Mortality as a result of human immunodeficiency virus (HIV) infection has declined significantly due to application of highly active antiretroviral therapy (HAART).1 However, an effect of this therapy is its promotion of immune reconstitution, which leaves patients more vulnerable to developing immune related illnesses, such as Crohn's disease (CD).2,3
CD causes an increase in tumour necrosis factor α (TNF‐α) that seems to play a fundamental role in the condition. Thus anti‐TNF‐α therapies represent a step forward in the management of CD.4 Elevated TNF‐α levels are also observed during all stages of HIV, and consequently the use of anti‐TNF‐α agents has been also suggested for HIV patients.5 To date, three controlled trials of anti‐TNF‐α based therapies6,7,8 in HIV patients have been reported. Each study registered a decrease in serum TNF‐α levels, and no change in the CD4 cell count or plasma HIV RNA, or any adverse side effects. However, the safety and efficacy of anti‐TNF‐α agents in CD in the context of chronic viral infections is unclear. This is the first report of a patient affected concomitantly by CD and HIV and treated with an anti‐TNF‐α agent (infliximab).
A 42 year old Caucasian woman was infected with HIV through heterosexual contact in 1997. HAART therapy had controlled the infection (CD4 counts usually >250; fig 1) and no opportunistic infections had appeared. In October 2003 she was diagnosed with inflammatory bowel disease (IBD) based on a flare up of rectal bleeding, diarrhoea, and fever. Left colonoscopy revealed multiple erosions and several deep geographic ulcerations. Histology was compatible with indeterminate colitis. Cytomegalovirus (CMV) and other viral, bacterial, and parasite infections were ruled out. At the time of IBD diagnosis, the patient's CD4 count was 555 cells/ml and her viral HIV load was <200 copies. The patient initially responded to corticosteroids.
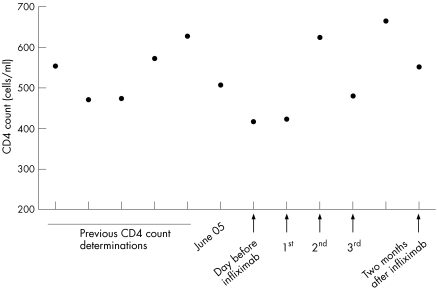
Figure 1 CD4 counts. Previous CD4 counts refer to years before and after diagnosis of Crohn's disease (CD). June 05 shows the CD4 count before the acute flare where it was decided to administer infliximab infusion. 1st, 2nd, and 3rd refer to infliximab infusions.
In August 2005, the subject suffered an acute IBD flare up with severe rectal bleeding, which was medically controlled with intravenous corticosteroids and antibiotics. Colonoscopy revealed IBD activity in the sigma and throughout the colon. Additionally, two fistulae orifices were observed in the anal canal. Nuclear magnetic resonance imaging showed a 5 cm inter‐sphincter (internal and external) fistula. This time the histology was compatible with CD. Again, CMV, other viruses, and infestation of bacteria or parasites were excluded. The last CD4 count before this episode had been 505 cells/ml (June 2005).
We decided to employ an anti‐TNF‐α agent (infliximab) for both inducing remission and treating the perianal disease. A mantoux test and a booster were performed, which were both negative. Infliximab was then administered according to the usual scheduled programme (at weeks 0, 2, and 6). We measured the CD4 count and viral HIV load before the first infusion of infliximab, 48 hours after each consecutive administration, and two months after the third infusion. No significant modification of the CD4 count was detected (see fig 1). Viral load never exceeded 200 copies. The patient experienced complete clinical and endoscopic remission, with closure of the fistulae.
The use of infliximab in subjects with HIV infection and CD seems to be safe and effective. The patient is now receiving maintenance therapy with infliximab. Regular CD4 counts will confirm the reliability of this therapeutic approach. However, if the CD4 count drops below 250 cell/ml, the programme would need to be re‐evaluated because of the risk of opportunistic infections.
Future experience will help to clarify the long term immunological effects of anti‐TNF‐α therapy in such circumstances. However, the benefits obtained with infliximab in a case of severe CD in the context of a well controlled HIV infection are highly relevant, particularly as they were not accompanied by deterioration in HIV infection
Footnotes
Conflict of interest: None declared.
References
- 1.Palella F J, Jr, Delaney K M, Folks T M.et al Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med 1998338853–860. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein B B, Gelb A, Tabanda‐Lichauco R. Crohn's ileitis in a patient with longstanding HIV infection. Am J Gastroenterol 199489937–939. [PubMed] [Google Scholar]
- 3.Moreno S D, Solis H J A, Medina A J.et al Subclinical Crohn's disease in acquired immunodeficiency syndrome. Rev Esp Enferm Dig 199384395–398. [PubMed] [Google Scholar]
- 4.van Dullemen H M, van Deventer S H, Hommes D W.et al Treatment of Crohn's disease with anti‐tumour necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995109129–135. [DOI] [PubMed] [Google Scholar]
- 5.Whalen C, Horsburgh C R, Hom D.et al Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 1995151125–135. [DOI] [PubMed] [Google Scholar]
- 6.Walker R E, Spooner K M, Kelly G.et al Inhibition of immunoreactive tumor necrosis factor‐alpha by a chimeric antibody in patients infected with human immunodeficiency virus type 1. J Infect Dis 199617463–68. [DOI] [PubMed] [Google Scholar]
- 7.Sha B E, Valdez H, Gelman R S.et al Effect of Etanecept (Enbrel) on interleukine‐6, tumor necrosis factor‐alpha and marker of immune activation in HIV‐infected subjects receiving interleukin 2. AIDS Res Hum Retroviruses 200218661–665. [DOI] [PubMed] [Google Scholar]
- 8.Wallis R S, Kyambadde P, Johnson J L.et al A study of the safety, immunology, virology and microbiology of adjunctive etanercept in HIV‐1‐associated tuberculosis. AIDS 200418257–264. [DOI] [PubMed] [Google Scholar]