Abstract
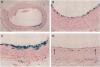
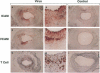
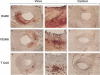
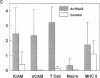
Adenovirus vectors are capable of high efficiency in vivo arterial gene transfer, and are currently in use as therapeutic agents in animal models of vascular disease. However, despite substantial data on the ability of viruses to cause vascular inflammation and proliferation, and the presence in current adenovirus vectors of viral open reading frames that are translated in vivo, no study has examined the effect of adenovirus vectors alone on the arterial phenotype. In a rabbit model of gene transfer into a normal artery, we examined potential vascular cell activation, inflammation, and neointimal proliferation resulting from exposure to replication-defective adenovirus. Exposure of normal arteries to adenovirus vectors resulted in: (a) pronounced infiltration of T cells throughout the artery wall; (b) upregulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in arterial smooth muscle cells; (c) neointimal hyperplasia. These findings were present both 10 and 30 d after gene transfer, with no evidence of a decline in severity over time. Adenovirus vectors have pleiotropic effects on the arterial wall and cause significant pathology. Interpretation of experimental protocols that use adenovirus vectors to address either biological or therapeutic issues should take these observations into account. These observations should also prompt the design of more inert gene transfer vectors.
Full text
PDF


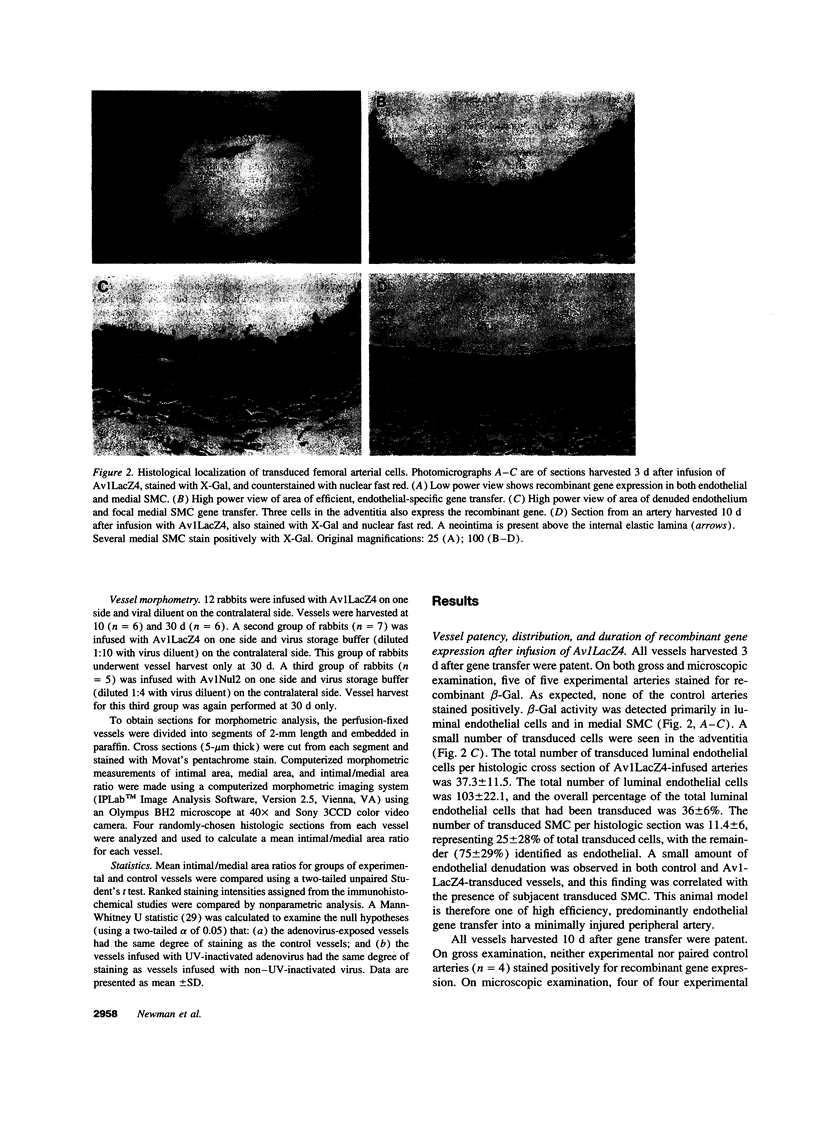
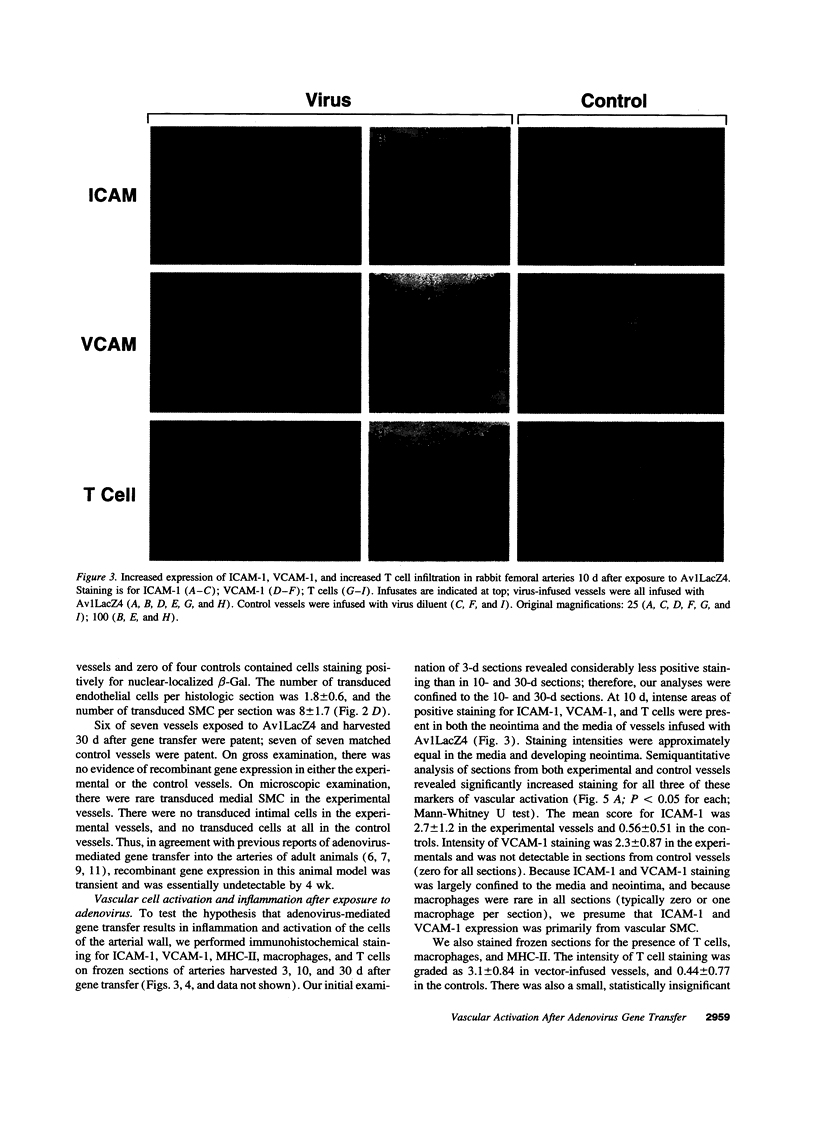
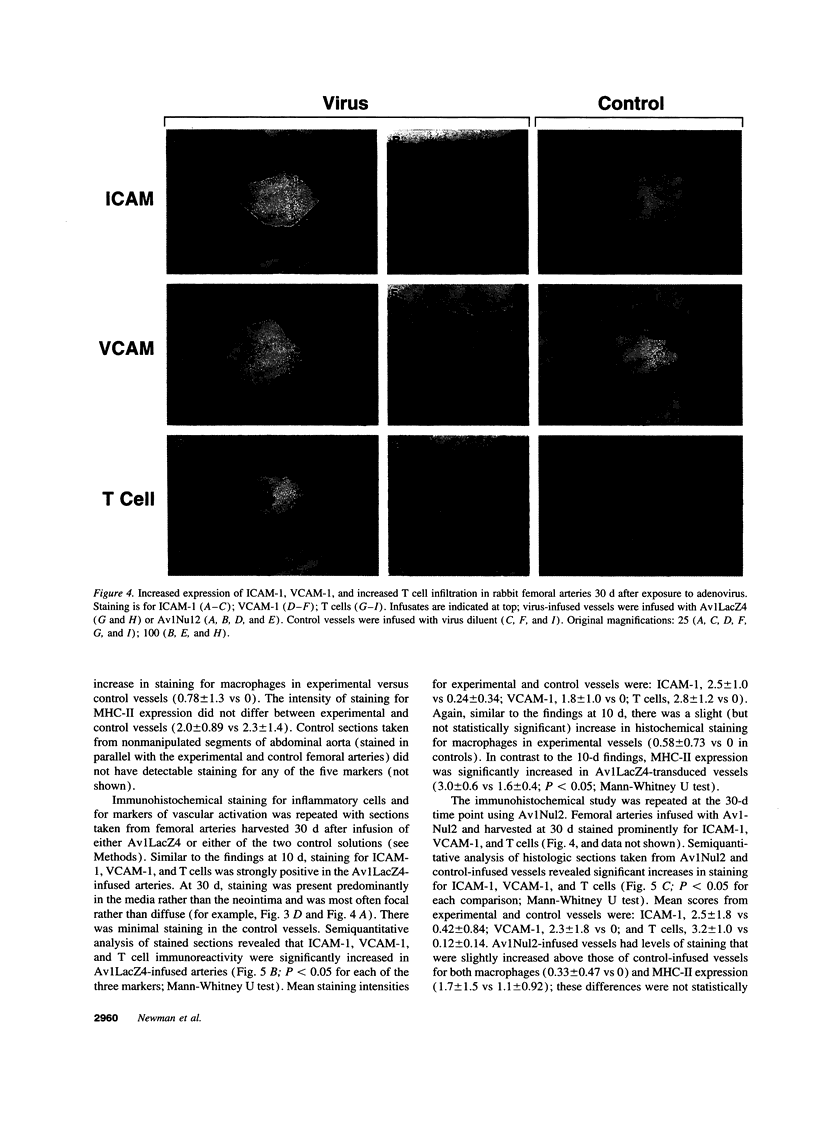
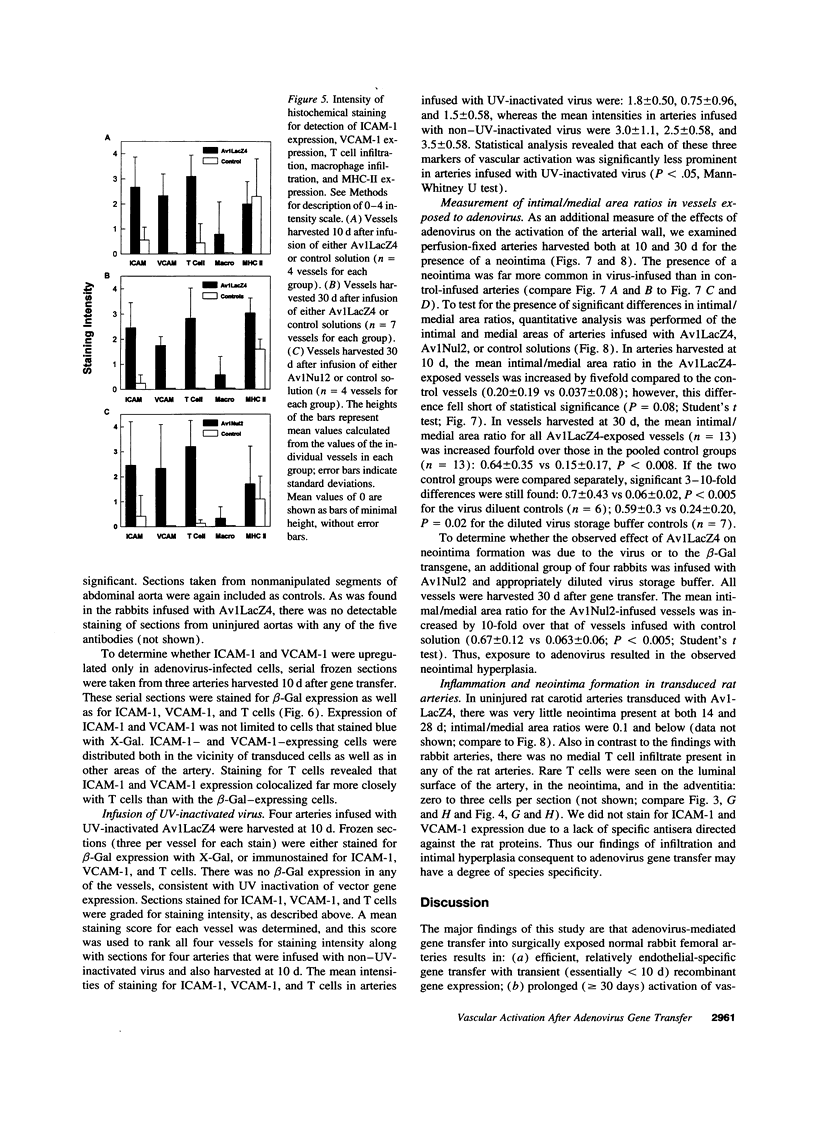

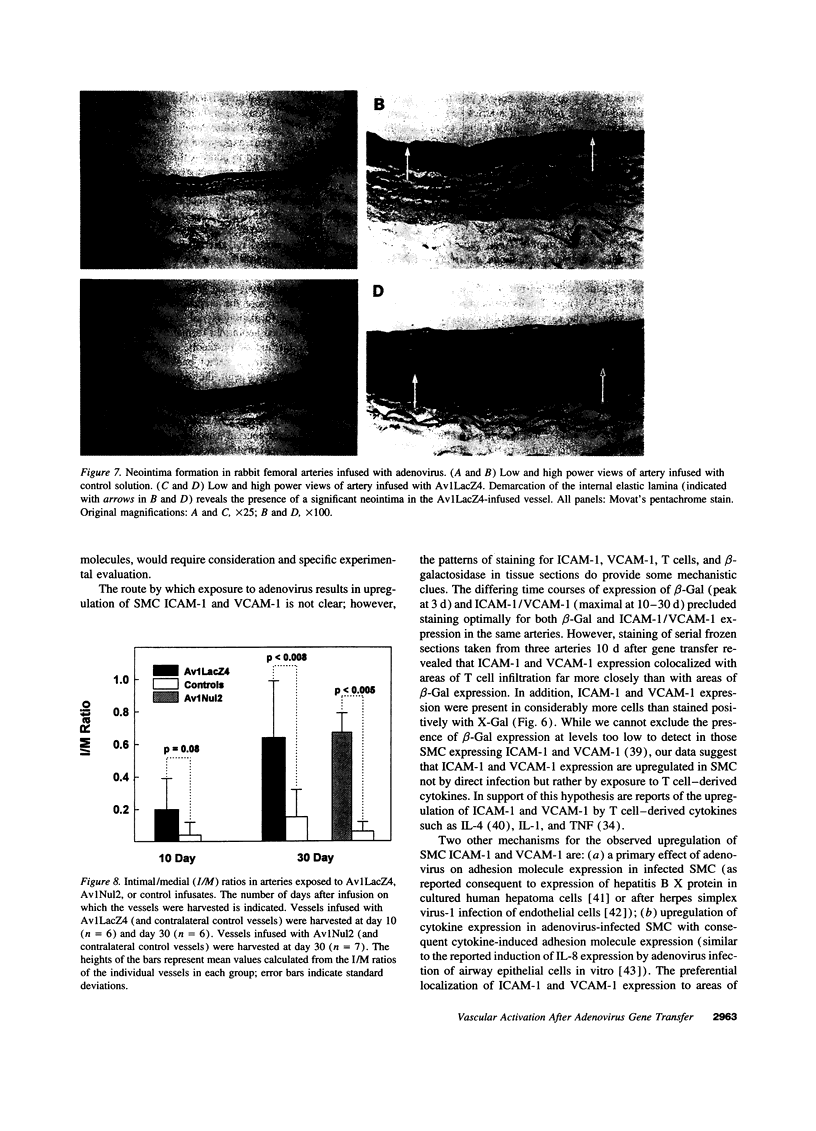


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin R., Wilmott R., Schwarz Y., Trapnell B., Stark J. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum Gene Ther. 1995 Feb;6(2):145–153. doi: 10.1089/hum.1995.6.2-145. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Blotnick S., Peoples G. E., Freeman M. R., Eberlein T. J., Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. L., Metzger M., Danel C., Rosenfeld M. A., Crystal R. G. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther. 1994 Jul;5(7):821–836. doi: 10.1089/hum.1994.5.7-821. [DOI] [PubMed] [Google Scholar]
- Chang M. W., Barr E., Seltzer J., Jiang Y. Q., Nabel G. J., Nabel E. G., Parmacek M. S., Leiden J. M. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995 Jan 27;267(5197):518–522. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Wilson J. M., Muller D. W. Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation. 1994 May;89(5):1922–1928. doi: 10.1161/01.cir.89.5.1922. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Ye X., Doranz B., Wilson J. M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7200–7203. doi: 10.1073/pnas.88.16.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. J., Steg P. G., Zheng L. P., Chen D., Kearney M., McGarr S. E., Barry J. J., Dedieu J. F., Perricaudet M., Isner J. M. Low-efficiency of percutaneous adenovirus-mediated arterial gene transfer in the atherosclerotic rabbit. J Clin Invest. 1995 Jun;95(6):2662–2671. doi: 10.1172/JCI117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B. A., Mazur W., Ali N. M., Geske R. S., Finnigan J. P., Rodgers G. P., Roberts R., Raizner A. E. Percutaneous transluminal in vivo gene transfer by recombinant adenovirus in normal porcine coronary arteries, atherosclerotic arteries, and two models of coronary restenosis. Circulation. 1994 Nov;90(5):2402–2413. doi: 10.1161/01.cir.90.5.2402. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R. J., Hirschowitz E. A., Brody S. L., Crystal R. G., Epstein S. E., Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R. J., Lemarchand P., Crystal R. G., Epstein S. E., Finkel T. Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation. 1993 Dec;88(6):2838–2848. doi: 10.1161/01.cir.88.6.2838. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hu K. Q., Yu C. H., Vierling J. M. Up-regulation of intercellular adhesion molecule 1 transcription by hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11441–11445. doi: 10.1073/pnas.89.23.11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Chused T. M., Wilkinson J. M., Leiserson W. M., Kindt T. J. Differentiation antigens identify subpopulations of rabbit T and B lymphocytes. Definition by flow cytometry. J Exp Med. 1983 Jan 1;157(1):34–46. doi: 10.1084/jem.157.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Wilson J. M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993 Jun;3(3):499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Trapnell B. C., Rade J. J., Virmani R., Dichek D. A. In vivo adenoviral vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ Res. 1993 Nov;73(5):797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- Lemarchand P., Jones M., Yamada I., Crystal R. G. In vivo gene transfer and expression in normal uninjured blood vessels using replication-deficient recombinant adenovirus vectors. Circ Res. 1993 May;72(5):1132–1138. doi: 10.1161/01.res.72.5.1132. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993 Dec;143(6):1551–1559. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Li H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. J Clin Invest. 1993 Aug;92(2):538–539. doi: 10.1172/JCI116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray P. B., Jr, Armstrong K., Zabner J., Miller D. W., Koretzky G. A., Couture L., Robillard J. E., Smith A. E., Welsh M. J. Adenoviral-mediated gene transfer to fetal pulmonary epithelia in vitro and in vivo. J Clin Invest. 1995 Jun;95(6):2620–2632. doi: 10.1172/JCI117964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G. Gene therapy for cardiovascular disease. Circulation. 1995 Jan 15;91(2):541–548. doi: 10.1161/01.cir.91.2.541. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Nabel G. J. Complex models for the study of gene function in cardiovascular biology. Annu Rev Physiol. 1994;56:741–761. doi: 10.1146/annurev.ph.56.030194.003521. [DOI] [PubMed] [Google Scholar]
- O'Brien K. D., Allen M. D., McDonald T. O., Chait A., Harlan J. M., Fishbein D., McCarty J., Ferguson M., Hudkins K., Benjamin C. D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993 Aug;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Poston R. N., Haskard D. O., Coucher J. R., Gall N. P., Johnson-Tidey R. R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992 Mar;140(3):665–673. [PMC free article] [PubMed] [Google Scholar]
- Printseva OYu, Peclo M. M., Gown A. M. Various cell types in human atherosclerotic lesions express ICAM-1. Further immunocytochemical and immunochemical studies employing monoclonal antibody 10F3. Am J Pathol. 1992 Apr;140(4):889–896. [PMC free article] [PubMed] [Google Scholar]
- Roessler B. J., Allen E. D., Wilson J. M., Hartman J. W., Davidson B. L. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest. 1993 Aug;92(2):1085–1092. doi: 10.1172/JCI116614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome J. J., Shayani V., Flugelman M. Y., Newman K. D., Farb A., Virmani R., Dichek D. A. Anatomic barriers influence the distribution of in vivo gene transfer into the arterial wall. Modeling with microscopic tracer particles and verification with a recombinant adenoviral vector. Arterioscler Thromb. 1994 Jan;14(1):148–161. doi: 10.1161/01.atv.14.1.148. [DOI] [PubMed] [Google Scholar]
- Ross R. The role of T lymphocytes in inflammation. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2879–2879. doi: 10.1073/pnas.91.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. D., French B. A. The advent of adenovirus. Gene therapy for cardiovascular disease. Circulation. 1993 Oct;88(4 Pt 1):1937–1942. doi: 10.1161/01.cir.88.4.1937. [DOI] [PubMed] [Google Scholar]
- Schulick A. H., Dong G., Newman K. D., Virmani R., Dichek D. A. Endothelium-specific in vivo gene transfer. Circ Res. 1995 Sep;77(3):475–485. doi: 10.1161/01.res.77.3.475. [DOI] [PubMed] [Google Scholar]
- Schulick A. H., Newman K. D., Virmani R., Dichek D. A. In vivo gene transfer into injured carotid arteries. Optimization and evaluation of acute toxicity. Circulation. 1995 May 1;91(9):2407–2414. doi: 10.1161/01.cir.91.9.2407. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Engelhardt J. F., Yang Y., Zepeda M., Weber-Pendleton S., Grossman M., Wilson J. M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993 Dec;4(6):771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- Steg P. G., Feldman L. J., Scoazec J. Y., Tahlil O., Barry J. J., Boulechfar S., Ragot T., Isner J. M., Perricaudet M. Arterial gene transfer to rabbit endothelial and smooth muscle cells using percutaneous delivery of an adenoviral vector. Circulation. 1994 Oct;90(4):1648–1656. doi: 10.1161/01.cir.90.4.1648. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Swanson S. J., Clinton S. K., Ganz P., Cybulsky M. I., Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993 Oct;88(4 Pt 1):1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- Willard J. E., Landau C., Glamann D. B., Burns D., Jessen M. E., Pirwitz M. J., Gerard R. D., Meidell R. S. Genetic modification of the vessel wall. Comparison of surgical and catheter-based techniques for delivery of recombinant adenovirus. Circulation. 1994 May;89(5):2190–2197. doi: 10.1161/01.cir.89.5.2190. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li Q., Ertl H. C., Wilson J. M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995 Apr;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Rosenfeld M. A., Seth P., Crystal R. G. Adenovirus-mediated augmentation of cell transfection with unmodified plasmid vectors. J Biol Chem. 1993 Feb 5;268(4):2300–2303. [PubMed] [Google Scholar]
- Zabner J., Couture L. A., Smith A. E., Welsh M. J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther. 1994 May;5(5):585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]
- von der Leyen H. E., Gibbons G. H., Morishita R., Lewis N. P., Zhang L., Nakajima M., Kaneda Y., Cooke J. P., Dzau V. J. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]