Abstract
Background
The majority of patients with hepatitis C virus (HCV) infection suffer from disabling fatigue, cognitive dysfunction, and quality of life reduction. Meanwhile, there is increasing evidence that HCV infection can affect brain function. Recent studies have shown that fatigue and psychomotor slowing may resolve in patients with hepatitis C after treatment with ondansetron. This observation indicates alteration of serotonergic neurotransmission in HCV infected patients with chronic fatigue.
Methods
Data from 20 HCV infected patients who were referred to our clinic because of disabling fatigue and cognitive decline of unknown cause were analysed retrospectively. Patients had undergone a diagnostic programme, including clinical and psychometric examination, electroencephalogram (EEG), magnetic resonance imaging of the brain, cerebrospinal fluid analysis, and I‐123‐beta‐CIT (2β‐carbomethoxy‐3‐β‐(4‐[123I]iodophenyl)tropane) single photon emission computerised tomography (SPECT) studies of serotonin and dopamine transporter binding capacity.
Results
All patients had pathological results on the fatigue impact scale. Two thirds of patients showed pathological attention test results. EEG, magnetic resonance imaging, and cerebrospinal fluid analysis were normal. Pathological dopamine transporter binding was present in 12/20 (60%) patients and pathological serotonin transporter binding in 8/19 (50%) patients. Patients with normal SPECT results did not significantly differ from controls with regard to psychometric test results. Interestingly, patients with both decreased serotonin and dopamine transporter binding showed significantly impaired performance in most of the tests applied. Comorbidity that could have impaired cerebral function was excluded in all patients.
Conclusion
Our findings indicate alteration of serotonergic and dopaminergic neurotransmission in HCV infected patients with chronic fatigue and cognitive impairment.
Keywords: hepatitis C, chronic fatigue, cognitive dysfunction, serotonin, dopamine
Chronic hepatitis C has been assumed to result in liver cirrhosis in approximately 30% of patients.1 Recent data however demonstrated a more benign course of the disease.2,3,4,5,6 In the majority of patients, extrahepatic manifestations of hepatitis C virus (HCV) infection can be observed. The most frequent are disabling chronic fatigue, cognitive decline, mood alterations, and musculoskeletal pain.7,8,9 Importantly, these manifestations are unrelated to the grade of liver disease. Up to 60% of women infected by anti‐D prophylaxis, for example, were found to suffer from impairment in well being even though they usually do not present significant liver disease decades after their infection.2,5,6 Goh and colleagues10 investigated whether the degree of fatigue in patients with chronic hepatitis C depends on the degree of liver disease or the presence of concomitant autoimmune disorder. They used the fatigue impact scale (FIS)11 to assess the severity of fatigue. They found significantly higher FIS scores in patients than in healthy controls. However, there was no difference between patients with and without virus replication, no correlation of the FIS score with the degree of liver dysfunction, and no difference between patients with and without concomitant autoimmune disorders. Other studies showed a significant reduction in quality of life in hepatitis C patients.12,13,14,15 Again, no correlation between grade of liver disease, virus replication rate, and quality of life scores was found.
The cause of fatigue, cognitive dysfunction, and mood disorder in HCV infected patients with only mild liver disease is not known. There is however growing evidence for involvement of the central nervous system (CNS) in the disorder.16,17,18,19,20
A clinical observation21 reported by Jones in 1999 implicated possible alteration of serotonergic neurotransmission. Jones observed that ondansetron, a 5‐hydroxytryptamine‐3 receptor antagonist, improved well being in HCV infected patients by a marked reduction in fatigue and an increase in the patient's psychomotor speed. His observation was recently confirmed by Piche and colleagues22 who found in a double blind placebo controlled study in 36 patients with chronic hepatitis C a significantly positive effect of ondansetron on chronic fatigue.
Inspired by these data on alteration of serotonergic neurotransmission in HCV infected patients with neuropsychiatric symptoms, we included I‐123‐beta‐2β‐carbomethoxy‐3‐β‐(4‐[123I]iodophenyl)tropane (CIT)‐single photon emission computerised tomography (SPECT) studies in the diagnostic workup of HCV infected patients with progressive neuropsychiatric disturbances who were referred to our clinic for diagnostic purposes.
Subjects and methods
Twenty patients (13 female; mean age 48.8 (5.3) years) with a history of HCV infection but only mild if any liver disease who had been referred to our clinic for clarification of disabling fatigue and cognitive decline of unknown cause underwent a diagnostic workup, including neurological examination, detailed neuropsychological examination, biochemical assessment, cerebrospinal fluid (CSF) analysis (n = 18), electroencephalogram (EEG), cerebral magnetic resonance imaging (MRI), and SPECT of mesencephalic/hypothalamic serotonin (SERT) and striatal dopamine (DAT) transporter binding capacity using I‐123‐beta‐CIT. In addition, patients completed questionnaires for assessment of health related quality of life and mood disturbances. Biochemical assessment included thyroid function (n = 20), hepatitis B (n = 20), and human immunodeficiency virus (HIV) serology (n = 14). Examinations were performed within approximately 72 hours while patients stayed at the hospital.
Data presented in this paper were retrospectively compiled and analysed. Data analysis was approved by the local ethics committee. In addition, patients gave informed consent.
Ten patients had been infected via application of anti‐D‐immunoglobulins, two patients via transfusion, three patients had a history of drug abuse more than 20 years ago, and in five patients the route of infection remained unclear. The genotype of the virus was 1b in 16 patients, 1a in two patients, and 3a in two patients. In all but two of the patients the infection and/or diagnosis of the infection had occurred more than 15 years ago. In one patient the time interval between infection/diagnosis and inclusion in this study was nine years while in another it remained unclear. Sixteen patients were polymerase chain reaction (PCR) positive at the time of admission and four were PCR negative. Two of the PCR negative patients had cleared the virus after interferon therapy more than one year previously (one former drug abuser and one patient from the anti‐D group). The two others—which had both been infected via anti‐D prophylaxis—had cleared the virus spontaneously. All but one of the patients did not take any CNS affecting medications. One patient was receiving citalopram 10 mg daily. Her serotonin transporter binding was normal. Therefore, she was not excluded from data analysis.
The psychometric test battery comprised the PSE syndrome test,24 the cancelling “d” test,25 and the tests “alertness”, “divided attention”, “incompatibility”, “modality comparison”, “attention shift”, and “Go/NoGo” from the TAP battery (Testbatterie zur Aufmerksamkeitsprüfung (battery of attention tests)).26 Furthermore, the hospital anxiety and depression scale (HADS),27 Beck's depression inventory (BDI),28 FIS,11 and the SF‐36 questionnaire29 were performed.
Individual neuropsychological results were evaluated by comparison with norm data provided by the test manuals. In addition, patient test results were compared with those of 14 healthy controls (10 females; mean age 51.5 (15.2) years).
All patients underwent a standard 12 channel EEG. EEG assessment was performed visually.
MRI of the brain was performed using a 1.5 T GE Signa Horizon. Conventional transverse and coronal T1 weighted images (TR 500 ms/TE 15 ms/slice thickness 5 mm/slice gap 1,5 mm) and sagittal T2 weighted fast IR images (TR 4000 ms/TE 40 ms/TI 130 ms/slice thickness 5 mm/slice gap 1.5 mm) were achieved to exclude cerebral lesions.
SPECT images (Multispect 3; Siemens, Erlangen, Germany) were obtained four hours after intravenous injection of 200 MBq I‐123‐Beta‐CIT to assess SERT in the hypothalamus/midbrain region and 24 hours post injection to measure striatal DAT. Imaging was performed according to the guidelines of the European Association of Nuclear Medicine.30 To detect count densities in regions of specific tracer binding (caudate and putamen on either side, hypothalamus/midbrain), small regions of interest of standardised shape and size were positioned over the respective structures on transaxial tomograms. Non‐specific binding was assessed using a more extended reference region which was drawn over the occipital or cerebellar cortex, respectively. As a measure of transporter binding capacity, ratios of count densities in specific binding areas compared with those in non‐specific binding regions were calculated. As we did not observe regionally pronounced impairment of striatal binding, a mean value for ratios obtained for the four subregions was used for further analysis of striatal binding. SPECT results in patients were compared with norm data achieved from the study of 20 healthy controls (11 females; mean age 50.2 (18.9) years) with respect to DAT binding and 16 healthy controls (eight females; mean age 47.3 (17.2) years) with respect to SERT binding previously. Further details of the reference groups are given in Berding and colleagues.31
Statistical analysis
To evaluate whether differences between SPECT results in patients and controls were significant, the t test for independent samples was used. A correlation analysis was performed to detect correlations between transporter binding and the various neuropsychological parameters. All p values were adjusted for multiple testing by applying the Bonferroni‐Holm procedure.32 In addition, the patient group was subdivided into four groups based on SPECT data: (1) normal DAT and SERT binding; (2) reduced SERT but normal DAT binding, (3) reduced DAT but normal SERT binding; (4) both reduced DAT and SERT binding. All subgroups were compared with controls using Dunnett's test for multiple comparisons. In addition, subgroups with reduced DAT but normal SERT binding3 and with reduced DAT and SERT binding,4 respectively, were compared with the patients subgroup with normal SPECT results using Dunnett's test for multiple comparisons.
Results
There were no significant differences in age and sex between patients in the respective comparison groups. Liver function was normal in all 20 patients. Liver biopsy was performed in seven patients. There was no indication of liver cirrhosis in any of the 20 patients based on both biopsy and APRI score23 (APRI score <1, n = 18; APRI score 1.1, n = 1, APRI score 1.9, n = 1, Ishak fibrosis score in this patient, 4). None of the patients had hepatitis B or HIV coinfection. Thyroid function was normal in all patients. Routine biochemical examinations did not reveal any metabolic dysfunction or immunological disease. CSF cell count and total protein, and EEG and MRI were normal in all patients.
FIS, mood, and quality of life scores
All patients had a pathological FIS score (mean 94.6 (25) (range 51–141); normal range <35). The HADS anxiety and depression scores were abnormal (>7 points) in 13 and 10 patients, respectively. BDI was normal in four patients (<10 points) while six scored as mild (11–17 points), six as moderate (18–23 points), and three as severe (>23 points) depression. SF‐36 indices differed significantly from those given in the manual for the corresponding age group of healthy controls (fig 1). Thereby patients scored worst with regard to the subscores “role physical”, “vitality”, and “role emotional”.
Figure 1 SF‐36 scores for patients compared with scores for the normal population, as given in the manual
The four PCR negative patients scored in the worst half of the mood and quality of life scores, showing an FIS score of 107–134, BDI 13–20 (n = 3), HADS‐A 4‐17, and HADS‐D 5–17. Their SF‐36 sum score was 81–304 (maximum achievable 800 points).
Psychometric test results
The sum score of the PSE syndrome test result was abnormal in two (one PCR negative) and the d2 test in five (two PCR negative) patients. Fourteen of 16 patients who underwent the TAP battery had pathological results in the “divided attention” test while approximately two thirds of patients achieved pathological results in the remaining subtests of the battery (table 1). Three of the four PCR negative patients underwent the TAP battery. In most of the tests at least two of them achieved pathological results while all three had pathological test results in the alertness test (simple and warned reaction time) and the modality comparison test.
Table 1 Number of patients with pathological results in the psychometric tests.
| Psychometric test | No of pathological results (%) (n) |
|---|---|
| Test d2 | |
| % errors | 21 (4/19) |
| Items—errors | 21 (4/19) |
| Combined | 26 (5/19) |
| Attention shift | |
| Reaction time | 50 (8/16) |
| Errors | 37.5 (6/16) |
| Combined | 56 (9/16) |
| Divided attention | |
| Reaction time | 75 (12/16) |
| Errors | 50 (8/16) |
| Missings | 69 (11/16) |
| Combined | 87.5 (14/16) |
| Simple RT | 75 (12/16) |
| Warned RT | 69 (11/16) |
| Phasic alertness | 19 (3/16) |
| Go/NoGo | |
| Reaction time | 60 (9/15) |
| Errors | 7 (1/15) |
| Missings | 20 (3/15) |
| Combined | 75 (10/15) |
| Modality comparison | |
| Reaction time | 62.5 (10/16) |
| Errors | 25 (4/16) |
| Missings | 12.5 (2/16) |
| Combined | 62.5 (10/16) |
| Incompatibility | |
| Reaction time | 47 (7/15) |
| Errors | 27 (4/15) |
| Combined | 60 (9/15) |
RT, reaction time.
SPECT results
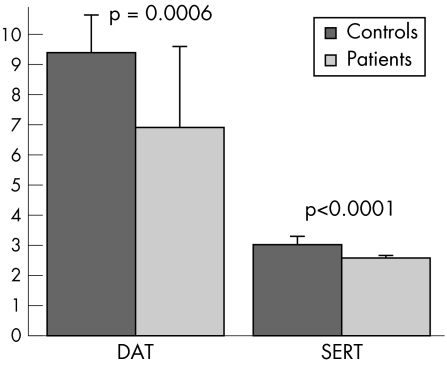
DAT and SERT binding were significantly reduced in patients compared with the reference group of healthy subjects (DAT: controls 9.39 (1.26), patients 6.91 (2.69), p<0.0006; SERT: controls 3.02 (0.29), patients 2.58 (0.08), p<0.0001) (figs 2, 3). Pathological DAT binding (that is, more than 2 SDs reduced) was present in 12/20 (60%) patients; pathological SERT binding was present in 8/19 (50%) patients. Six patients had pathological SERT and DAT binding and six had entirely normal results. SERT and DAT binding were pathological in three of the four PCR negative patients, while both were normal in the fourth.
Figure 2 Dopamine transporter binding (DAT) and serotonin transporter binding (SERT) in patients and controls.
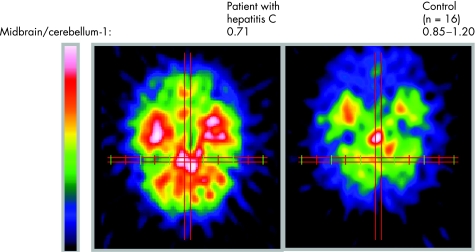
Figure 3 Comparison of tracer binding four hours after injection in a patient with hepatitis C (left) and a healthy control (right), representing serotonin transporter binding. The ratio of specific binding (in the midbrain) to unspecific binding (in the cerebellum) was 0.71 in this patient. In healthy controls the ratio was 0.85–1.20.
There was no correlation between the SPECT data (DAT and SERT) and FIS, or mood and quality of life scores. In addition, there was no correlation between the SPECT results and the psychometric data after adjustment of p values for multiple testing.
After subdivision of the patient group according to their SPECT results however, it became obvious that patients with altered DAT or DAT and SERT binding differed significantly from controls in most of the psychometric tests (tables 2 and 3; fig 4) while there was no difference in psychometric results between patients with normal SPECT and controls. There was also no significant difference between the subgroups with regard to FIS, HADS, BDI, and SF36 results.
Table 2 Mean (SD) psychometric tests results for patients and controls.
| Psychometric test | Controls | HCV patients with normal SPECT | HCV patients with altered DAT but normal SERT binding | HCV patients with altered DAT and SERT binding |
|---|---|---|---|---|
| d2 (% of errors) | 4.6 (2.4) | 4.5 (3.0) | 7.8 (7.7) | 18.5 (9.4) |
| Modality comparison errors (n) | 0.7 (1.1) | 0.25 (0.5) | 2.0 (4.0) | 4.0 (4.1) |
| Modality comparison RT (ms) | 477.3 (64.2) | 529.6 (85.3) | 554 (115.5) | 743.5 (201.1) |
| Attention shift RT (ms) | 929.7 (223.0) | 963.8 (236.8) | 1310.4 (283.7) | 1909.2 (1201.0) |
| Attention shift errors (n) | 3.1 (2.2) | 2.3 (2.6) | 14.5 (16.8) | 19.2 (16.8) |
| d2 items—errors (n) | 448.3 (104.4) | 380.2 (88.4) | 345.8 (85.3) | 218.2 (80.2) |
| Simple RT (ms) | 286.8 (34.8) | 366.3 (85.9) | 516.0 (215.7) | 542.9 (253.6) |
| Warned RT (ms) | 281.2 (36.8 ) | 340.8 (53.6) | 472.8 (264.5) | 470.7 (227.1) |
| Divided attention missings (n) | 2.5 (2.4 ) | 3.3 (1.0) | 11.8 (11.2) | 12.4 (9.1) |
| Modality comparison missings (n) | 0.3 (1.1) | 0.75 (0.5) | 2.25 (4.5) | 2.0 (2.3) |
| Divided attention RT (ms) | 731.9 (74.2) | 807.1 (152.4) | 1112.8 (390.6) | 807.5 (85.2) |
| Divided attention errors (n) | 2.8 (2.3) | 5.5 (7.8) | 10.8 (11.7) | 5.6 (6.8) |
HCV, hepatitis C virus; DAT, dopamine transporter binding; SPECT, single photon emission computerised tomography; SERT, serotonin transporter binding; RT, reaction time.
Table 3 Comparison of psychometric test results of the different patient and control groups using Dunnett's test for multiple comparison.
| Psychometric test | Controls/HCV patients with normal SPECT | Controls/HCV patients with altered DAT binding but normal SERT binding | Controls/HCV patients with altered DAT and SERT binding |
|---|---|---|---|
| d2 (% of errors) | NS | NS | p<0.0001 |
| Modality comparison errors | NS | NS | p = 0.0003 |
| Modality comparison RT | NS | NS | p = 0.0002 |
| Attention shift RT | NS | NS | p = 0.0049 |
| Attention shift errors | NS | p = 0.0014 | p<0.0001 |
| d2 items—errors | NS | p = 0.0159 | p = 0.0001 |
| Simple RT | NS | p = 0.0049 | p = 0.0010 |
| Warned RT | NS | p = 0.0241 | p = 0.0162 |
| Divided attention missings | NS | p = 0.0200 | p = 0.0081 |
| Modality comparison missings | NS | P = 0.0219 | P = 0.0285 |
| Divided attention RT | NS | p = 0.0011 | NS |
| Divided attention errors | NS | p = 0.0101 | NS |
HCV, hepatitis C virus; DAT, dopamine transporter binding; SPECT, single photon emission computerised tomography; SERT, serotonin transporter binding; RT, reaction time.
Figure 4 Test d2 results give an example of the performance differences between the four patient groups and controls for cognitive function. HCV, hepatitis C virus; DAT, dopamine transporter binding; SERT, serotonin transporter binding.
Patients with decreased DAT and SERT binding differed significantly from controls with regard to the following test results: number of evaluated items minus errors in the cancelling d test, percentage of errors in the cancelling d test, number of errors in the “modality comparison” and “attention shift” test, number of missings in the “divided attention” and “modality comparison” test, reaction time in the subtests “modality comparison” and “attention shift”, and simple and warned reaction time in the “alertness test” of the TAP. Patients with significantly decreased DAT but normal SERT binding also did worse than controls but did not reach the level of significance in as many tests as those with altered DAT and SERT binding (table 3). After Bonferroni‐Holm correction for multiple comparisons,32 they achieved significantly worse results than controls (nominal α = 0.0042 for the smallest p value) only with regard to the number of errors in the attention shift test and reaction time in the divided attention test. Patients with altered DAT and SERT binding differed significantly from controls after Bonferroni‐Holm correction with regard to the test d2 results (items—errors, percentage of errors), number of errors in the modality comparison test and the attention shift test, and simple reaction time in the alertness test of the TAP battery.
As only two patients had significantly decreased SERT binding but normal DAT binding, comparison of their psychometric results with the control group was not performed.
When data from the patient groups with altered DAT and altered DAT and SERT were compared with those of patients with normal SPECT results, significant differences were found for the cancelling d test results (percentage of errors p = 0.011; number of evaluated items minus errors p = 0.015) for the group with DAT and SERT alteration.
Discussion
Patients evaluated in this study complained of a decline in cognitive function. In addition, they felt abnormally fatigued, and some also reported mood alterations with increased anxiety and depression. They were referred to our clinic to clarify the cause of their neuropsychiatric symptoms. There was no indication in any of the patients of the presence of liver cirrhosis and/or hepatic encephalopathy. Other causes of cerebral dysfunction were also excluded via a comprehensive diagnostic workup.
Patients scored significantly worse than healthy controls in the FIS, HADS, and SF‐36. As these measures rely on self report, the abnormal findings could result from anxiety due to knowledge of the HCV infection rather than somatic alterations. However, a study analysing HCV positive patients unaware of their diagnosis also found them to be impaired compared with HCV negative controls.14
Previous studies have indicated that approximately half of HCV positive patients with only mild liver disease complain of abnormal fatigue, depression, and cognitive dysfunction, irrespective of their PCR status.2,5,6,10 Evaluation of cognitive function in HCV positive patients with normal or only mildly impaired liver function has shown that they suffer from deficits in attention, learning ability, and memory predominantly.17,18 Magnetic resonance spectroscopic studies revealed significant alterations in the choline/creatine and/or N‐acetyl‐aspartate/creatine ratio as an indication of cerebral metabolic alterations whereas MRI was normal.16,17,18,19 In agreement with this, Kramer and colleagues20 were able to show a significant increase in P300 latency, a measure of stimulus evaluation processing, in non‐cirrhotic hepatitis C patients compared with healthy controls. Thus there is increasing evidence that hepatitis C virus infection is accompanied by cerebral dysfunction in some patients.
Our present findings emphasise this assumption. We have recently shown that cognitive decline in patients with hepatitis C is especially due to deficits in attention, higher executive functions, learning ability, and memory.18 This was confirmed in the present study. All but two of the 16 evaluated patients showed pathological results in the divided attention test, and approximately two thirds had pathological results in the simple and warned reaction time test, intermodal comparison test, incompatibility test, and attention shift test. In particular, we observed an above normal reaction time in most patients. Furthermore, especially in the divided attention test and the attention shift test, the number of errors and missings were increased compared with controls. Therefore, increased reaction times could not be attributed to motor slowing but to attention deficits. This assumption is supported by the fact that none of the patients gained pathological results in the “serial dotting” subtest of the PSE syndrome test, a test of pure motor speed.
The observations of Jones21 and Piche and colleagues22 that disabling fatigue can be significantly improved in patients with chronic hepatitis C by treatment with the 5‐hydroxytryptamine‐3 receptor antagonist ondansetron suggests a role for the serotonergic system in the pathophysiology of chronic fatigue and cognitive decline in HCV infected patients. Serotonergic neurotransmission can be studied in vivo by SPECT. I‐123‐Beta‐CIT is normally used for evaluation of striatal dopamine transporter binding. This tracer however also binds to serotonin transporters. Due to the fact that high densities of SERT and DAT are present in different anatomical locations and because of the different binding kinetics of I‐123‐beta‐CIT to DAT or SERT, early scans—performed approximately four hours after injection of the tracer—can be used to study SERT binding in the hypothalamus/midbrain region while later scans—approximately 24 hours after injection—represent DAT binding in the striatum.33,34
Using I‐123‐beta‐CIT‐SPECT we were able to show decreased binding capacity of the midbrain serotonin transporters and/or the striatal dopamine transporters in 14 of the 20 patients examined. Interestingly, those patients with altered monoamine transporter binding performed significantly worse than healthy controls in most of the psychometric tests. Based on these data we conclude that psychometric alterations in HCV infected patients are related to alterations in neurotransmission. FIS scores did not significantly differ between the patient groups. Thus fatigue seems to be caused by additional factors apart from monoaminergic dysfunction. This assumption is also supported by the fact that ondansetron treatment is effective in only about a third of patients.22
Decreased β‐CIT binding, as shown in our patients, may be due to structural and/or functional alteration of the serotonin and dopamine transporter or a decrease in the number of serotonergic or dopaminergic neurones within the region of interest. As we cannot differentiate between these different pathologies and also we do not know about the presence and function of the respective receptors, the mechanisms of monoaminergic dysfunction in HCV exposed patients cannot be described in detail.
Dopamine has been shown to be a “key regulator to adapt action, emotion, motivation, and cognition”.35 With regard to cognition, alteration of striatal dopaminergic neurotransmission is considered to result in deficits in mental flexibility, working memory, learning ability, sustained attention, attention shift, and higher executive functions.36,37,38
In common with dopamine, serotonin is also involved in the modulation of perception, attention, emotion, and cognition.39 The vast majority of serotonergic neurones are located in the brainstem. The rostral group, which includes the dorsal and median raphe nuclei, is reciprocally connected to several cortical areas, especially the medial prefrontal cortex.40,41 This region is associated with a large number of cognitive functions and is involved in the planning and execution of complex tasks. Dorsal raphe neurones are also involved in the modulation of the sleep‐waking cycle, major depression, suicidal behaviour, and aggressive and anxiety behaviour.40 According to Severson and colleagues,42 serotonergic neurones located in the midbrain maintain pH homeostasis by inducing arousal, anxiety, and changes in cerebrovascular tone. Structural or functional alterations of these neurones are considered to be the cause of otherwise unrelated diseases such as sudden infant death syndrome, panic disorder, and migraine.
It must be emphasised that four of the 20 patients examined were PCR negative. These four patients did not differ from the PCR positive patients with regard to their clinical presentation, neuropsychological findings, or SPECT results. Thus it could be questioned whether the neuropsychiatric symptoms and alterations in neurotransmission found in our patients are a consequence of their HCV infection. However, it is well known from previous studies that chronic fatigue, cognitive dysfunction, and a decrease in quality of life are independent of the grade of liver disease or the virus replication rate.10,12,13,14,15 Also, there is increasing evidence that the virus may enter the brain via lympho‐ or monocytic blood cells.43 In common with HIV infection, the “Trojan horse” hypothesis must be considered in HCV infection. The virus enters the brain in blood derived macrophagic cells, is hosted by microglial cells, and alters the function of these microglial cells thereby inducing neuronal dysfunction.44 Radkowski and colleagues45 and Forton and colleagues46 provided evidence that the CNS is a site of HCV replication and that within the CNS virus replication occurs at a lower level than in the liver. In addition, they showed that different HCV quasispecies may be present within brain tissue and serum. Thereby the responsiveness of brain and liver hosted virus to any antiviral therapy may be different, and while there is no evidence of viral replication in serum, the virus may persist in the brain and alter neuronal function.
In conclusion, some HCV infected patients with normal liver function show alterations in both the midbrain serotonergic and striatal dopaminergic systems, irrespective of their PCR status. In addition, these patients show alterations of mood and cognition consistent with impaired dopaminergic and serotonergic function (that is, depression, anxiety, and marked attention deficits). Thus the present findings implicate a role for altered monoaminergic neurotransmission in the pathophysiology of cerebral dysfunction concomitant with HCV infection. Even the less frequently reported symptoms nausea, migraine‐like headaches, increased or decreased food intake, or aggressive behaviour, which are part of the “encephalopathy” associated with HCV infection, may be explained by these findings. Further studies should be performed aiming for both detection of the causes of these alterations at the cellular level and development of adequate treatment strategies.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Abbreviations
BDI - Beck's depression inventory
CSF - cerebrospinal fluid
CNS - central nervous system
DAT - dopamine transporter binding
EEG - electroencephalogram
FIS - fatigue impact scale
HADS - hospital anxiety and depression scale
HCV - hepatitis C virus
HIV - human immunodeficiency virus
I‐123‐beta‐CIT - 2β‐carbomethoxy‐3‐β‐(4‐[123I]iodophenyl)tropane
MRI - magnetic resonance imaging
PCR - polymerase chain reaction
SPECT - single photon emission computerised tomography
SERT - serotonin transporter binding
TAP - Testbatterie zur Aufmerksamkeitsprüfung (battery of attention tests)
Footnotes
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Lauer G, Walker B. Hepatitis C Virus. N Engl J Med 200134541–52. [DOI] [PubMed] [Google Scholar]
- 2.Kenny‐Walsh E for the Irish Hepatology Research Group Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. N Engl J Med 19993401228–1233. [DOI] [PubMed] [Google Scholar]
- 3.Rodger A J, Roberts S, Lanigan A.et al Assessment of long‐term outcomes of community‐acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 200032582–587. [DOI] [PubMed] [Google Scholar]
- 4.Seeff L B, Miller R N, Rabkin C S.et al 45‐year follow‐up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000132105–111. [DOI] [PubMed] [Google Scholar]
- 5.Wiese M, Berr F, Lafrenz M.et al Low frequency of cirrhosis in a hepatitis C (genotype 1b) single‐source outbreak in Germany: a 20‐year multicenter study. Hepatology 20003291–96. [DOI] [PubMed] [Google Scholar]
- 6.Wiese M, Grüngreiff K, Güthoff W.et al Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany—a 25 year multicenter study. J Hepatol 200543590–598. [DOI] [PubMed] [Google Scholar]
- 7.Barkhuizen A, Rosen H R, Wolf S.et al Muskuloskeletal pain and fatigue are associated with chronic hepatitis C. A report of 239 hepatology clinic patients. Am J Gastroenterol 1999941355–1360. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Cacoub P, Ratziu V.et al Fatigue in patients with chronic hepatitis C. J Viral Hepat 20029295–303. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub P, Ratziu V, Myers R P.et al Impact of treatment on extra hepatic manifestations in patients with chronic hepatitis C. J Hepatol 200236812–818. [DOI] [PubMed] [Google Scholar]
- 10.Goh J, Coughlan B, Quinn J.et al Fatigue does not correlate with the degree of hepatitis C or the presence of autoimmune disorder in chronic hepatitis C infection. Eur J Gastroenterol Hepatol 199911833–838. [DOI] [PubMed] [Google Scholar]
- 11.Fisk J D, Ritvo P G, Ross L.et al Measuring the functional impact of fatigue: validation of the fatigue impact scale. Clin Infect Dis 199418(suppl 1)S79–S83. [DOI] [PubMed] [Google Scholar]
- 12.Carithers R L, Sugano D, Bayliss M. Health assessment for chronic HCV infection: results of quality of life. Dig Dis Sci 19964175–80S. [DOI] [PubMed] [Google Scholar]
- 13.Foster G R, Goldin R D, Thomas H C. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 199827209–212. [DOI] [PubMed] [Google Scholar]
- 14.Rodger A J, Jolley D, Thompson S C.et al The impact of diagnosis of hepatitis C virus on quality of life. Hepatology 1999301299–1301. [DOI] [PubMed] [Google Scholar]
- 15.Barrett S, Goh J, Coughlan B.et al The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut 200149423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forton D M, Allsop J M, Main J.et al Evidence for a cerebral effect of the hepatitis C virus. Lancet 200135838–39. [DOI] [PubMed] [Google Scholar]
- 17.Forton D M, Thomas H C, Murphy C A.et al Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology 200235433–439. [DOI] [PubMed] [Google Scholar]
- 18.Weissenborn K, Krause J, Bokemeyer M.et al Hepatitis C virus infection affects the brain—evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol 200441845–851. [DOI] [PubMed] [Google Scholar]
- 19.McAndrews M P, Farcnik K, Carlen P.et al Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology 200541801–808. [DOI] [PubMed] [Google Scholar]
- 20.Kramer L, Bauer E, Funk G.et al Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol 200237349–354. [DOI] [PubMed] [Google Scholar]
- 21.Jones E A. Relief from profound fatigue associated with chronic liver disease by long‐term ondansetron therapy. Lancet 1999354397. [DOI] [PubMed] [Google Scholar]
- 22.Piche T, Vanbiervliet G, Cherikh F.et al Effect of ondansetron, a 5‐HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut 2005541169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wai C T, Greenson J K, Fontana R J.et al A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 200338518–526. [DOI] [PubMed] [Google Scholar]
- 24.Schomerus H, Weissenborn K, Hamster W.et alPSE‐Syndrom‐Test. Psychodiagnostisches Verfahren zur quantitativen Erfassung der (minimalen) portosystemischen Enzephalopathie. Frankfurt: Swets Test Services, 1999
- 25.Brickenkamp R.Test d2. Aufmerksamkeits‐Belastungs‐Test. Göttingen‐Toronto‐Zürich: Verlag für Psychologie J Hogrefe, 1981
- 26.Zimmermann P, Fimm B.Neuropsychologische Testbatterie zur Erfassung von Aufmerksamkeitsdefiziten—Revidierte Fassung. Freiburg: Psychologisches Institut der Universität Freiburg, 1989
- 27.Zigmond A S, Snaith R P. The hospital anxiety and depression scale. Acta Psychiatr Scand 198367361–370. [DOI] [PubMed] [Google Scholar]
- 28.Beck A T, Ward C H, Mendelson M.et al An inventory for measuring depression. Arch Gen Psychiatry 19614561–571. [DOI] [PubMed] [Google Scholar]
- 29.Bullinger M, Kirchberger I.Der SF‐36 Fragebogen zum Gesundheitszustand (SF‐36). Handbuch für die deutschsprachige Fragebogenversion. Göttingen: Hogrefe—Verlag für Psychologie, 1998
- 30.Tatsch K, Asenbaum S, Bartenstein P.et al European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using (123)I‐labelled dopamine transporter ligands. Eur J Nucl Med Mol Imaging 200229BP30–BP35. [PubMed] [Google Scholar]
- 31.Berding G, Brucke T, Odin P.et al [123I]beta‐CIT SPECT imaging of dopamine and serotonin transporters in Parkinson's disease and multiple system atrophy. Nuklearmedizin 20034231–38. [PubMed] [Google Scholar]
- 32.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist 1979665–70. [Google Scholar]
- 33.Brucke T, Kornhuber J, Angelberger P.et al SPECT imaging of dopamine and serotonin transporters with [123I]beta‐CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 199394137–146. [DOI] [PubMed] [Google Scholar]
- 34.Kuikka J T, Tiihonen J, Bergstrom K A.et al Imaging of serotonin and dopamine transporters in the living human brain. Eur J Nucl Med 199522346–350. [DOI] [PubMed] [Google Scholar]
- 35.Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol 200316(suppl 2)S3–S9. [PubMed] [Google Scholar]
- 36.Volkow N D, Logan J, Fowler J S.et al Association between age‐related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 200015775–80. [DOI] [PubMed] [Google Scholar]
- 37.Volkow N D, Gur R C, Wang G ‐ J.et al Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998155344–349. [DOI] [PubMed] [Google Scholar]
- 38.Bäckman L, Ginovart N, Dixon R A.et al Age‐related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 2000157635–637. [DOI] [PubMed] [Google Scholar]
- 39.Roth B L, Hanizavareh M, Blum A E. Serotonin receptors represent highly favourable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology 200417417–24. [DOI] [PubMed] [Google Scholar]
- 40.Hornung J P. The human raphe nuclei and the serotonergic system. J Clin Neuroanat 200326331–343. [DOI] [PubMed] [Google Scholar]
- 41.Celada P, Puig V, Casanovas J M.et al Controls of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin‐1A, GABAA, and glutamate receptors. J Neuroscience 2001219917–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Severson C, Wang W, Pieribone V A.et al Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 200361139–1140. [DOI] [PubMed] [Google Scholar]
- 43.Laskus T, Radkowski M, Adair D M.et al Emerging evidence of hepatitis C virus neuroinvasion. AIDS 200519(suppl 3)S140–S144. [DOI] [PubMed] [Google Scholar]
- 44.Kramer‐Hämmerle S, Rothenaigner I, Wolff H.et alCells of the central nervous system as targets and reservoir of the human immunodeficiency virus, Virus Research 2005111194–213. [DOI] [PubMed] [Google Scholar]
- 45.Radkowski M, Wilkinson J, Nowicki M.et al Search for hepatitis c virus negative‐strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J Virol 200276600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forton D M, Karayiannis P, Mahmud N.et al Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol 2004785170–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.