Abstract
Background
Increased infertility in women has been reported after ileal pouch‐anal anastomosis (IPAA) for ulcerative colitis but reported infertility rates vary substantially.
Aims
(1) To perform a systematic review and meta‐analysis of the relative risk of infertility post‐IPAA compared with medical management; (2) to estimate the rate of infertility post‐IPAA; and (3) to identify modifiable risk factors which contribute to infertility.
Methods
Medline, EMBASE, Current Contents, meeting abstracts, and bibliographies were searched independently by two investigators. The titles and abstracts of 189 potentially relevant studies were reviewed; eight met the criteria and all data were extracted independently. Consensus was achieved on each data point, and fixed effects meta‐analyses, a funnel plot, and sensitivity analyses were performed.
Results
The initial meta‐analysis of eight studies had significant heterogeneity (p = 0.004) due to one study with very high preoperative infertility (38%). When this study was omitted, the relative risk of infertility after IPAA was 3.17 (2.41–4.18), with non‐significant heterogeneity. The weighted average infertility rate in medically treated ulcerative colitis was 15% for all seven studies, and the weighted average infertility rate was 48% after IPAA (50% if all eight studies are included). We were unable to identify any procedural factors that consistently affected the risk of infertility.
Conclusions
IPAA increases the risk of infertility in women with ulcerative colitis by approximately threefold. Infertility, defined as achieving pregnancy in 12 months of attempting conception, increased from 15% to 48% in women post‐IPAA for ulcerative colitis. This provides a basis for counselling patients considering colectomy with IPAA. Further studies of modifiable risk factors are needed.
Keywords: ulcerative colitis, ileal pouch‐anal anastomosis, infertility, fertility
Ulcerative colitis (UC) that is resistant to steroid therapy leaves physicians and patients with limited options. Surgical proctocolectomy with J pouch is often recommended but both ciclosporin and infliximab have been successfully used as rescue therapies. The recent approval of infliximab for chronic maintenance use in moderate to severe ulcerative colitis by the US Food and Drug Administration has led patients and physicians to closely re‐examine the risks and benefits of colectomy in comparison with medical therapy.1,2,3,4 Total colectomy is reasonably safe, and is substantially less expensive than maintenance infliximab for UC. Colectomy eliminates future colitis flares and has been shown to improve patient quality of life.4 However, complications from colectomy and reconstruction can have significant effects on the long term physical and psychosocial functioning of patients.
One potential complication that makes colectomy less attractive for young female patients is the risk of infertility. Infertility has been reported frequently in women with UC after colectomy with ileal pouch‐anal anastomosis (IPAA).6,7,8,9,10,11,12,13,14 However, the reported estimates of both the relative risk of infertility after IPAA and the rate of infertility after IPAA vary widely. These inconsistent data make it difficult to provide patients with clear expectations of the infertility risk of this procedure.
Our purpose was to perform a systematic review and meta‐analysis of the published literature regarding infertility in women who have undergone IPAA for UC. Our specific study objectives were: (1) to estimate a summary relative risk of infertility after IPAA to better inform patients about the risk of infertility; (2) to identify a reasonable estimate of infertility rates after IPAA; and (3) to identify any consistent patient or procedure characteristics associated with increased infertility rates.
Materials and methods
Literature search
A computer assisted search with the OVID interface to Medline, EMBASE, and Current Contents was conducted to identify potentially relevant published papers. A search of these databases from 1966 to 31 December 2005 was performed using the exploded (exp) medical subject heading (MeSH) terms exp inflammatory bowel disease AND exp fertility AND exp proctocolectomy OR IPAA OR surgery. The results were limited to human studies. Manual searches of reference lists from potentially relevant papers were also performed to identify any additional studies that may have been missed using the computer assisted strategy.
Study selection criteria and data extraction
Two investigators (AW, JW) independently reviewed the titles and abstracts of all citations identified by the literature search. Potentially relevant studies were retrieved and selection criteria applied. Selection criteria were: (1) availability of data from both subjects medically treated for UC and subjects post‐IPAA for UC; (2) availability of raw data on infertility defined as lack of conception despite trying to conceive; (3) publication in full manuscript form; and (4) data not duplicated in another manuscript. Eligible articles were reviewed in a duplicate independent manner by two investigators (AW, JW). Agreement between investigators was greater than 95%, and disagreement in data extraction was resolved by consensus.
Meta‐analysis of relative risk (RR) and calculation of infertility rates
Meta‐analysis using the metan command in Stata 9.1 with a fixed effects model was performed. If heterogeneity was identified, studies having outlying values were excluded one at a time to determine if they contributed to the heterogeneity. Summary results are reported for all studies and for the homogeneous subset of studies. A meta‐analysis was also performed with a random‐effects model to determine whether it would dramatically change the outcome. The presence of publication bias was evaluated with a funnel plot (metafunnel command), and a sensitivity analysis was performed to determine whether undue influence of a single study was present (metainf command).
Two studies7,8 reported only aggregate data from subjects post‐IPAA, without reporting whether their preoperative diagnosis was UC or familial adenomatous polyposis (FAP). As the prevalence of UC is approximately 100/100 000 and the prevalence of FAP is approximately 2/100 000 (50‐fold less), we assumed that the data in these two studies were representative of patients with UC.15,16 To assess the validity of this assumption, a second sensitivity analysis was performed by excluding these studies. To obtain the weighted average infertility rates for the medically treated and post‐IPAA groups, the raw data from each of the studies were combined in a calculation of [infertility rate = total subjects reporting infertility/total subjects attempting conception] in Microsoft Excel.
Results
Characteristics of selected studies
Searches of the Medline, EMBASE, and Current Contents databases, and of the bibliographies of relevant manuscripts, yielded 189 potentially relevant articles. Reviews of the titles and abstracts of these publications identified 10 relevant articles published between 1990 and 2004. After review of the full manuscripts, one manuscript was excluded because subjects who underwent surgery had ileostomies rather than IPAA.17 A second manuscript was excluded14 because it appeared to duplicate data presented in a later publication,11 leaving eight studies for systematic review and meta‐analysis (table 1).
Table 1 Fertility data from included studies.
| Study | Population | Year | UC infertile | UC fertile | Medical infertility rate (%) | IPAA infertile | IPAA fertile | IPAA infertility rate (%) |
|---|---|---|---|---|---|---|---|---|
| Wikland6 | Swedish | 1989 | 8 | 19 | 29.6 | 8 | 6 | 57.2 |
| Oresland7 | Swedish | 1994 | 0 | 7 | 0.0 | 13 | 1 | 92.9 |
| Counihan8 | US | 1994 | 5 | 105 | 4.5 | 18 | 92 | 16.4 |
| Sjögren9 | Swedish | 1995 | 5 | 25 | 16.7 | 5 | 25 | 16.7 |
| Hudson10 | Scottish | 1997 | 15 | 78 | 16.1 | 6 | 5 | 54.5 |
| Olsen11 | Swedish | 2002 | 19 | 65 | 22.6 | 122 | 27 | 81.8 |
| Gorgun12 | US | 2004 | 45 | 72 | 38.4 | 70 | 50 | 58.3 |
| Johnson13 | Canada | 2004 | 8 | 52 | 13.3 | 59 | 94 | 38.6 |
| Overall | 105 | 423 | 19.9 | 301 | 300 | 50.1 | ||
| Overall (excluding Gorgun) | 60 | 351 | 14.6 | 231 | 250 | 48.0 |
UC, ulcerative colitis; IPAA, ileal pouch‐anal anastomosis.
Wikland and colleagues6 published a retrospective study in 1990, conducted in Sweden. The authors used an interview and gynaecological examination to identify gynaecological problems in patients after IPAA. Their population included 41 women who had a proctocolectomy for UC (n = 41). Age range was 20–60 years but response rate, duration of disease, and details of surgical technique were not provided. Infertility was either defined as failure of conception to occur while not using contraception (before surgery definition) or while attempting to become pregnant (after surgery definition). Thirty per cent (8/27) of subjects attempting conception reported infertility during medical treatment and 57% (8/14) of those attempting conception reported infertility after IPAA. This study was weakened by the distinct definitions of infertility in the pre‐ and postoperative groups, and reliance on distant recall for medically treated infertility rates.
Oresland and colleagues7 published a retrospective study in 1994, conducted in Sweden, using an interview, gynaecological examination, and hysterosalpingography in subjects who lived close to the hospital and had a pelvic pouch for at least 18 months. The surgical technique used endoanal mucosectomy, a hand sewn anastomosis, and either a two or three stage procedure. A letter of invitation was sent out to 60 pelvic pouch patients in the age range 23–38 years and mean time elapsed from their operation was 38 months (18–80). A response rate of 21/60 (35%) was obtained. The authors indicated that their patients had surgery for UC or FAP, but did not divide the data by preoperative diagnosis. The study criteria for infertility were not specifically defined, but in women who reported trying to get pregnant, 0% (0/7) reported infertility during medical treatment and 93% (13/14) reported infertility after IPAA. Hysterosalpingograms were performed in all 21 subjects; 52% were found to have fallopian tube occlusion and 48% had their fallopian tubes adhered to the pelvic floor. Only 33% had normal fallopian tubes. This study was limited by the lack of preoperative hysterosalpingograms for comparison, reliance on recall for preoperative data, and lack of detail on preoperative diagnosis.
Counihan and colleagues8 published a study in 1994 from the Lahey Clinic in Burlington, Massachusetts, USA, reviewing records and questionnaires collected between 1980 and 1991. The authors collected data from 203 patients with UC and FAP who underwent IPAA. The authors did not report data for subjects with UC separately from data for subjects with FAP. Mean age was 31 years (range 14–61) at the time of construction of their pouch, and subjects were followed for a mean duration of 41 months (range 1–132). Their response rate was 51%; the surgical technique was not defined. They defined infertility as the inability to conceive after one year of unprotected sexual intercourse. In the medically treated women having unprotected intercourse, the infertility rate was 5% (5/110), and in the women post‐IPAA having unprotected intercourse the infertility rate was 16% (18/110). This study was limited by the absence of separate data on subjects with UC and reliance on distant recall for medically treated infertility rates.
Sjögren and colleagues9 published a retrospective study in 1995 conducted in Sweden that compared infertility rates in medically treated patients before colectomy and in the same patients after colectomy with IPAA for UC. The women answered a detailed questionnaire and had follow up interviews. The sample of patients included 30 women who had colectomy for UC at a single centre. Each had a three stage operation with proctomucosectomy and an S pouch. Age range for patients was 19–54 years and median duration of inflammatory bowel disease was nine years. There was no definition of infertility provided. In this group of patients, 17% (5/30) reported unwanted infertility during medical treatment and 17% (5/30) reported unwanted infertility after IPAA. The authors concluded that fertility was largely unimpaired by anatomical and postoperative factors. This study was limited by the lack of a specific definition for infertility and the reliance on distant recall for medically treated infertility data.
Hudson and colleagues10 published a retrospective study in 1997 conducted in north eastern Scotland using an anonymous questionnaire on fertility in 466 women with inflammatory bowel disease. They had a response rate of 88%, and collected information on 232 patients with UC, aged 16–45 years, but no information was provided regarding duration of disease or colectomy technique. They defined infertility as lack of conception in couples who had two years of unprotected intercourse. The authors concluded that in those attempting conception, the rate of infertility in medically treated subjects was 16% (15/93) compared with 54% (6/11) in subjects post‐IPAA. This differed substantially from the infertility rate of 108/710 (15%) found in their non‐UC population controls.
Olsen and colleagues11 published a retrospective study in 2002 conducted in Sweden and Denmark using telephone interviews. The authors evaluated both fertility and fecundability in 290 women with UC before and after restorative proctocolectomy. Unlike the other studies reviewed, the authors' primary endpoint was fecundability, defined as the probability of becoming pregnant per month of unprotected intercourse. The authors also reported fertility (the cumulative incidence of pregnancy after 12 months) in a table, and these data were used for meta‐analysis as all other studies reported their results in terms of fertility. They evaluated subjects' fertility history during the time they were 15–39 years old. No information was provided regarding duration of their disease or surgical technique. The survey response rate was 85%, and pregnancies resulting from in vitro fertilisation were excluded. The authors found an infertility rate of 22% (19/84) in medically treated patients and 82% (122/149) in subjects after IPAA.
Of note, Olsen et al published a previous study14 that did use fertility as the primary end point in a similar sample of subjects. However, this study was excluded from the meta‐analysis because: (1) the subject sample appeared to overlap with that of the later paper in Gastroenterology, (2) there were differences in the methods of data collection in UC subjects and non‐UC controls, and (3) there was less rigorous data collection and data analysis in this study than in Olsen's later 2002 manuscript.11
Gorgun and colleagues12 published a retrospective study in 2004 conducted at the Cleveland Clinic, based on a questionnaire and chart review of patients after IPAA. Their patient sample included women who underwent pelvic pouch operations between 1983 and 2001 and were between the ages of 15 and 44 years at the time of operation. The questionnaire was mailed to 500 females with restorative proctocolectomy and IPAA. Three hundred subjects (60%) completed and returned the questionnaire. Infertility was defined as the inability to conceive after one year of unprotected sexual intercourse. The authors presented no detailed data on surgical technique but provided separate data on the subset of patients with UC. In those attempting conception, 38% (45/117) of the medically treated subjects were infertile and 58% (70/120) were infertile after IPAA. This was the only study to evaluate the effects of potentially modifiable risk factors.
Gorgun and colleagues12 evaluated a number of patient and procedure associated covariates in a logistic regression model, with infertility as the dependent outcome variable. They reported that intraoperative transfusions were significantly associated with infertility, while oophoropexy, anti‐adhesion barriers, age at operation, and intraoperative factors such as pouch type, adhesiolysis, and prior colectomy, did not have any association with subsequent infertility.
Johnson and colleagues13 published a retrospective study in 2004 that was conducted in Toronto. The authors mailed questionnaires to 237 women with UC who were medically treated and 323 women with UC who had an IPAA. Data on duration of disease but not surgical technique were provided. Their sample was restricted to females who were at least 18 years old at the time of study and were younger than 44 years of age at the time of IPAA or diagnosis of UC. The questionnaire response rates were 60.3% in the medically treated group and 78.6% in the post‐IPAA group. Infertility was defined as failure to become pregnant during 12 months of unprotected intercourse in patients aged 18–44 years who were married or cohabiting with a male partner. The authors found an infertility rate of 13% (8/60) in the medically treated subjects attempting conception and an infertility rate of 39% (59/153) in the post‐IPAA subjects attempting conception.
Meta‐analysis results
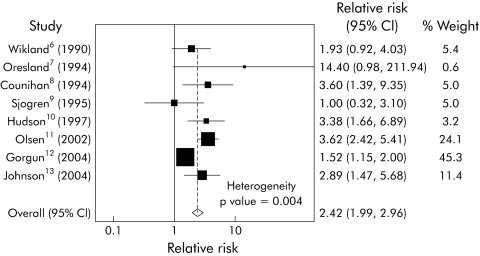
Our initial fixed effects meta‐analysis (fig 1) had a heterogeneity χ2 value of 21.18 (p = 0.004), making it inappropriate to combine these studies in a single estimate (fig 1). Inspection of the forest plot, in which only the Cleveland Clinic study does not overlap with the summary estimate, and inspection of the infertility rates in table 1 led us to consider whether this study was an important outlier. Gorgun and colleagues12 reported a substantially higher infertility rate (38%) in medically treated patients with UC than any of the other studies.
Figure 1 Significant heterogeneity was found in the studies fulfilling the selection criteria. A forest plot of the initial fixed effects meta‐analysis is shown, and the Gorgun study is the only study whose 95% confidence interval (95% CI) does not have any overlap with the summary estimate.
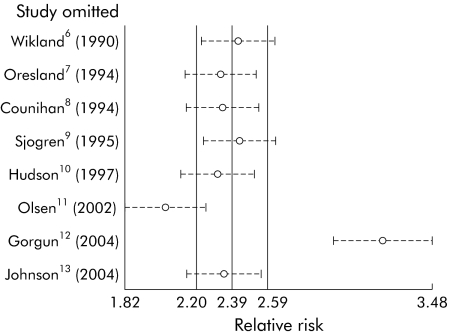
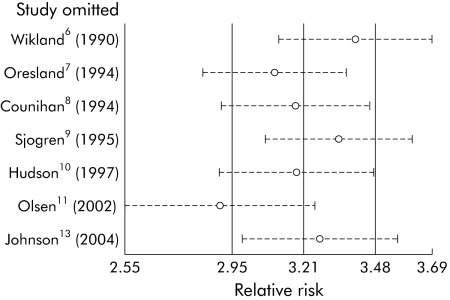
In order to test the effect of the Cleveland Clinic study, we performed a sensitivity analysis, removing one study at a time from our initial model, and found that omission of this study had a large effect on the overall estimate of RR of infertility associated with IPAA (fig 2). This was likely due to the high infertility rate in medically treated patients, suggesting that the medically treated population sample at the Cleveland Clinic was significantly different from the population of subjects in the other studies. Choosing to omit this study dramatically improved the homogeneity of the meta‐analysis.
Figure 2 Sensitivity analysis of the effects of individual studies. Because the Gorgun study was an apparent outlier, we performed a sensitivity analysis to determine its effect on the summary estimate. Each study was omitted one at a time, and the resulting summary estimates are displayed. The Gorgun study has a dramatic effect on the summary estimate.
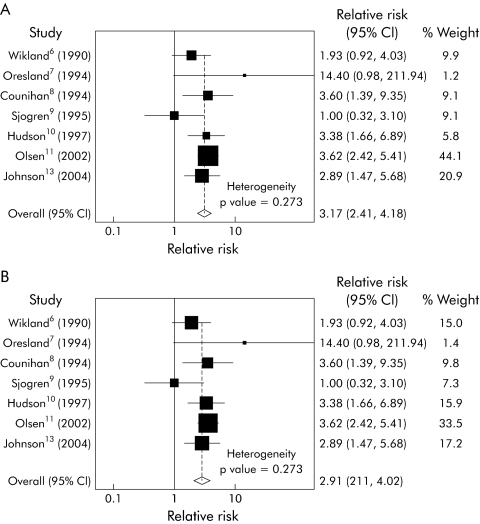
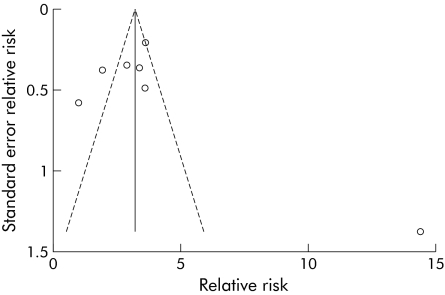
We repeated the fixed effects meta‐analysis after removing the Cleveland Clinic study (fig 3A) and found that the heterogeneity was significantly reduced (heterogeneity χ2 = 7.55, p = 0.273). The Mantel‐Haenszel pooled RR for infertility after IPAA was 3.17 (95% CI 2.41–4.18). We also performed a random effects meta‐analysis (fig 3B) to determine whether this would significantly change the pooled risk ratio. The random effects pooled RR was 2.91 (95% CI 2.11–4.02), which was not substantially different from the fixed effects result. We then performed a funnel plot (fig 4) to detect any significant publication bias or outliers. We found no evidence of asymmetry to suggest publication bias, but did find that the Oresland study was an obvious outlier.
Figure 3 Meta‐Analysis of remaining seven studies. (A) Fixed effects model, (B) random effects model. Because of the differences between the Gorgun study and the others, we considered whether exclusion of this study would allow us to create a valid summary estimate. (A) A fixed effects model of the remaining seven studies was used to generate a summary estimate, with substantial improvement in heterogeneity. (B) A random effects model of the seven studies was also used to determine the robustness of the fixed effect approach, and only a modest reduction in the effect was found, from 3.17 to 2.91.
Figure 4 Funnel plot. In order to detect publication bias, a funnel plot of the included studies was performed with pseudo 95% confidence limits. Overall symmetry is seen but the Oresland study is a noticeable outlier far to the right on the funnel plot.
We then repeated the sensitivity analysis of the effect of each study in the new model using the remaining seven studies. Omission of the Oresland study did not have a significant effect, as it is only given 1.2% weighting in the meta‐analysis due to its low precision. Omission of the Olsen study was the only omission that changed the estimate of the effect size (fig 5), and excluding this study only slightly reduced the effect to a Mantel‐Haenszel pooled RR of infertility of 2.82 (95% CI 1.94–4.12).
Figure 5 Only omission of the Olsen study changed the estimate of effect size. Because the Oresland study was an outlier, we repeated the sensitivity analysis with a fixed effects model. The Oresland study did not produce a significant change in the estimate of effect size, as it contributed relatively little to the final weighted estimate (1.2%). Only omission of the relatively large Olsen study produced a substantial shift in the effect size estimate, with a resulting relative risk of 2.82 (95% confidence interval 1.94, 4.12).
We also considered whether the two studies that did not exclude subjects with FAP would have an effect on the estimate, and performed a sensitivity analysis to address this. After omission of these two studies, we performed a fixed effects model meta‐analysis, and the Mantel‐Haenszel pooled RR of infertility was only slightly reduced to 2.98 (95% CI 2.24–3.98). The forest plot is not shown.
Weighted average infertility rate
In the seven studies included in the final meta‐analysis, we calculated weighted average infertility rates in each group. In the 411 medically treated subjects who attempted conception, there were a total of 60 who reported infertility (as defined in each study), for an overall infertility rate of 14.6%. In the 481 subjects who had IPAA and attempted conception, there were 231 who reported infertility, for an overall infertility rate of 48.0%. We also performed the calculations with the outlying Cleveland Clinic study included, which produced an infertility rate of 19.9% (105/528) in the medically treated subjects and 50.1% (301/601) in the post‐IPAA subjects.
Discussion
We used raw data from the available literature to perform a meta‐analysis of the RR of infertility in UC patients who had undergone IPAA compared with those on medical therapy. We found that the RR of infertility was threefold greater after IPAA, and that half of the surgically treated women who attempt to conceive will be unsuccessful after 12 months.
We were unable to identity any patient or procedure related variables that consistently affected risk, including adhesion barriers, intraoperative lysis of adhesions, pouch type (S v J), and pouch anti‐adhesives. In a single study, Gorgun et al did show that intraoperative transfusion during IPAA was associated with an increased risk of infertility.12 The limited data available from Oresland's study7 suggest that post‐IPAA infertility may be largely due to scarring of the fallopian tubes after IPAA surgery, and this is supported by Asztely's previous study of hysterosalpingography in women after proctocolectomy.18 We would like to advocate collection of potential risk factors for infertility in future studies, both to identify the women at highest risk of infertility and any potentially modifiable risk factors.
Identification of intraoperative transfusion as a risk factor raises the question of whether there are modifiable procedure related variables that could affect fertility outcome. Transfusion, rather than directly causing infertility, may be a proxy for difficult pelvic dissection. An increased surface area of dissection would contribute to increased development of fibrin bridges, which are considered precursors to the formation of adhesions.19,20 It is reasonable to believe that patients undergoing a two stage operation (versus three stage) might have a lower likelihood of pelvic scarring and infertility. It is also possible that hand sewn anastomoses may pose greater risk than stapled anastomoses, due to increased distal dissection and tissue manipulation, potentially increasing the formation of adhesions. It is unclear whether future advancements in laparoscopic colectomy or adhesion barriers may decrease the rate of infertility after proctocolectomy. If infertility is associated specifically with fallopian tube occlusion, it is conceivable that wrapping the fallopian tubes in an adhesion barrier may reduce the risk of infertility. On the other hand, anecdotal reports of increased tissue inflammation at the site of intraoperative hyaline membrane placement suggest that further study is required before a change in surgical technique can be recommended.
This study had several limitations. Firstly, subjects in individual studies were not randomised to medical therapy or colectomy, potentially resulting in selection bias. Patients undergoing colectomy may have been more debilitated, resulting in higher baseline fertility impairment. Secondly, we identified variable response rates in each study. Subjects who attributed their infertility to colectomy may have been more likely to respond to surveys, as suggested by the higher response rate of the IPAA group in the study of Johnson and colleagues.13 Thirdly, several studies included only women post‐IPAA, and therefore relied more on distant recall for data on infertility and attempted conception during medical therapy. Reliance on recall may have systematically affected the medical therapy data more than the post‐IPAA data on infertility. Fourthly, two studies included an unknown number of patients with FAP rather than UC. However, our sensitivity analysis showed that this did not have a substantial effect on the summary estimate. In addition, we only evaluated studies of IPAA surgery. A recent small study of fertility after colectomy and ileorectal anastomosis (IRA) found a postoperative infertility rate of only 33%.21 Larger studies are needed to determine whether IRA offers a better approach for young women, as this approach leaves the inflamed rectum in place, which has its own drawbacks. We were also limited by the heterogeneity of the study designs and definitions of infertility. Despite these differences, after excluding the Cleveland Clinic study, we were able to identify a set of seven homogeneous studies to produce a summary estimate of RR. While the Cleveland Clinic study was an outlier, it had the same direction of effect: colectomy with IPAA significantly increases the risk of infertility in women with UC. It is plausible that these patients were referred to a tertiary centre due to more severe preoperative illness, resulting in the appearance of a decreased effect of surgery on infertility in this study.
Our study found that colectomy with IPAA increased the risk of infertility by approximately threefold in women with UC compared with medical management. Infertility increased from approximately 15% in UC before proctocolectomy with J pouch to approximately 50% after the surgery. Additional research is needed to identify modifiable procedure related risks and to determine whether interventions to reduce scarring of the fallopian tubes may have an ameliorative effect. We wish to emphasise that in patients with more severe disease, postoperative infertility rates may be higher, as suggested by the Cleveland Clinic study. These findings may influence physicians to more strongly consider potentially hazardous rescue therapies, including ciclosporin and infliximab, in young women with severe UC before committing to colectomy. For those patients who experience infertility after colectomy and reconstruction, in vitro fertilisation should be considered, as this has been successful in these patients.11 We advocate the presentation of all medical and surgical options, including a clear statement of the infertility risk associated with proctocolectomy, to patients with severe UC to encourage informed decision making.
Acknowledgements
PH is supported by the NIH K12 (#RR017607‐01) Research Award and a Crohn's and Colitis Foundation of America Senior Research Award. JW is supported by the Robert Wood Johnson Foundation. AMM is supported in part by Limited Project Grant LPG‐O78 from the American Society of Colon and Rectal Surgeons Research Foundation.
The authors would like to acknowledge the contribution of Andrea Todisco, MD, who kindly translated the Minerva Chirurgica manuscript from the original Italian.
Abbreviations
UC - ulcerative colitis
IPAA - ileal pouch‐anal anastomosis
FAP - familial adenomatous polyposis
RR - relative risk
IRA - ileorectal anastomosis
Footnotes
Dr Higgins receives funding for research on inflammatory bowel disease from the National Institutes of Health, the American Gastroenterological Association, and the Crohn's and Colitis Foundation of America. Dr Higgins is also the local site investigator at the University of Michigan for industry funded leucopheresis device trials for ulcerative colitis and Crohn's disease, and receives funding to conduct these trials from Otsuka America.
Conflict of interest: None declared.
References
- 1.Rutgeerts P, Sandborn W J, Feagan B G.et al Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 20053532462–2476. [DOI] [PubMed] [Google Scholar]
- 2.Jarnerot G, Hertervig E, Friis‐Liby I.et al Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo‐controlled study. Gastroenterology 20051281805–1811. [DOI] [PubMed] [Google Scholar]
- 3.Travassos W J, Cheifetz A S. Infliximab: Use in inflammatory bowel disease. Curr Treat Options Gastroenterol 20058187–196. [DOI] [PubMed] [Google Scholar]
- 4.Parsi M A, Lashner B A. Safety of infliximab: primum non nocere. The safety profile of infliximab in patients with Crohn's disease: the Mayo Clinic experience in 500 patients, Inflamm Bowel Dis 200410486–487. [DOI] [PubMed] [Google Scholar]
- 5.Muir A J, Edwards L J, Sanders L L.et al A prospective evaluation of health‐related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol 2001961480–1485. [DOI] [PubMed] [Google Scholar]
- 6.Wikland M, Jansson I, Asztely M.et al Gynaecological problems related to anatomical changes after conventional proctocolectomy and ileostomy. Int J Colorectal Dis 1990549–52. [DOI] [PubMed] [Google Scholar]
- 7.Oresland T, Palmblad S, Ellstrom M.et al Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int J Colorectal Dis 1994977–81. [DOI] [PubMed] [Google Scholar]
- 8.Counihan T C, Roberts P L, Schoetz D J., Jret al Fertility and sexual and gynecologic function after ileal pouch‐anal anastomosis. Dis Colon Rectum 1994371126–1129. [DOI] [PubMed] [Google Scholar]
- 9.Sjögren B, Poppen B. Sexual life in women after colectomy‐proctomucosectomy with S‐pouch. Acta Obstet Gynecol Scand 19957451–55. [DOI] [PubMed] [Google Scholar]
- 10.Hudson M, Flett G, Sinclair T S.et al Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet 199758229–237. [DOI] [PubMed] [Google Scholar]
- 11.Olsen K O, Juul S, Berndtsson I.et al Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology 200212215–19. [DOI] [PubMed] [Google Scholar]
- 12.Gorgun E, Remzi F H, Goldberg J M.et al Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery 2004136795–803. [DOI] [PubMed] [Google Scholar]
- 13.Johnson P, Richard C, Ravid A.et al Female infertility after ileal pouch‐anal anastomosis for ulcerative colitis. Dis Colon Rectum 2004471119–1126. [DOI] [PubMed] [Google Scholar]
- 14.Olsen K O, Joelsson M, Laurberg S.et al Fertility after ileal pouch‐anal anastomosis in women with ulcerative colitis. Br J Surg 199986493–495. [DOI] [PubMed] [Google Scholar]
- 15.Loftus E V., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 20041261504–1517. [DOI] [PubMed] [Google Scholar]
- 16.Bulow S. Results of national registration of familial adenomatous polyposis. Gut 200352742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaglia M, Bronsino E, Canino V.et al The impact of conventional proctocolectomy on sexual function. Minerva Chir 199348903–910. [PubMed] [Google Scholar]
- 18.Asztely M, Palmblad S, Wikland M.et al Radiological study of changes in the pelvis in women following proctocolectomy. Int J Colorectal Dis 19916103–107. [DOI] [PubMed] [Google Scholar]
- 19.Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg 1997163169–174. [PubMed] [Google Scholar]
- 20.Thompson J. Pathogenesis and prevention of adhesion formation. Dig Surg 199815153–157. [DOI] [PubMed] [Google Scholar]
- 21.Mortier P E, Gambiez L, Karoui M.et al Colectomy with ileorectal anastomosis preserves female fertility in ulcerative colitis. Gastroenterol Clin Biol 200630594–597. [DOI] [PubMed] [Google Scholar]