 30 μM of Y‐27632 (fig 5B).
Figure 5 Basal perfusion pressure of in situ perfused livers (A), and effect of Rho‐kinase inhibition with Y‐27632 on basal perfusion pressure (B). Data are means with SEM, n = 9–10 in each group. BDL, bile duct ligated; sham, sham operated.
Effect of Rho‐kinase inhibition on methoxamine induced increase in intrahepatic resistance
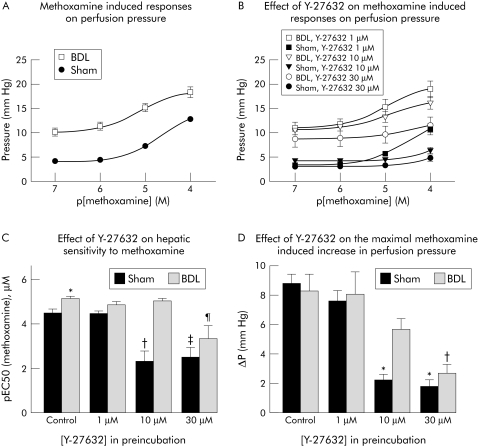
To investigate the role of the RhoA/Rho‐kinase pathway in vasoconstrictor mediated regulation of intrahepatic microvascular tone, we studied the effect of Y‐27632 on the methoxamine induced increase in intrahepatic perfusion pressure. As shown in fig 6A, the addition of methoxamine to the perfusate elicited dose dependent increases in perfusion pressure in both groups. The sensitivity of livers from BDL rats to methoxamine was significantly greater than in sham operated rats, as shown by the increase in EC50 (fig 6C, left columns). This underlines the well known hyperreactivity of the hepatic vascular resistance of cirrhotic livers to vasoconstrictors. However, the changes in perfusion pressure (ΔPmax) elicited by the maximum concentration of methoxamine (100 μM) were similar in both groups (fig 6, A and D, left panels), although the perfusion pressures reached in BDL rats were higher than in sham operated rats, owing to the higher basal intrahepatic perfusion pressure.
Figure 6 (A) Concentration–response curves for the effect of the α1 adrenoceptor agonist methoxamine on the perfusion pressure of in situ perfused livers from sham operated (sham) and bile duct ligated (BDL) rats. (B) Effect of Rho‐kinase inhibition with Y‐27632 on the dose dependent methoxamine induced increase in perfusion pressure of in situ perfused livers. (C) Effect of Rho‐kinase inhibition with Y‐27632 on the methoxamine sensitivity of in situ perfused livers from sham operated and BDL rats. *p<0.002 v sham without Y‐27632; †p<0.0004 v sham without Y‐27632; ‡p<0.0006 v sham without Y‐27632; ¶p<0.003 v BDL without Y‐27632. (D) Reduction of methoxamine (100 μM) induced increase in perfusion pressure by Rho‐kinase inhibition with Y‐27632 in livers from sham operated and BDL rats perfused in situ. Data are means with SEM, n = 9–10 in each group. *p<0.0001 v sham without Y‐27632; †p<0.0005 v BDL without Y‐27632. p[methoxamine], negative decadic logarithm of a given concentration of methoxamine.
Next, we tested the effect of different concentrations of Y‐27632 on the dose dependent methoxamine induced increase in intrahepatic resistance. The addition of 1 μM Y‐27632 had no effect on the methoxamine induced increase in intrahepatic perfusion pressure in sham operated and BDL rats (fig 6, panels B and D). However, in sham operated rats, 10 μM and 30 μM Y‐27632 significantly inhibited the ΔPmax induced by 100 μM methoxamine (fig 6, panels A and D). In contrast, in BDL rats, only 30 μM Y‐27632 significantly inhibited the methoxamine (100 μM) stimulated increase in perfusion pressure (fig 6D). Y‐27632 10 μM and 30 μM significantly increased the EC50 to methoxamine of perfused livers from sham operated rats (fig 6C). By contrast, in BDL rats, only the 30 μM concentration of Y‐27632 was able to affect the EC50 to methoxamine (fig 6C).
Haemodynamic in vivo effects of Rho‐kinase inhibition
To obtain further insight into the regulation of portal pressure through the RhoA/Rho‐kinase pathway in vivo, we studied the haemodynamic effects of bolus injection of different doses of Y‐27632.
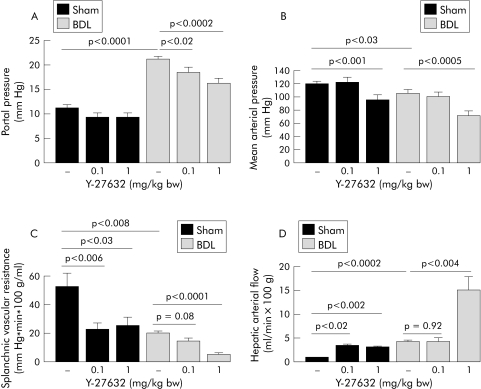
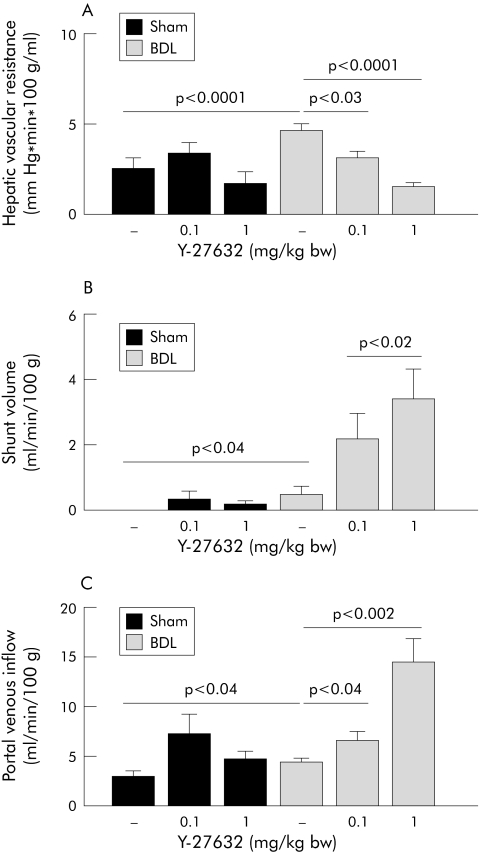
As expected, BDL rats had increased portal pressure, low arterial pressure, decreased splanchnic vascular resistance, increased intrahepatic resistance, and increased porto‐systemic shunt flow. Bolus injection of Y‐27632 (0.1 mg/kg) reduced portal pressure in BDL but not in sham operated rats (fig 7A). Interestingly, this was paralleled by a significant decrease in hepatic vascular resistance in BDL rats but not in sham operated rats (fig 8A). In contrast, splanchnic vascular resistance and hepatic arterial flow were unaffected in BDL rats, but reduced in sham operated rats after bolus injection of 0.1 mg/kg Y‐27632 (fig 7, panels C and D). The shunt volume in BDL rats was significantly increased after Y‐27632 (fig 8B). In both groups, the systemic administration of 0.1 mg/kg Y‐27632 did not change mean arterial pressure (fig 7B).
Figure 7 (A) Dose dependent in vivo effects of intravenous application of the Rho‐kinase inhibitor Y‐27632 on portal pressure in sham operated (sham) and bile duct ligated (BDL) rats, determined 45 minutes after bolus injection. (B) Dose dependent in vivo effects of intravenous application of the Rho‐kinase inhibitor Y‐27632 (0.1 and 1 mg/kg bw) on mean arterial pressure in sham operated and BDL rats, determined 45 minutes after bolus injection. Data are means with SEM, n = 11–27 in each group. (C) Dose dependent in vivo effects of intravenous application of the Rho‐kinase inhibitor Y‐27632 (0.1 and 1 mg/kg bw) on splanchnic vascular resistance in sham operated and BDL rats, determined 45 minutes after bolus injection. (D) Dose dependent in vivo effects of intravenous application of the Rho‐kinase inhibitor Y‐27632 (0.1 and 1 mg/kg bw) on hepatic arterial flow in sham operated and BDL rats, determined 45 minutes after bolus injection. Data are means with SEM, n = 11–27 in each group. bw, body weight.
Figure 8 Dose dependent in vivo effects of intravenous application of the Rho‐kinase inhibitor Y‐27632 (0.1 and 1 mg/kg bw) on hepatic vascular resistance (A), shunt volume (B), and portal venous flow (C) in sham operated (sham) and bile duct ligated (BDL) rats, determined 45 minutes after bolus injection. Data are means with SEM, n = 11–16 in each group.
Bolus injection of Y‐27632 in a dose of 1 mg/kg elicited large and sustained decreases in portal pressure in BDL rats but had no effect on portal pressure in sham operated rats (fig 7A). The reduction in portal pressure in response to the high dose of Y‐27632 was associated with a further reduction in hepatic vascular resistance (but also with further increases in portal venous inflow and shunt volume) in BDL rats, but not in sham operated rats (fig 8). In both groups, 1 mg/kg of Y‐27632 induced large sustained decreases in mean arterial pressure (fig 7B), significant decreases in splanchnic vascular resistance, and significant increases in hepatic arterial flow (fig 7, panels C and D).
Discussion
The results of the present study provide evidence for the contribution of an abnormal increase in intrahepatic Rho‐kinase signalling to the increased intrahepatic resistance and the increased sensitivity of the hepatic resistance to vasoconstrictors observed in rats with secondary biliary cirrhosis. An increased resistance of the intrahepatic microcirculation to portal flow contributes essentially to portal hypertension. Thus the intrahepatic regulation of liver blood flow by abnormal vasoconstrictor and vasodilator mediated signalling in cirrhosis has been studied widely.5,6,9,10,11,12,13,14,15,16,17,18,19,20,21,22 To date, two mechanisms have been revealed which contribute functionally to the increased vascular tone in the cirrhotic liver. Increased intrahepatic resistance in cirrhosis is mediated by a decreased formation and action of the vasodilator nitric oxide (NO), and an increased sensitivity to vasoconstrictors (for example, α1 adrenoceptor agonists and endothelin).5,6,9,10,11,12,13,14,15,16,17,18,19,20,21,22 This increased sensitivity to vasoconstrictors is not completely understood. Vasoconstrictor mediated signalling downstream of the level of G protein coupled vasopressor receptors could play a role. Therefore, we investigated the RhoA/Rho‐kinase pathway in the vasoconstrictor mediated intrahepatic regulation of liver blood flow. It has been shown that this pathway is essentially involved in contraction of vascular smooth muscle.23,24,25,26,27,28,29,30 Furthermore, a role for RhoA/Rho‐kinase mediated signalling in activation and contraction of hepatic stellate cells as well as in hepatic fibrogenesis has also been demonstrated.31,32,33,34,35,36,37,38,39,40,41
First, we investigated expression of RhoA and Rho‐kinase in livers from sham operated and BDL rats. There was a strong upregulation of RhoA and Rho‐kinase protein expression as well as mRNA expression in livers of rats with secondary biliary cirrhosis. The functional activity of Rho‐kinase can be assessed as the phosphorylation state of its substrate moesin.43,44,45,46,47,48 The hepatic upregulation of RhoA and Rho‐kinase in cirrhotic rats indeed resulted in an increased moesin phosphorylation, reflecting an increased activity of Rho‐kinase in these animals. Most of the phosphorylated moesin found by immunohistochemical staining was localised to the walls of intrahepatic branches of portal venules and hepatic arteries, and to a lesser extent in perisinusoidal cells. Moesin phosphorylation in presinusoidal portal venules was highly increased in BDL rats. A similar increase in moesin phosphorylation was found by western blot analysis in liver homogenates from patients with alcohol induced cirrhosis. This indicates that these processes are not restricted to the animal model used here, but may probably be of clinical relevance.
To assess the functional relevance of the increased hepatic expression of RhoA and Rho‐kinase and the subsequent increase in Rho‐kinase activity for the hepatic vascular resistance of BDL rats, we tested the effect of the Rho‐kinase inhibitor Y‐27632 on the basal perfusion pressure of in situ perfused livers. Basal perfusion pressure at a constant flow (that is, the intrahepatic resistance to portal flow) was increased in BDL rats. Inhibition of Rho‐kinase by Y‐27632 reduced the basal perfusion pressure in BDL rats but not in sham operated rats. Thus the intrahepatic microcirculation in BDL rats was more susceptible to Rho‐kinase inhibition than that of sham operated rats. As these findings were obtained under conditions excluding the influence of circulating vasoactive mediators, they possibly reflect an increased contribution of Rho‐kinase to the increased basal vascular tone of the intrahepatic microvasculature in BDL rats.
Next, we studied the dose dependent changes in perfusion pressure after stimulation with the α1 adrenoceptor agonist methoxamine in both groups. Livers from BDL rats were hypersensitive to methoxamine, as shown by the decreased EC50. This increased sensitivity of livers from BDL rats to methoxamine shows the exaggerated response of cirrhotic livers to vasoconstrictors. To study the role of the RhoA/Rho‐kinase pathway in the regulation of vasoconstrictor induced intrahepatic vascular tone, we examined the effect of different doses of Y‐27632 on the methoxamine stimulated changes in perfusion pressure. In sham operated rats, Rho‐kinase inhibition with Y‐27632 at a concentration of 10 μM was already able to decrease the pEC50 for methoxamine. In contrast, at least 30 μM of Y‐27632 was necessary to elicit the same effect in BDL rats. Furthermore, contractions elicited by methoxamine were less susceptible to Rho‐kinase inhibition in BDL rats than in sham operated rats.
These data permit several conclusions. First, contractile G‐protein coupled receptors (for example, α1 adrenoceptors) in the intrahepatic microvasculature are coupled to the RhoA/Rho‐kinase pathway. Thus the RhoA/Rho‐kinase pathway is indeed involved in the adrenergic regulation of intrahepatic vascular tone. Second, in BDL rats compared with sham operated rats, activation of the RhoA/Rho‐kinase pathway in response to α1 adrenergic stimulation of intrahepatic microcirculation is probably increased.
To investigate the role of the Rho‐kinase signalling in intrahepatic resistance to liver blood flow and portal pressure in vivo, we studied the effects of systemic administration of the Rho‐kinase inhibitor Y‐27632 in anaesthetised rats. Portal pressure was dose dependently reduced in response to bolus injection of Y‐27632 in cirrhotic but not in non‐cirrhotic rats. Simultaneously, intrahepatic vascular resistance was decreased in BDL rats in response to Y‐27632 but not in sham operated rats.
Taken together, in BDL rats Y‐27632 decreased portal vascular resistance and hepatic vascular resistance after intravenous application; furthermore, Y‐27632 reduced the intrahepatic perfusion pressure in the in situ perfused cirrhotic liver. These effects were much less pronounced in the sham operated rat. Because Y‐27632 also efficiently reduced the perfusion pressure of in situ perfused livers, we assume that the portal pressure lowering effect of Y‐27632 observed in vivo is at least partially mediated by intrahepatic actions of the inhibitor. However, it cannot be excluded that Y‐27632 also directly acts at the portal vein itself. The decrease in hepatic vascular resistance of BDL rats was accompanied by an increased porto‐systemic shunting. As microspheres were injected into the portal vein through the superior mesenteric vein to study porto‐systemic shunting, we assume that Y‐27632 decreased intrahepatic resistance at least in part by opening intrahepatic shunts. Hepatic arterial flow was increased in BDL rats despite an increase in moesin phosphorylation in intrahepatic hepatic arteries, suggesting further regulatory pathways in these vessels counterbalancing an increased Rho‐kinase activity. A contribution of hepatic arteries to the regulation of intrahepatic resistance is unclear. By contrast, portal venules are a principal site of regulation of intrahepatic resistance and portal pressures. This is emphasised by the pronounced effect of Y‐27632 in livers perfused in situ.
The decrease in portal pressure was paralleled by a decrease in splanchnic vascular resistance. This decrease in splanchnic resistance might be explained by the vasodilating properties of Y‐27632 on these vessels. Interestingly, the net decrease was greater in sham operated rats than in BDL rats, suggesting a reduced Rho‐kinase activity in the splanchnic vasculature of the cirrhotic rats, which might contribute to the abnormally persistent vasodilatation of these vessels. The decrease in splanchnic vascular resistance increased portal tributary flow. This should normally increase portal pressure. However, in our BDL rats, portal pressure was decreased despite an increase in portal tributary blood flow, suggesting that the Y‐27632 induced decrease in intrahepatic vascular resistance overcomes the increase in portal tributary flow. Again, this highlights the role of the hyperactivation of the RhoA/Rho‐kinase pathway in the hepatic vascular bed for portal hypertension of BDL rats. It remains to be shown whether similar abnormalities in Rho‐kinase signalling also contribute to the increased hepatic vascular resistance in other models of cirrhosis of the liver. As an increase in moesin phosphorylation was also found in livers from patients with alcohol induced cirrhosis, it seems possible that the Rho‐kinase mediated increase in hepatic vascular resistance is not restricted to biliary cirrhosis in rats, but is rather a common feature of cirrhosis of the liver. Arterial pressure and splanchnic vascular resistance were also reduced by Y‐27632. However, at the lower dose, Y‐27632 reduced portal pressure in BDL rats without affecting arterial pressure. Thus most of the portal pressure lowering effect of Y‐27632 seems to be mediated by decreasing intrahepatic resistance. Nevertheless, liver‐specific drugs inhibiting the RhoA/Rho‐kinase signalling preferentially in the cirrhotic liver should be developed before testing such drugs for pharmacological treatment of portal hypertension. Such Rho‐kinase inhibiting drugs have the advantage that they also decrease hepatic fibrogenesis.38,39,40,41
In summary, increased intrahepatic resistance in rats with secondary biliary cirrhosis is associated with an upregulation of RhoA and Rho‐kinase signalling. Inhibition of this pathway in the liver can reduce portal pressure in rats with secondary biliary cirrhosis.
Acknowledgements
The study was supported by grants of the Deutsche Forschungsgemeinschaft (HE 2402/5‐01) and the Ernst und Berta Grimmke‐Stiftung. The authors thank G Hack and D Bammer for excellent technical assistance. QZ is a fellow of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, PR China.
Abbreviations
BDL - bile duct ligation
EC50 - concentration producing a half maximal effect
HSC - hepatic stellate cell
PSS - porto‐systemic shunting
RT‐PCR - real time reverse transcription polymerase chain reaction
Y‐27632 - (+)‐(R)‐trans‐4‐(1‐aminoethyl)‐N‐(4‐pyridyl)cyclohexane carboxamide dihydrochloride, monohydrate
Footnotes
Conflict of interest: None declared.
References
- 1.Hernandez‐Guerra M, Garcia‐Pagan J C, Bosch J. Increased hepatic resistance. A new target in the pharmacological therapy of portal hypertension. J Clin Gastroenterol 200539S131–S137. [DOI] [PubMed] [Google Scholar]
- 2.Van de Casteele M, Sägesser H, Zimmermann H.et al Characterisation of portal hypertension models by microspheres in anaesthetised rats: a comparison of liver flow. Pharmacol Ther 20019035–40. [DOI] [PubMed] [Google Scholar]
- 3.Menon K V N, Kamath P S. Regional and systemic hemodynamic disturbances in cirrhosis. Clin Liver Dis 20015617–627. [DOI] [PubMed] [Google Scholar]
- 4.Groszmann R J, Abraldes J G. Portal hypertension. From bench to bedside. J Clin Gastroenterol 200539S125–S130. [DOI] [PubMed] [Google Scholar]
- 5.Shah V. Cellular and molecular basis of portal hypertension. Clin Liver Dis 20015629–644. [DOI] [PubMed] [Google Scholar]
- 6.Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology 1997252–5. [DOI] [PubMed] [Google Scholar]
- 7.Reynaert H, Thompson M G, Thomas T.et al Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut 200250571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockey D C. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis 200121337–349. [DOI] [PubMed] [Google Scholar]
- 9.Wiest R, Groszmann R J. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 200235478–491. [DOI] [PubMed] [Google Scholar]
- 10.Shah V, Haddad F, Garcia‐Cardena G.et al Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of hepatic resistance. J Clin Invest 19971002923–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudenhoefer A A, Loureiro‐Silva M R, Cadelina G W.et al Bioactivation of nitroglycerin and vasomotor response to nitric oxide are impaired in cirrhotic livers. Hepatology 200236381–385. [DOI] [PubMed] [Google Scholar]
- 12.Gupta T, Toruner M, Chung M.et al Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology 199828926–931. [DOI] [PubMed] [Google Scholar]
- 13.Shah V, Toruner M, Haddad F.et al Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental liver cirrhosis. Gastroenterology 19991171222–1228. [DOI] [PubMed] [Google Scholar]
- 14.Loureiro‐Silva M R, Cadelina G W, Groszmann R J. Deficit in nitric oxide production in cirrhotic rat livers is located in the sinusoidal and postsinusoidal areas. Am J Physiol Gastrointest Liver Physiol 2002284G567–G574. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Shao R, Qian H.et al Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest 2000105741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockey D C, Chung J J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology 1998114344–351. [DOI] [PubMed] [Google Scholar]
- 17.Ghandi C R, Sproat L A, Subbotin V M. Increased hepatic endothelin‐1 levels and endothelin receptors density in cirrhotic rat livers. Life Sci 19965855–62. [DOI] [PubMed] [Google Scholar]
- 18.Pinzani M, Milani S, Franco R.et al Endothelin‐1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology 1996110534–548. [DOI] [PubMed] [Google Scholar]
- 19.Elliot A J, Vo L T, Grossmann V L.et al Endothelin‐induced vasoconstriction in isolated perfused liver preparations from normal and cirrhotic rats. J Gastroenterol Hepatol 199712314–318. [DOI] [PubMed] [Google Scholar]
- 20.Grossmann H J, Grossmann V L, Bhathal P S. Enhanced vasoconstrictor response of the isolated perfused cirrhotic rat liver to humoral vasoconstrictior substances found in portal venous blood. J Gastroenterol Hepatol 19927283–287. [DOI] [PubMed] [Google Scholar]
- 21.Graupera M, Garcia‐Pagan J C, Titos E.et al 5‐Lipoxygenase inhibition reduces intrahepatic vascular resistance of cirrhotic rat livers: a possible role of cysteinyl‐leukotrienes. Gastroenterology 2002122387–393. [DOI] [PubMed] [Google Scholar]
- 22.Graupera M, Garcia‐Pagan J C, Abraldes J G.et al Cyclooxygenase‐derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology 200337172–181. [DOI] [PubMed] [Google Scholar]
- 23.Somlyo A P, Somlyo A V. Signal transduction by G‐proteins, Rho‐kinase and protein phosphatase to smooth muscle and non‐muscle myosin II. J Physiol (Lond) 2000522177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uehata M, Ishizaki T, Satoh H.et al Calcium sensitization of smooth muscle mediated by a Rho‐associated protein kinase in hypertension. Nature 1997389990–994. [DOI] [PubMed] [Google Scholar]
- 25.Sakurada S, Okamoto H, Takuwa N.et al Rho activation in agonist‐stimulated vascular smooth muscle. Am J Physiol Cell Physiol 2001281C571–C578. [DOI] [PubMed] [Google Scholar]
- 26.Somlyo A P, Somlyo A V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G‐proteins, kinases, and myosin phosphatase. Physiol Rev 2003831325–1358. [DOI] [PubMed] [Google Scholar]
- 27.Somlyo A P, Wu X, Walker L A.et al Pharmacomechanical coupling: the role of calcium, G‐proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol 1999134201–234. [DOI] [PubMed] [Google Scholar]
- 28.Bishop A L, Hall A. Rho GTPases and their effector proteins. Biochem J 2000348241–255. [PMC free article] [PubMed] [Google Scholar]
- 29.Etienne‐Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002420629–635. [DOI] [PubMed] [Google Scholar]
- 30.Riento K, Ridley A J. Rocks: multifunctional kinases in cell behaviour. Nature Rev Mol Cell Biol 20034446–456. [DOI] [PubMed] [Google Scholar]
- 31.Yanase M, Ikeda H, Matsui A.et al Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho‐kinase. Biochem Biophys Res Commun 200027772–78. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto H, Nakamuta M, Tada S.et al A p160ROCK‐specific inhibitor, Y‐27632, attenuates rat hepatic stellate cell growth. J Hepatol 200032762–770. [DOI] [PubMed] [Google Scholar]
- 33.Lee J S, Decker N K, Chatterjee S.et al Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol 20051661861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rombouts K, Knittel T, Machesky L.et al Actin filament formation, reorganization and migration are impaired in hepatic stellate cells under influence of trichostatin A, a histone deacetylase inhibitor. J Hepatol 200237788–796. [DOI] [PubMed] [Google Scholar]
- 35.Kato M, Iwamoto H, Higashi N.et al Role of Rho small GTP binding protein in the regulation of actin cytoskeleton in hepatic stellate cells. J Hepatol 19993191–99. [DOI] [PubMed] [Google Scholar]
- 36.Mizunuma K, Ohdan H, Tashiro H.et al Prevention of ischemia‐induced hepatic microcirculatory disruption by inhibiting stellate cell contraction using rock inhibitor. Transplantation 200375579–586. [DOI] [PubMed] [Google Scholar]
- 37.Kawada N, Seki S, Kuroki T.et al ROCK inhibitor Y‐27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin‐1. Biochem Biophys Res Commun 1999266296–300. [DOI] [PubMed] [Google Scholar]
- 38.Muata T, Arii S, Nakamura T.et al Inhibitory effect of Y‐27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. Hepatology 200135474–481. [DOI] [PubMed] [Google Scholar]
- 39.Kanno K, Tazuma S, Nishioka T.et al Angiotensin II participates in hepatic inflammation and fibrosis through MCP‐1 expression. Dig Dis Sci 200550942–948. [DOI] [PubMed] [Google Scholar]
- 40.Tada S, Iwamoto H, Nakatuma M.et al A selective ROCK inhibitor, Y‐27632, prevents dimethylnitrosamine‐induced hepatic fibrosis in rats. J Hepatol 200134529–536. [DOI] [PubMed] [Google Scholar]
- 41.Murata T, Arii S, Mori A.et al Therapeutic significance of Y‐27632, a Rho‐kinase inhibitor, on the established liver fibrosis. J Surg Res 200311464–71. [DOI] [PubMed] [Google Scholar]
- 42.Heller J, Shiozawa T, Trebicka J.et al Acute haemodynamic effects of losartan in anaesthetized cirrhotic rats. Eur J Clin Invest 2003331006–1012. [DOI] [PubMed] [Google Scholar]
- 43.Fukuta Y, Oshiro N, Kaibuchi K. Activation of moesin and adducin by Rho‐kinase downstream of Rho. Biophys Chem 199982139–147. [DOI] [PubMed] [Google Scholar]
- 44.Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho‐associated kinase (Rho‐kinase) plays a crucial role in the formation of microvilli‐like structures. J Biol Chem 199827334663–34666. [DOI] [PubMed] [Google Scholar]
- 45.Fukuta Y, Kimura K, Oshiro N.et al Association of the myosin‐binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho‐associated kinase and myosin phosphatases. J Cell Biol 1998141409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Retzer M, Essler M. Lysophosphatidic acid‐induced platelet shape change proceeds via Rho/Rho kinase‐mediated myosin light‐chain and moesin phosphorylation. Cell Signal 200012645–648. [DOI] [PubMed] [Google Scholar]
- 47.Shaw R J, Henry M, Solomon F.et al RhoA‐dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts. Mol Cell Biol 19989403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsui T, Maeda M, Doi Y.et al Rho‐kinase phosphorylates COOH‐terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head‐to‐tail association. J Cell Biol 1998140647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leifeld L, Trautwein C, Dumoulin F L.et al Enhanced Expression of CD80 (B7‐1), and CD40 and Their Ligands CD28 and CD154 in Fulminant Hepatic Failure. Am J Pathol 19991541711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kojima H, Yamao J, Tsujimoto T.et al Mixed endothelin receptor antagonist, SB209670, decreases portal pressure in biliary cirrhotic rats in vivo by reducing portal venous system resistance. J Hepatol 20003243–50. [DOI] [PubMed] [Google Scholar]
- 51.Seyde W C, Longnecker D E. Anesthetic influence on regional hemodynamics in normal and hemorrhaged rats. Anesthesiology 198461686–698. [DOI] [PubMed] [Google Scholar]
- 52.Hakkinen J P, Miller M W, Smith A H.et al Measurement of organ blood flow with coloured microspheres in the rat. Cardiovasc Res 19952974–79. [PubMed] [Google Scholar]
- 53.Geraghty J G, Angerson W J, Carter D C. Portal venous pressure and portosystemic shunting in experimental portal hypertension. Am J Phyiol 1989257G52–G57. [DOI] [PubMed] [Google Scholar]