Abstract
Background
An uncontrolled pilot study demonstrated that daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), might be effective for the treatment of active ulcerative colitis.
Methods
A randomised, double blind, placebo controlled trial was conducted to evaluate the efficacy of daclizumab induction therapy in patients with active ulcerative colitis. A total of 159 patients with moderate ulcerative colitis were randomised to receive induction therapy with daclizumab 1 mg/kg intravenously at weeks 0 and 4, or 2 mg/kg intravenously at weeks 0, 2, 4, and 6, or placebo. The primary end point was induction of remission at week 8. Remission was defined as a Mayo score of 0 on both endoscopy and rectal bleeding components and a score of 0 or 1 on stool frequency and physician's global assessment components. Response was defined as a decrease from baseline in the Mayo score of at least 3 points.
Results
Two per cent of patients receiving daclizumab 1 mg/kg (p = 0.11 v placebo) and 7% of patients receiving 2 mg/kg (p = 0.73) were in remission at week 8, compared with 10% of those who received placebo. Response occurred at week 8 in 25% of patients receiving daclizumab 1 mg/kg (p = 0.04) and in 33% of patients receiving 2 mg/kg (p = 0.30) versus 44% of those receiving placebo. Daclizumab was well tolerated. The most frequently reported adverse events in daclizumab treated patients compared with placebo treated patients were nasopharyngitis (14.6%) and pyrexia (10.7%).
Conclusion
Patients with moderate ulcerative colitis who are treated with daclizumab are not more likely to be in remission or response at eight weeks than patients treated with placebo.
Keywords: ulcerative colitis, medical treatment, controlled trials, CD‐25, daclizumab
The majority of patients with ulcerative colitis (UC) can be successfully treated with aminosalicylates for mild to moderate disease and with short courses of steroids followed by immunosuppressive maintenance therapy for those failing mesalamine. However, a subgroup of patients who do not respond to standard medical treatment experience persistent and debilitating symptoms.
Current evidence suggests that humoral immunity with antibody secreting plasma cells and T helper cells leads to uncontrolled tissue damage in the mucosa and submucosa of patients with UC.1 The T cell derived cytokine interleukin 2 (IL‐2) induces lymphocyte proliferation and differentiation in immune disorders. A role for this cytokine in the pathogenesis of UC is inferred from studies, which demonstrate that the calcineurin inhibitor ciclosporin is effective for the treatment of severely active UC.2,3,4 Ciclosporin is widely used as an immunosuppressive agent in solid organ transplantation and immune mediated disorders to inhibit the production of IL‐2. IL‐2 interacts with specific receptors on lymphocytes. Activated T cells express the high affinity α chain of the IL‐2 receptor (CD25) responsible for intracellular IL‐2 signalling.
Two drugs, daclizumab (Zenapax), a humanised IgG1 monoclonal antibody, and basiliximab (Simulect), a chimeric IgG1 monoclonal antibody, both directed against the α chain of the IL‐2 receptor (CD25), have recently been developed.5 These agents are currently indicated for the prevention of renal allograft rejection in patients receiving concomitant immunosuppression with ciclosporin and steroids, with or without azathioprine or mycophenolate mofetil.6,7,8 The CD25 specific hypervariable or Fab variable regions have been grafted onto the constant regions of human IgG1 antibodies.9 The use of these antibodies in organ transplantation has been characterised by a favourable safety profile.
A small uncontrolled pilot study suggested that daclizumab 1 mg intravenously at weeks 0 and 4 might be effective for the treatment of active UC.10 Similarly, a small uncontrolled study suggested that basiliximab might be effective in patients with active steroid refractory UC.11 We conducted a 20 week randomised, double blinded, placebo controlled, multicentre trial in which patients with moderate UC received induction therapy with daclizumab 1 mg/kg intravenously at weeks 0 and 4, or 2 mg/kg intravenously at weeks 0, 2, 4, and 6, or placebo.
Methods
Patients
This randomised, double blinded, placebo controlled, multicentre trial was conducted in Belgium, Canada, and the USA at 40 sites between April 2003 and May 2004. The institutional review board or ethics committee at each site approved the protocol. All patients gave written informed consent.
Criteria for eligibility included patients 12 years of age or older with UC of at least four months' duration which was moderately active, as defined by a Mayo Clinic score12 of 5–10 (inclusive). Men and women of childbearing potential consented to the use of double barrier contraception for the duration of the study. Concurrent therapies for UC, including stable doses of 5‐aminosalicylates, methylprednisolone 32 mg/day or less (or equivalent dose of other corticosteroid), azathioprine, and 6‐mercaptopurine were permitted. Concomitant therapy with methotrexate, ciclosporin, tacrolimus, antibiotics, or rectally administered corticosteroids or 5‐aminosalicyate containing medications was not permitted. Patients were eligible if they had a haemoglobin level ⩾8.5 g/dl, white blood cell count ⩾3.0×109/l, lymphocytes ⩾0.5×109/l, platelets ⩾100×109/l, neutrophils ⩾1.5×109/l, aspartate aminotransferase and alanine aminotransferase within three times the upper limit of normal, and creatinine ⩽1.6 mg/dl. Patients were excluded if they had Crohn's disease or indeterminate colitis, a positive pregnancy test or breast feeding in female patients, imminent need for surgery for UC or toxic megacolon, positive stool culture for enteric pathogens (including C difficile), prior treatment with daclizumab, known human immunodeficiency virus, hepatitis B or C infection, prior malignancy within five years or current malignancy (excluding adequately treated non‐melanoma skin carcinoma or in situ carcinoma of the cervix), use of any investigational drug within 30 days prior to screening, use of a monoclonal antibody within 12 weeks of screening (including infliximab), infection with varicella, herpes zoster, or severe viral infection within six weeks of screening, exposure to varicella within 21 days of screening, or vaccination with live virus within four weeks prior to screening.
Study design
Eligible patients were randomly assigned in a 1:1:1 ratio to receive either an intravenous infusion of daclizumab (Protein Design Labs, Inc., Fremont, California, USA) at a dose of: 1 mg/kg at weeks 0 and 4 alternating with placebo at weeks 2 and 6 (the dosing regimen used in the uncontrolled pilot study in patients with UC); or 2 mg/kg at weeks 0, 2, 4, and 6, or placebo at weeks 0, 2, 4 and 6, and followed through to week 20. Randomisation was performed at each site by an unblinded study pharmacist and treatment allocation was stratified according to: (1) investigational site; and (2) baseline use of oral corticosteroid therapy. Both patients and investigative staff (except for the study pharmacist at each site) were blinded to treatment assignment. The dose of all concurrent medications remained constant through to week 8. After week 8, the daily dose for patients receiving oral corticosteroids could be tapered at the investigator's discretion.
Patient schedule and efficacy and safety evaluations
Patients were assessed at weeks 0, 2, 4, 6, 8, 12, 16, and 20. The Mayo score12 was determined at weeks 0 and 8. The components of the scoring system are: stool frequency, rectal bleeding, endoscopic findings, and the physician's global assessment. Each subscore is rated on a scale from 0 to 3, indicating normal to severe activity. Remission was defined as a score of 0 on the rectal bleeding and endoscopy subscores and a score of 0 or 1 on the stool frequency and physician's global assessment subscores. Response was defined as a decrease from baseline in the total Mayo score by at least 3 points. Clinical response was defined as a decrease from baseline in the combined rectal bleeding, stool frequency, and physician's global assessment subscores by at least 2 points. Endoscopic response was defined as a decrease from baseline in the endoscopic subscore by at least one point. All randomised patients were required to have mucosal biopsies for histological assessment during endoscopy performed at baseline and at week 8. Histological assessment of inflammation was performed with the validated grading scale of Geboes, an instrument with six domains (structural (architectural change), chronic inflammatory infiltrate, lamina propria neutrophils and eosinophils, neutrophils in epithelium, crypt destruction, and erosions or ulceration). Scores can range from 0 to 5.4, with higher scores indicating more severe histological inflammation.13
Data for all 159 randomised patients were included in the safety analysis. At each visit, adverse events and concomitant medications were recorded and, at some visits, samples were collected for laboratory evaluations. Safety evaluations included vital signs, physical examination, haematology, serum biochemistry, and urinalysis. Samples for determination of antibodies to daclizumab were collected at weeks 0, 4, 8, 12, 16, and 20.
Immunohistochemical analysis of lamina propria T cells
At 15 selected clinical sites, additional mucosal biopsies were obtained to determine lamina propria lymphocyte CD25 expression and saturation with daclizumab, T cell activation (Ki67 staining), and T cell apoptosis (caspase 3 staining). Immunohistochemisty was performed on 5 µm thick cryostat sections, using a three step indirect immunoperoxidase technique. Expression of CD25 in mucosal biopsies was investigated using antibody 2A3 (347640; Becton Dickinson, San Jose, California, USA) (anti‐CD25 daclizumab competing antibody) and 143‐13 (Neomarker Lab, Fremont, California, USA; Vision MS‐785‐P) (anti‐CD25 daclizumab non‐competing antibody) in a dilution of 1/.5 and 1/10, respectively.
Expression of Ki67 in mucosal biopsies was assessed using the primary antibody MiB1 (Ki67, IgG1, kappa; Dako, Glostrup, Denmark) in a dilution 1/100. Expression of T cells was assessed using the CD5 (leu1) antibody (Becton Dickinson) in a dilution 1/2.5. Expression of caspase 3 in mucosal biopsies was assessed using a polyclonal antibody against caspase which detects the large fragment of activated caspase‐3 (17–20 kDa) (ASP175; Cell Signaling Technology, Beverly, Massachusetts, USA) in a dilution 1/20. Detection was performed using swine antirabbit/biotin (1/500; Dako) followed by a wash in phosphate buffered saline for five minutes and incubation for 30 minutes with the avidin‐biotin complex/horseradish peroxidase (Dako). Subsequently, sections were washed for five minutes in phosphate buffered saline and treated for 10 minutes with DAB. These slides were faintly counterstained with Harris haematoxylin, rinsed with distilled water, and coverslipped with glycerol. Controls that were negative consisted of omission of the primary or secondary antibody and the use of the chromogen alone. In addition, staining with an irrelevant, isotype matched, antibody directed against cytokeratin 10 was performed. Positive controls for CD5 consisted of lymph nodes; for Ki67, the positive controls were the proliferative compartment of intestinal crypts. For CD25, skin biopsies from patients with psoriasis were used, and for caspase 3, samples from colorectal cancers were used. The number of positive cells was counted in 10 fields of 144 µm2 using a calibrated eyepiece. The percentage of T cells staining for CD25 was counted by dividing the number of CD25 positive cells by the number of CD5 positive cells. To confirm that CD25 staining actually stained T cells, double stainings for CD25 and CD5 were performed in two cases.
Evaluation of CD25 expression on peripheral lymphocytes and peripheral T cell counts
At 15 selected clinical sites, anticoagulated peripheral blood samples were collected at screening and at weeks 0, 4, 8, 12, 16, and 20 for determination of the relative proportions of lymphocytes, T cells (CD3), T cell subsets (CD4 and CD8), and CD25 expression on all lymphocytes, T cells, CD4, and CD8 T cells using fluorescence activated cell sorter (FACS) analysis (FACSCalibur; Becton Dickinson). At the same time points, the percentage of CD25+ cells and mean fluorescence intensity were determined with two murine IgG1 antibodies specific for CD25‐anti‐CD25 antibody 2A3 that competes with daclizumab for binding to CD25, and non‐competitive anti‐CD25 antibody MA251. The percentage of CD25 (2A3)+ cells indicates the percentage of CD25+ cells that have no DAC bound to CD25, and the percentage of CD25 (MA251)+ cells indicates the percentage of cells expressing CD25 on the cell surface. We considered CD4 and CD8 cells saturated with DAC when the level of CD4+/CD25 (2A3)+ was at or below 1%.
Absolute CD3, CD4, and CD8 T cell counts were calculated at screening and at weeks 0, 4, 8, and 20 based on lymphocyte counts measured on a haematology analyser, and the percentage of CD3+, CD3+CD4+, and CD3+CD8+ T cells determined by FACS.
Statistical analysis
The primary efficacy end point was remission at week 8. The major secondary end points were response at week 8, clinical response at week 8, endoscopic response at week 8, and Mayo scores and total histopathology disease severity scores at weeks 0 and 8. All randomised patients who had valid baseline assessments and initiated infusions of study drug were analysed, regardless of the number of administrations of study drug that they completed (“intention to treat” approach). Patients who prematurely withdrew from the study for efficacy reasons were included in the analyses using “last observation carried forward” methods. Early terminations due to safety or issues that were not treatment related, such as relocation, were included in the analyses up to the last valid visit prior to withdrawal.
To compare the proportion of patients achieving a specified end point (that is, remission, response, clinical response, endoscopic response) between treatment groups, a two sided Fisher's exact test was used. Continuous response parameters were compared using a t test with unequal variance.
We estimated that 50 patients were needed in each of the daclizumab groups and 50 patients in the placebo group in order to have 80% power to detect a true difference in the proportion of patients with moderate UC who achieved remission at week 8, assuming the proportion in either of the two active groups was 35% and that placebo was 10%. We planned to recruit a total of 150 patients.
Results
Characteristics of patients
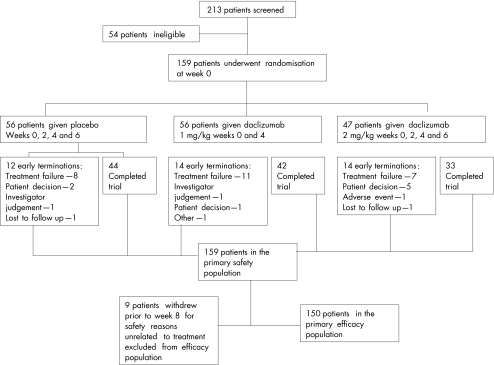
A summary of patient disposition is provided in fig 1. Fifty six patients with moderate UC were randomised to receive intravenous therapy with placebo at weeks 0, 2, 4, and 6; 56 were randomised to receive daclizumab 1 mg/kg intravenously at weeks 0 and 4; and 47 were randomised to receive daclizumab 2 mg/kg intravenous at weeks 0, 2, 4, and 6. There were 150 patients included in the primary efficacy analysis. Of the nine patients not included in the efficacy analysis, eight terminated early due to safety or issues that were not treatment related, and one patient had an incomplete Mayo score at week 8.
Figure 1 Overview of patient enrolment, treatment assignments, and subjects available for final evaluation.
The 150 patients had either completed treatment or had withdrawn prior to week 8 due to inefficacy. The safety population comprised all 159 randomised patients who received at least one dose of study medication. Baseline characteristics were similar in the three treatment groups (table 1).
Table 1 Baseline characteristics of all randomised patients.
| Characteristic | Placebo | Daclizumab 1 mg/kg | Daclizumab 2 mg/kg | p Value |
|---|---|---|---|---|
| No of patients | 56 | 56 | 47 | |
| Male sex (n (%)) | 33 (58.9) | 29 (51.8) | 25 (53.2) | 0.778 |
| White race (n (%)) | 47 (83.9) | 45 (80.4) | 38 (80.9) | 0.902 |
| Age (y) (mean (SD)) | 40.7 (13.23) | 47.4 (14.09) | 42.6 (15.37) | 0.041 |
| Body weight (kg) (mean (SD)) | 80.5 (19.24) | 76.6 (19.45) | 79.8 (19.29) | 0.536 |
| Disease duration (y) (mean (SD)) | 6.8 (7.35) | 7.8 (6.62) | 8.3 (8.06) | 0.563 |
| Involved colonic area (n (%)) | ||||
| Left sided | 46 (82.1) | 46 (82.1) | 33 (70.2) | 0.411 |
| Extensive | 10 (17.9) | 10 (17.9) | 13 (27.7) | |
| Mayo clinic score (mean (SD)) | 8.0 (1.66) | 7.8 (1.61) | 8.0 (1.74) | 0.735 |
| Concomitant medication (n (%)) | ||||
| Corticosteroids | 19 (33.9) | 21 (37.5) | 17 (36.3) | 0.936 |
| Azathioprine/6‐MP | 12 (21.4) | 12 (21.4) | 9 (19.1) | 0.966 |
| Current smoker (n (%)) | 6 (10.7) | 3 (5.4) | 4 (8.5) | 0.589 |
6‐MP, 6‐mercaptopurine.
Efficacy
Overall, 12 patients (21%) in the placebo group withdrew from the study prematurely compared with 14 patients (25%) and 14 patients (30%) in the daclizumab 1 mg/kg and 2 mg/kg groups, respectively, primarily due to treatment failure.
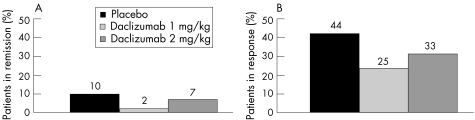
At week 8, 2% of patients in the daclizumab 1 mg/kg group (1/54; p = 0.11 v placebo) and 7% of patients in the daclizumab 2 mg/kg group (3/43; p = 0.73 v placebo) were in remission, compared with 10% of patients in the placebo group (5/53) (fig 2A). Similarly, 25% of patients in the daclizumab 1 mg/kg group (13/53; p = 0.04 v placebo) and 33% of patients in the daclizumab 2 mg/kg group (14/43; p = 0.29 v placebo) were in response at week 8, compared with 44% of patients in the placebo group (23/52) (fig 2B). Also, for clinical and endoscopic Mayo score response and for mean clinical at week 8, no differences between the three groups were observed (table 2)
Figure 2 Proportions of patients with remission (A) and response (B) at week 8.
Table 2 Secondary efficacy end points.
| End point | Placebo | Daclizumab 1 mg/kg | p Value | Daclizumab 2 mg/kg | p Value |
|---|---|---|---|---|---|
| Clinical response at week 8 (n (%)) | 26/53 (49.1) | 18/54 (33.3) | 0.12 | 16/43 (37.2) | 0.30 |
| Endoscopic response at week 8 (n (%)) | 27/53 (50.9) | 15/53 (28.3) | 0.03 | 14/42 (33.3) | 0.10 |
| Mayo clinic score | |||||
| Baseline score (n, mean (SEM)) | 56, 8.0 (0.2) | 56, 7.8 (0.2) | 0.097 | 47, 8.0 (0.3) | 0.849 |
| Week 8 score (n, mean (SEM)) | 48, 5.8 (0.5) | 43, 6.4 (0.5) | 35, 5.6 (0.5) | ||
| Change from baseline (n, mean (SEM)) | 48, −2.3 (0.4) | 43, −1.3 (0.4) | 35, −2.1 (0.4) | ||
| Total histopathology disease severity score | |||||
| Baseline score (n, mean (SEM)) | 55, 3.8(0.2) | 52, 4.3 (0.2) | 45, 4.1 (0.3) | ||
| Week 8 score (n, mean (SEM)) | 42, 3.3 (0.3) | 39, 3.6 (0.4) | 0.962 | 33, 3.0 (0.4) | 0.619 |
| Change from baseline (n, mean (SEM)) | 42, −0.6 (0.2) | 37, −0.6 (0.3) | 33, −0.9 (0.4) |
At baseline, 57 of 159 patients (36%) were receiving corticosteroids. An exploratory analysis in this patient subgroup demonstrated that 0% of patients in the daclizumab 1 mg/kg group (0/21; p = 0.45 . placebo) and 6% of patients in the daclizumab 2 mg/kg group (1/16; p = 1.00 v placebo) were in remission, compared with 6% of patients in the placebo group (1/17).
At baseline, most patients had histological signs of active disease: placebo, 92% (n = 12); daclizumab 1 mg/kg, 100% (n = 12); daclizumab 2 mg/kg, 92% (n = 13). Histological severity scores at week 8 were similar in all three treatment groups. Also, the majority of patients had moderate to severe histological disease regardless of treatment assignment (placebo, 75%; daclizumab 1 mg/kg, 75%; daclizumab 2 mg/kg, 79%).
Safety
The incidence of adverse events was similar in the placebo (75%) and two daclizumab groups (76.6% in the 2 mg/kg group and 83.9% in the 1 mg/kg group). A similar number of patients discontinued treatment because of an adverse event in the placebo group (0%) as in the 1 mg/kg and 2 mg/kg daclizumab groups (0% and 2%, respectively). The most frequently reported adverse events in the three groups were similar (table 3). One patient receiving 2 mg/kg daclizumab experienced a moderate infusion reaction after the first dose of study drug. The patient continued in the trial and had no further events. Serious adverse events occurred in 3.6% of patients in the placebo group, and in 12.5% and 4.3% in the 1 mg/kg and 2 mg/kg daclizumab treatment groups, respectively (table 3). Most of the serious adverse events involved exacerbation of underlying UC. No deaths occurred during the study and no patient developed solid tumour or haematological malignancies. There were no clinically significant changes in laboratory values in either treatment group.
Table 3 Summary of safety analyses for all randomised patients up to week 20.
| Preferred term | Daclizumab 2 mg/kg (n = 47) | Daclizumab 1 mg/kg (n = 56) | Placebo (n = 56) |
|---|---|---|---|
| Nasopharyngitis | 6 (12.8%) | 9 (16.1%) | 5 (8.9%) |
| Pyrexia | 6 (12.8%) | 5 (8.9%) | 0 |
| Abdominal pain | 6 (12.8%) | 3 (5.4%) | 5 (8.9%) |
| Sinusitis | 6 (12.8%) | 1 (1.8%) | 2 (3.6%) |
| Headache | 5 (10.6%) | 10 (17.9%) | 10 (17.9%) |
| Nausea | 5 (10.6%) | 7 (12.5%) | 6 (10.7%) |
| Influenza | 5 (10.6%) | 2 (3.6%) | 5 (8.9%) |
| Colitis ulcerative | 4 (8.5%) | 9 (16.1%) | 3 (5.4%) |
| Pruitus | 1 (2.1%) | 7 (12.5%) | 1 (1.8%) |
Adverse events reported in ⩾10% daclizumab treated subjects. Intention to treat subjects (n = 159).
The incidence of infections was similar in the placebo and in the two daclizumab groups. Pneumonia occurred in two patients in the daclizumab 1 mg/kg group. None of the patients developed opportunistic infections.
Pharmacokinetics
Peripheral T cells counts and CD25 expression on peripheral T cells
Administration of daclizumab at either 1 or 2 mg/kg resulted in an immediate binding of DAC to CD25 on peripheral lymphocytes that was demonstrated by absent staining with 2A3 antibody at two hours after the first dose. In the high dose group, the percentage of CD25 (2A3)+ T cells remained at or below 1% throughout day 84, indicating almost three months of CD25 saturation on peripheral T cells, and in some patients CD25 saturation lasted throughout day 140. In the low dose group, all patients (except for one patient) demonstrated CD25 saturation throughout day 56, in some patients CD25 desaturation occurred on day 84, and by day 112 the percentage of CD25 (2A3)+ T cells returned to pretreatment values, indicating complete receptor desaturation. Staining with the non‐competitive anti‐CD25 antibody MA251 confirmed the presence of CD25 on circulating T cells. In both dosing regimens, the percentage of CD25 (MA251)+ T cells and expression of CD25 were reduced between days 28 and 84 compared with pretreatment values. The maximum reduction was observed between days 28 and 84 and was reversible by day 140.
Immunohistochemical analysis of lamina propria T cells
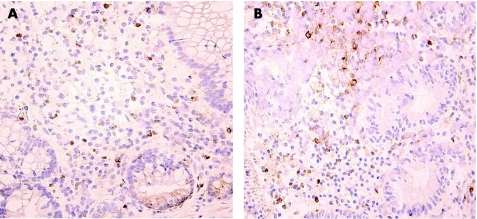
Immunohistochemical analysis showed, on average, a low number of CD5+ T cells in the mucosa of patients and a low percentage of CD25+ T cells (29–39%) prior to treatment. The numbers of both 143‐13 positive cells and 2A‐3 positive T cells were not significantly changed at day 56 in comparison with pretreatment values in any of the treatment groups, revealing CD25 expression on T cells and lack of CD25 saturation with daclizumab (fig 3). In only four patients, no CD25 staining was detected with 2A3 anti‐CD25 antibody on day 56 although T cells were stained with 143‐13 antibody. These data indicate that in these subjects CD25 was expressed on T cells and saturated with daclizumab. Average counts of Ki67+ and caspase‐3+ T cells demonstrate no effect of treatment when pre‐ and post‐treatment samples were compared.
Figure 3 CD25 expression in colonic biopsies before and after daclizumab treatment. Immunohistochemistry using a non‐competing antibody (143‐13) and showing (brown colour) CD25 positive lymphocytes before (A) and after (B) treatment with high dose daclizumab. CD25 staining remained unchanged using both a competing (2A‐3) and non‐competing anti‐ CD25 antibody (143‐13). Note that at baseline only a minority of mononuclear cells were CD25+. Pictures are representative of all patients evaluated.
Discussion
The results of this randomised placebo controlled trial indicate that daclizumab is generally well tolerated in a patient population with active UC. However, despite the favourable open label experience with both the humanised anti‐CD25 antibody daclizumab and the chimeric antibody basiliximab,10,11 the results of this placebo controlled trial show no evidence of a clinical benefit of daclizumab at two different dose levels to treat active moderate UC. The remission rate was low in the placebo group (10%), indicating that lack of efficacy was not due to high placebo remission rates. The open label trial with basiliximab indicated that anti‐CD25 antibodies specifically restored T cell steroid sensitivity, and suggested that UC patients failing steroids benefit from treatment with this monoclonal antibody.11 In contrast, in the present trial, both the high and low dose of daclizumab failed to demonstrate an increased remission or clinical response rate in the subgroup of patients with active disease while receiving adequately dosed corticosteroids at baseline (steroid refractory).
Several factors may account for the discrepancy between the favourable open label experience with CD25 inhibiting agents and the absence of efficacy in this placebo controlled trial. Firstly, open label trials are prone to placebo effects, specifically in patients with relapsing disorders such as UC. Secondly, although both basiliximab and daclizumab target the same receptor and have some overlapping properties, differences in receptor binding affinity and in mechanistic properties have been noted in vitro. Daclizumab appears not only to have less cytolytic activity than basiliximab, presumably due to its lower affinity, but daclizumab also may have unique synergistic activity when combined with calcineurin inhibitors.7,14 Finally, our data indicate that daclizumab most likely did not saturate CD25 on T cells in the inflamed colon even if we used a high dose regimen with higher dose and dosing frequency than what has been used in other indications. Saturation of the CD25 IL‐2 receptors on peripheral T cells occurred with both daclizumab regimens used in this trial throughout the dosing period, but immunohistochemistry of the colonic biopsies showed unchanged CD25 T cell expression after treatment in most patients. This was true for both the daclizumab competing and non‐competing antibodies, indicating that there is a possibility that daclizumab did not penetrate into the lamina propria of the inflamed colon. Finally, inhibition of the IL‐2 receptor may not be sufficient to prevent lymphocyte activation and proliferation in active UC. Most of the clinical experience with daclizumab has concentrated on the prevention of organ rejection, and other molecular pathways may drive lymphocyte activation in the actively inflamed colon. However, a recent placebo controlled trial in asthma showed that daclizumab significantly improved pulmonary function and prolonged time to severe asthma exacerbation.15
IL‐2 and IL‐2 receptor knockout mice develop spontaneous colitis.16 Absence of CD4+CD25+ regulatory T cells probably contributes to the colitis in these animals. In our trial, exacerbation of colitis was not specifically observed in daclizumab treated patients. Although there was a trend towards a higher incidence of worsening UC in the low dose group, it is unlikely that this trend is caused by CD25 inhibition, as it was not observed in the high dose group with persistent and total CD25 saturation. Also, local CD25 expression on lymphocytes in the colonic wall did not change with treatment. Overall, daclizumab was well tolerated in this population and there was no indication of increased incidence of adverse events. More specifically, infectious complications were not more frequently observed in the actively treated group. In summary, in the present placebo controlled dose ranging trial to treat active UC, daclizumab 1 mg/kg intravenously for four weeks or 2 mg/kg intravenously for two weeks was well tolerated but failed to show clinical efficacy.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Acknowledgements
The authors sincerely thank Dr Karel Geboes, Division of Pathology, University of Leuven, for analysis of biopsy specimens and for performing immunohistochemistry.
This study was designed by Protein Design Labs and three of the authors of this manuscript (GVA, WJS, BGF). Protein Design Labs and selected investigators, including those who designed the study, analysed and interpreted the data, wrote this manuscript, and agreed to submit this manuscript for publication. The principal investigator (GVA) approved the content of the manuscript prior to submission.
Abbreviations
IL‐2 - interleukin 2
UC - ulcerative colitis
FACS - fluorescence activated cell sorter
Footnotes
Financial support was received from Protein Design Labs, Fremont, California, USA.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastrorenterology 1998115182–205. [DOI] [PubMed] [Google Scholar]
- 2.Lichtiger S, Present D H, Kornbluth A.et al Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Eng J Med 19943301841–1845. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn W J. A critical review of cyclosporin therapy in inflammatory bowel disease. Inflamm Bowel Dis 1995148–63. [Google Scholar]
- 4.D'Haens G, Lemmens L, Geboes K.et al Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 20011201323–1329. [DOI] [PubMed] [Google Scholar]
- 5.Willerford D M, Chen J, Ferry J A.et al Interleukin‐2 receptor alpha chain regulates the size and content of the peripheral lymphoid component. Immunity 19953521–530. [DOI] [PubMed] [Google Scholar]
- 6.Beniaminovitz A, Itescu S, Lietz K.et al Prevention of rejection in cardiac transplantation by blockade of the interleukin‐2 receptor with a monoclonal antibody. N Engl J Med 2000342613–619. [DOI] [PubMed] [Google Scholar]
- 7.Maes B, Vanrenterghem Y. Anti‐interleukin‐2 receptor monoclonal antibodies I renal transplantation. Nephrol Dial Transplant 1999142824–2826. [DOI] [PubMed] [Google Scholar]
- 8.Vicenti F, Kirkman R, Light S.et al Interleukin‐2‐receptor blockade daclizumab to prevent acute rejection in renal transplantation. N Engl J Med 1998338161–165. [DOI] [PubMed] [Google Scholar]
- 9.McClellan M, Keller S, Zhao V.et al Daclizumab inhibits mitogen‐stimulated Th1 and Th2 cytokine production from human PBMC. J Allergy Clin Immunol 2002109S24 [Google Scholar]
- 10.Van Assche G, Dalle I, Noman M.et al A pilot study on the use of the humanized anti interleukin‐2 receptor antibody daclizumab in active ulcerative colitis. Am J Gastroenterol 200398369–376. [DOI] [PubMed] [Google Scholar]
- 11.Creed T J, Norman M R, Probert C S.et al Basiliximab (anti‐CD25) in combination with steroids may be an effective new treatment for steroid‐resistant ulcerative colitis. Aliment Pharmacol Ther 20031865–75. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder K W, Tremaine W J, Ilstrup D M. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 19873171625–1629. [DOI] [PubMed] [Google Scholar]
- 13.Geboes K, Ridell R, Ost A. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 200047404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger J G, Walters I B, Miyazawa M.et al Successful in vivo blockade of CD25 (high‐affinity interleukin 2 receptor) on T cells by administration of humanized anti‐Tac antibody to patients with psoriasis. J Am Acad Dermatol 200043448–458. [DOI] [PubMed] [Google Scholar]
- 15.Busse W W, Baker J W, Charous B L.et al Preliminary safety and efficacy of patients with moderate to severe chronic persistent asthma. JACI 2004113S286–S287. [Google Scholar]
- 16.Poussier P, Ning T, Chen J.et al Intestinal inflammation in IL‐2R/IL‐2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology 2000118880–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.