Abstract
Aim
The aim of this study was to investigate the hypothesis that the opioid system is involved in the development of hepatic fibrosis.
Methods
The effect of naltrexone (an opioid receptor antagonist) on hepatic fibrosis in bile duct ligated (BDL) or sham rats was assessed by histology and hepatic hydroxyproline levels. Liver matrix metalloproteinase 2 (MMP‐2) was measured by zymography, and α smooth muscle actin (α‐SMA) and CD45 (leucocyte common antigen) by immunohistochemistry. The redox state of the liver was assessed by hepatic glutathione (GSH)/oxidised glutathione (GSSG) and S‐nitrosothiol levels. Subtypes of opioid receptors in cultured hepatic stellate cells (HSCs) were characterised by reverse transcriptase‐polymerase chain reaction, and the effects of selective δ opioid receptor agonists on cellular proliferation, tissue inhibitor of metalloproteinase 1 (TIMP‐1), and procollagen I expression in HSCs determined.
Results
Naltrexone markedly attenuated the development of hepatic fibrosis as well as MMP‐2 activity (p<0.01), and decreased the number of activated HSCs in BDL rats (p<0.05). The development of biliary cirrhosis altered the redox state with a decreased hepatic GSH/GSSG ratio and increased concentrations of hepatic S‐nitrosothiols, which were partially or completely normalised by treatment with naltrexone, respectively. Activated rat HSCs exhibited expression of δ1 receptors, with increased procollagen I expression, and increased TIMP‐1 expression in response to δ1 and δ2 agonists, respectively.
Conclusions
This is the first study to demonstrate that administration of an opioid antagonist prevents the development of hepatic fibrosis in cirrhosis. Opioids can influence liver fibrogenesis directly via the effect on HSCs and regulation of the redox sensitive mechanisms in the liver.
Keywords: liver fibrosis; opioids, glutathione; tissue inhibitor of metalloproteinase; hepatic stellate cells; cirrhosis
Increased opioid neurotransmission is well recognised in patients with liver disease. There are increased plasma concentrations of opioid peptides in patients with primary biliary cirrhosis, and administration of opioid antagonists may cause an opioid withdrawal‐like syndrome.1 We have recently shown that the opioid antagonist naltrexone decreases liver injury in rats and mice with acute biliary obstruction.2,3 Although the mechanism by which naltrexone decreases liver injury is unknown, one potential mechanism may include prevention of a decrease in hepatic glutathione (GSH) levels, which become suppressed by endogenous opioids.3,4 Evidence to support this concept comes from studies in which intracerebroventricular injection of an opioid agonist decreases hepatic GSH synthesis and results in elevation in liver enzyme activities.5,6 This suggests that central mechanisms are involved in the regulation of the redox state of hepatocytes. Thus the contribution of endogenous opioids to the regulation of the redox state of the liver may be important in various disease states and could potentially be involved in the mechanism(s) by which neural injury affects liver function and inflammation.7 Oxidative stress and the altered redox state of the liver are also thought to be important in the pathogenesis of hepatic fibrosis.
Chronic liver injury leading to hepatic fibrosis occurs in response to a variety of insults, which trigger a wound healing‐like reaction, and has the potential of being reversible. During chronic liver injury, hepatic stellate cells (HSCs) become activated and express a combination of matrix metalloproteinases (MMPs) and their specific inhibitors (tissue inhibitors of metalloproteinase (TIMPs)) which have the ability to induce global quantitative and qualitative changes in the liver extracellular matrix. This pattern is characterised by increased expression of TIMP‐1, pro‐MMP‐2 (gelatinase), and membrane‐type MMP‐1, which leads to degradation of the sub‐endothelial basement membrane, loss of normal liver architecture, and inhibition of degradation of fibrillar liver collagen by collagenases (MMP‐1/MMP‐13).8
More recent studies have shown that some of the metalloproteinases (for example, MMP‐9) are activated by S‐nitrosation,9 a process which can be regulated through concentrations of low molecular weight thiols. Therefore, altered concentrations of hepatic GSH may affect MMPs activity either indirectly through inhibition of reactive oxygen species formation, or directly via modification of MMP precursors10,11 or through the altered redox state of the cellular milieu which can affect S‐nitrosation reactions.9
The aim of this study was to investigate the hypothesis that administration of naltrexone (a long acting opioid receptor blocker) prevents hepatic fibrosis, and to investigate some of the mechanisms involved.
Methods
Reagents
Delta‐1 (δ1) and δ2 opioid receptor agonist (DPDPE and deltorphin II, respectively) were purchased from Tocris (Bristol, UK). All other materials were purchased from Sigma (Pool, UK) unless otherwise specified in the text.
Animals
Male Sprague‐Dawley rats (200–230 g) were used in this study. All animal procedures were in accordance with “Guide for the Care and Use of Laboratory Animals” (NIH US publication No 85–23, revised 1985) and Home Office (UK) recommendations. Animals were randomly divided into two groups of age matched rats (n = 16–29). One group underwent a sham procedure (controls) and the other group underwent bile duct ligation, as described previously.2,3 These two groups were then divided into group A (saline treated) or group B (naltrexone treated).
Drug administration and sample collection
Group A rats (sham controls, n = 8; bile duct ligated (BDL) controls, n = 15) underwent daily administration of sterile saline solution (1 ml/kg/day). Group B rats (sham/naltrexone, n = 8; BDL/naltrexone, n = 14) were injected with subcutaneous naltrexone hydrochloride (20 mg/kg/day subcutaneously daily) for 28 days post‐sham procedure or bile duct ligation.2,3 On day 28, animals were sacrificed by exsanguination (cardiac puncture) under sodium pentobarbital (50 mg/kg intraperitoneally) anaesthesia. Liver samples were collected and frozen in liquid nitrogen and stored at −80°C.
Hepatic hydroxyproline content
Hepatic hydroxyproline, a marker of fibrosis, was measured using a modified chloramine T/diaminobenzidine (DAB) assay.12 In brief, wet liver tissue was weighed (∼250 mg), homogenised in 6 M HCl, and hydrolysed at 110°C for 18 hours. Post‐hydrolysis, the pH was adjusted to 7.0 using 6 M NaOH, and the hydrolysate filtered through a 0.45 μm Millipore filter. Samples were then incubated with chloramine T for five minutes (2.5 mM final concentration) and paradimethyl‐amino‐benzaldehyde was added (final concentration 410 mM) for 30 minutes at 60°C. The concentration of hydroxyproline in each sample was determined by spectrophotometry at 560 nm using a standard curve generated from known quantities of hydroxyproline. Each liver sample was measured in triplicate, and the mean value of hydroxyproline used for analysis. Results were expressed as µg/g of wet tissue.
Matrix metalloproteinase 2 zymography
MMP‐2 activity was determined by gelatine zymography, as previously described.13 In brief, liver samples were homogenised in a glass homogeniser with lysis buffer (1% Triton X‐100, 500 mM, Tris/HCl, pH 7.6, 200 mM NaCl, and 10 mM CaCl2) and centrifuged at 10 000 g for 15 minutes. Protein content was assayed by the Lowry assay. Twenty micrograms of protein were subjected to sodium dodecyl sulphate (SDS)‐polyacrylamide gel electrophoresis in 10% polyacrylamide gel impregnated with 1 mg/ml gelatine. After electrophoresis, gels were washed twice, for 30 minutes each time, in buffer containing 1% Triton‐100 to displace SDS, and the gels were developed for 48 hours in 50 mM Tris buffer containing 5 mM CaCl2 (pH 7.4). The gels were stained with 1% Coomassie brilliant blue and then destained. Conditioned medium from 12‐O‐tetradecanoylphorbol‐13‐acetate stimulated HT1080 cells was used as a positive control.
Hepatic GSH/GSSG level
Glutathione levels were measured by fluorometry (both reduced (GSH) and oxidised (GSSG) forms). In brief, ∼250 mg of liver tissue were homogenised on ice in 3.75 ml of phosphate‐EDTA buffer. The homogenate was centrifuged at 10 000 g for 30 minutes at 4°C. The homogenate (0.5 ml) was diluted further in 4.5 ml of phosphate‐EDTA buffer (1:10). The resulting mixture (0.1 ml) was added to 1.8 ml of phosphate‐EDTA buffer (pH 8.0) followed by 100 μl of O‐phthalaldehyde. The analysis was performed fluorometrically at 420 nm, with excitation at 350 nm. GSSG was measured by the same procedure following incubation of tissue homogenates with N‐ethylmaleimide (5 mM) for 30 minutes at room temperature to alkylate and block any free thiol residues.
Measurement of hepatic S‐nitrosothiol concentrations
A reductive chemiluminescense based assay was used for measurement of hepatic concentrations of S‐nitrosothiols.14 In a separate set of experiments, immediately after scarification of the animals, all organs were perfused through the left ventricle with ice cold perfusion buffer (phosphate saline buffer, pH 7.4 containing a thiol blocking agent N‐ethylmaleimide and diethylentriamine‐pentaacetic acid, 5 and 1 mM, respectively). After homogenisation of ∼500 mg of the snapped frozen tissue in ice cold perfusion buffer, tissue concentrations of S‐nitrosothiols were determined by a copper (II)/iodine/iodine mediated cleavage of S‐nitrosothiols to nitric oxide (NO), which was then quantified by its gas phase chemiluminescent reaction with ozone in an NO analyser (NOA; Sievers, Boulder, Colorado, USA) by a method developed in our laboratory.13 Quantitation of S‐nitrosothiols were made with respect to a standard curve generated from known amounts of SNO‐albumin. Results were expressed as pmol/g of wet tissue.
Biochemical markers of liver injury and nitrite/nitrate measurement
Bilirubin and enzyme activities of alkaline phosphatase (ALP) and alanine aminotransferase (ALT) were determined in plasma using a commercially available kit. Plasma nitrite/nitrate was measured by a chemiluminescence based assay.15 In brief, plasma was filtered through a 30 kDa cut off filter (Milipore, USA) and nitrate was converted to nitrite using nitrate reductase. Samples were then injected into a reaction chamber containing acetic acid and potassium iodide (50 mg/ml) at a ratio of 4:1. This reduces nitrite to NO, which is purged from the refluxing solution by nitrogen and reacts with ozone before analysis by chemiluminescence. Measurements were calibrated against standard curves of sodium nitrate.
Hepatic stellate cell culture study
Cell culture
Hepatic stellate cells (HSCs) were isolated from normal livers of 350 g adult male Sprague‐Dawley rats by sequential perfusion with collagenase and pronase, followed by discontinuous density centrifugation in 11.5% Optiprep (Life Technologies, UK). HSCs were cultured on plastic in Dulbecco's modified Eagle's medium supplemented with penicillin 100 U/ml, streptomycin 100 μg/ml, L‐glutamine 2 mM, and 16% fetal calf serum, and were maintained at 37°C in an atmosphere of 5% CO2. Activated HSCs were generated by continuous culture of freshly isolated cells on plastic for 7–10 days.
Opioid receptors expression by reverse transcriptase‐polymerase chain reaction (RT‐PCR)
Total RNA was purified from isolated cells or frozen rat brain using the total RNA purification kit (Qiagen, UK) following the manufacturer's instructions. First strand cDNA was generated by using 1 μg of deoxyribonuclease treated RNA, 1 μl of random hexamer primer (p(dN)6), and ribonuclease free water (Qiagen), heated at 70°C for five minutes, and then placed on ice. RNasin (ribonuclease inhibitor), 100 U of Moloney murine leukaemia virus reverse transcriptase, Moloney murine leukaemia virus buffer, and 0.4 mmol/l deoxynucleoside triphosphates were added, and the mix was incubated at 42°C for one hour.
Oligonucleotide primers used for PCR amplification of rat opioid receptors and β‐actin were as follows16:
-
Rat µ opioid receptor (NM013071), PCR product: 638 bp
-
-
sense: 5′‐ACA TCC CTC CAC GGC TAA TAC
-
-
antisense: 5′‐AAC AAT ACA AAA CCA AGA AAC
-
-
-
Rat δ1 opioid receptor (NM012617), PCR product: 356 bp
-
-
sense: 5′‐ATC TTC ACG CTC ACC ATG AT
-
-
antisense: 5′‐CGG TCC TTC TCC TTG GAG CC
-
-
-
Rat κ opioid receptor (NM017167), PCR product: 352 bp
-
-
sense: 5′‐CCG CTG TCT ACT CTG TGG TGT
-
-
antisense: 5′‐ATG TTG ATG ATC TTT GCT TTC
-
-
-
Rat β‐actin PCR product: 500 bp
-
-
sense: 5′‐AGA GGG AAA TCG TGC GTG ACA
-
-
antisense: 5′‐ACA TCT GCT GGA AGG TGG ACA
-
-
PCR reactions comprised of 1 µl of cDNA template, 100 ng each of sense and antisense oligonucleotide primers, 2.5 µl of optimised TaqPCR buffer (Promega, UK), 0.4 mmol/l dNTP mixture, and 2 U of Taq polymerase in a total reaction volume of 25 µl. After an initial five minute incubation at 94°C, PCRs were performed using a one minute annealing step (δ1 and β‐actin at 55°C, κ and μ at 50°C), followed by a one minute elongation step at 72°C and a 45 second denaturation step at 94°C. Forty PCR cycles were performed for amplification of all opioid receptor cDNAs, 25 cycles for β‐actin cDNA, followed by a final elongation for 10 minutes at 72.0°C. PCR products were separated by electrophoresis through a 1% agarose gel and detected by ethidium bromide staining.
Proliferation assay
The effect of δ1 and δ2 opioid receptor agonists on proliferation of activated rat HSCs was measured using [3H] thymidine incorporation.17 Experiments were conducted in duplicate in 24 well plates. Activated rat HSCs between passage 1 from three separate rat preparations were trypsinised and plated at 50 000 cells/well and grown in media containing 16% fetal calf serum for 24 hours followed by complete serum deprivation for 24 hours. Cells were then cultured for an additional 16 hours in serum supplemented (16%) or serum free media in the presence of DPDPE or deltorphin II (selective δ1 and δ2‐ opioid receptor agonists, respectively) at a concentration of 1 μmol/l,18,19 or 10 ng/ml recombinant human platelet derived growth factor (PDGF‐BB) (Calbiochem, UK) prior to addition of 1 µCi of [methyl‐3H] thymidine (Amersham Biosciences, Little Chalfont, UK). Cells were incubated for a further 24 hours before washing with Hanks balanced salt solution and fixing in ice cold methanol. The fixed cells were solubilised in 0.25 M sodium hydroxide/0.2% (w/v) SDS and neutralised with 5 mol/l hydrochloric acid, the lysates were added to OptiPhase HiSafe 3 scintillation fluid (Wallac, Turku, Finland), and scintillation was counted using a MicroBeta 1450 scintillation counter. We used fetal calf serum as a stimulus for cellular proliferation in these experiments.
Tissue inhibitor of metalloproteinase 1 (TIMP‐1) and procollagen I expression by quantitative Taqman RT‐PCR
Primary cultured activated HSCs between days 7 and 10 from five separate rat preparations were cultured in serum free media for 24 hours followed by exposure for a further 12 hours to δ1 or δ2 opioid receptor agonists (1 μmol/l) with or without the presence of serum (16%). Serum was used as a stimulus for TIMP‐1 and procollagen I expression to evaluate the possible potentiation of its effect by δ agonists. Cells were then harvested, total RNA was isolated, and first strand cDNA was generated as described previously in this paper. 18S ribosomal RNA Taqman primers and probe were purchased from Applied Biosystems (UK). For rat procollagen I, forward primer 5′‐ttc acc tac agc acg ctt gtg‐3′, reverse primer 5′‐gat gac tgt ctt gcc cca agt t‐3′, probe 5′‐atg gct gca cga gtc aca ccg‐3′, quencher‐N,N,N′,N′‐tetra methyl‐6‐carboxy rhodamine, and flourophore‐6‐carboxy fluorescein were used. For rat TIMP‐1, forward 5′‐agc ctg tag ctg tgc ccc aa‐3′, reverse 5′‐aac tcc tcg ctg cgg ttc tg‐3′, and probe 5′‐aga ggc tct cca tgg ctg ggg gtg ta‐3′ were used. Taqman quantitative RT‐PCRs were composed of cDNA; 0.3 μmol/l of forward, reverse, and probe primers; and 12.5 μl of Taqman master mix (Applied Biosystems) in a final volume of 25 μl. Reaction conditions were 50°C for two minutes and 95°C for 10 minutes, followed by denaturing for 15 seconds at 95°C and annealing and extension at 60°C for one minute for 40 cycles. The relative level of transcriptional difference between treated groups and untreated serum free group was calculated using the equation
[1/(2A)] ×100
where A indicates the cycle threshold (ct) of the treatment group minus the ct of the serum free untreated group (baseline group for comparison) after the 18S RNA ct value had been deducted from the target gene for each sample.
Histological study
Formalin fixed paraffin embedded section of the right and left liver lobes were cut into 3 μm sections for histological staining.
Masson's Trichrome staining
Samples were stained with haematoxylin‐eosin and Masson's Trichrome. Histology was evaluated by a single pathologist who was blinded to the treatment groups and laboratory data. Fibrosis was staged 0–4 based on Scheuer's scoring system (0, no fibrosis; 1, expansion of portal tract without linkage; 2, portal expansion with portal to portal linkage; 3, extensive portal to portal and focal portal to central linkage; 4, cirrhosis).20 Haematoxylin‐eosin and Trichrome stained liver specimens from control and BDL animals were evaluated by light microscopy for measuring ductal proliferation in 50 portal areas. Ductular proliferation was scored using the following grading system as described previously21: 0, <10% of portal areas involved; 1, 10–50% of portal areas involved; 2, >50% of portal areas involved; 3, circumferential involvement of at least 50% of the portal area without significant expansion of portal tract; 4, circumferential involvement of at least 50% of the portal area with significant expansion of portal tract; and 5, same as 4 plus bridging of the portal tracts.
Sirius red staining for quantitative assessment of liver fibrosis
Liver sections were deparaffinised and rehydrated. They were then incubated for one hour at room temperature with aqueous saturated solution of picric acid containing 0.1% Sirius red (direct 80). Stained slides were washed in two changes of acidified water and dehydrated in three changes of 100% ethanol, mounted, and examined by light microscopy. Red stained collagen fibres were then quantified by digital image analysis. In brief, the image was captured by ×40 power using a charge coupled device (CCD) camera, and the degree of collagen staining was analysed using UTHSCSA shared image analysis system (http://ddsdx.uthscsa.edu/dig/itdesc.html). The image consisted of 400 000 pixels and the areas of collagen staining were differentially selected using threshold adjustment on a gray scale picture. The fraction of positively stained pixels relative to the whole number of pixels was expressed as percentage of collagen staining. Quantitative histomorphometry was averaged from four randomly selected fields per slide and also per region.
Immunohistochemical study of α smooth muscle actin (α‐SMA)
Immunohistochemical staining for α‐SMA, a marker for HSC activation, was performed as previously described.22 Antigen retrieval was achieved by microwaving in citric saline for 15 minutes. Endogenous peroxidase activity was blocked by hydrogen peroxide pretreatment for 10 minutes and was then further blocked by using the avidin/biotin blocking kit (Vector Laboratories, UK). Monoclonal mouse antirat α‐SMA primary antibodies (Serotec, Oxford, UK) were diluted 1:160 and incubated for 1.5 hours at room temperature; secondary and anti‐immunoglobulin G horseradish peroxidase conjugated tertiary antibody was incubated for 20 minutes (Vector Laboratories, Peterborough, UK). α‐SMA expression was visualised by 3,3′‐diaminobenzidine tetrahydrochloride staining.
Immunohistochemical study of leucocyte infiltration (CD45)
The CD45 antigen family (also known as leucocyte common antigen) is a group of high molecular weight glycoproteins that are expressed on the plasma membranes of leucocytes.23 An indirect immunoperoxidase method was used for detection of CD45 antigen in paraffin embedded liver tissues. Multiple 5 µm thick sections were cut and stored at 4°C until use. Endogenous peroxidase was blocked by hydrogen peroxide pretreatment for 15 minutes. Sections were then incubated with a 1:100 dilution of primary antibody (anti‐CD45 antibody; Dako, Denmark) overnight at 4°C. Slides were washed and then incubated with secondary antibody for 30 minutes at room temperature. After further washing in phosphate buffered saline‐Tween 20, the reaction product was visualised using DAB (100 mg DAB in 100 ml of phosphate buffered saline and 66 µl H2O2). After five minutes, sections were washed and counterstained in haematoxylin and mounted with Entellan on poly‐l‐lysine‐coated slides. As negative controls, all specimens were incubated with an isotype matched control antibody under identical conditions. Stained slides were then coded for blind reading by a single pathologist. The amount of leucocyte infiltration was assessed using the histological activity index, as described by Ishak and colleagues.24
Statistical analysis
Results are expressed as mean (SEM). Statistical evaluation of data from the in vivo experiments was performed by analysis of variances (ANOVA) followed by the Tukey post hoc test. Data from the in vitro study were analysed using a two tailed paired t test. A p value <0.05 was considered statistically significant.
Results
Effect of naltrexone on liver function tests
Five animals died in the BDL/saline (n = 3) and BDL/naltrexone (n = 2) groups, before day 28, and were excluded from analysis. As expected, bile duct ligation caused an increased concentration of plasma bilirubin. The activities of plasma ALP and ALT increased following bile duct ligation but were partially attenuated by treatment with naltrexone in the BDL animals (table 1).
Table 1 Plasma levels of bilirubin, nitrate+nitrite, alkaline phosphatase activity (ALP), and alanine transaminase activity (ALT) in control (CTR) and bile duct ligated (BDL) rats given naltrexone or saline.
| CTR | BDL | |||
|---|---|---|---|---|
| Saline | Naltrexone | Saline | Naltrexone | |
| Plasma bilirubin(mg/dl) | 0.47 (0.05) | 0.44 (0.06) | 13.45 (1.65)*** | 11.42 (1.07)*** |
| Plasma ALP activity (U/l) | 376 (36) | 300 (39) | 917 (45)*** | 701 (32)†† |
| Plasma ALT activity (U/l) | 38.4 (2.9) | 40.1 (3.5) | 100.6 (9.5)*** | 63.7 (8.6)‡ |
| Plasma nitrite+nitrate (μM) | 15.2 (2.1) | 13.5 (3.1) | 21.7 (1.9) | 22.1 (4.7) |
***p<0.001 compared with control groups; ††p<0.01 compared with BDL/saline group; ‡p<0.05 compared with BDL/saline group.
Opioid receptor blockade decreased liver fibrosis and HSC activation without a significant effect on liver inflammation in BDL rats
Prolonged biliary obstruction resulted in marked morphological changes in liver tissue, including extensive proliferation of the bile ducts, enlargement of the portal tracts (with inflammation and necrosis) and the formation of extensive periportal fibrosis as well as pericentral collagen deposition (fig 1). Semiquantitative evaluation of histological changes in the liver is shown in table 2, which confirms significant fibrosis and inflammation following bile duct ligation and marked attenuation of fibrosis but not inflammation by opioid receptor blockade in BDL rats (p<0.01).
Figure 1 Hepatic fibrosis was reduced in naltrexone treated rats compared with untreated bile duct ligated (BDL) rats. Twenty eight days after the surgical procedure, liver tissue was obtained from BDL and sham operated (CTR) saline treated and naltrexone treated rats. Collagen fibres were stained with Masson's Trichrome. Bile duct ligation was associated with extensive bridging fibrosis (portal to portal and portal to central linkage with fibrotic bands) and bile duct proliferation. In naltrexone treated BDL animals, the degree of fibrosis was significantly reduced but bile duct proliferation remained the main histological feature.
Table 2 Histological assessment of fibrosis and inflammation in control (CTR) and bile duct ligated (BDL) rats given naltrexone or saline.
| CTR | BDL | |||
|---|---|---|---|---|
| Saline (n = 8) | Naltrexone (n = 8) | Saline (n = 12) | Naltrexone (n = 12) | |
| Fibrosis | ||||
| Stage 0 | 8 | 8 | 0 | 4 |
| Stage 1 | 0 | 0 | 2 | 6 |
| Stage 2 | 0 | 0 | 4 | 2 |
| Stage 3 | 0 | 0 | 6 | 0 |
| Stage 4 | 0 | 0 | 0 | 0 |
| Mean (SEM) | 0.00 (0.00) | 0.00 (0.00) | 2.33 (0.22)* | 0.83 (0.20)*† |
| Inflammation | ||||
| Score 0 | 8 | 8 | 0 | 2 |
| Score 1 | 0 | 0 | 8 | 6 |
| Score 2 | 0 | 0 | 2 | 3 |
| Score 3 | 0 | 0 | 2 | 1 |
| Score 4 | 0 | 0 | 0 | 0 |
| Inflammatory score | ||||
| Mean (SEM) | 0.00 (0.09) | 0.00 (0.00) | 1.50 (0.23)* | 1.25 (0.25)* |
*p<0.05 compared with control groups; †p<0.05 compared with BDL/saline group.
To confirm the results obtained from Masson's Trichrome staining, a collagen specific staining method (Sirius red) was used and the degree of fibrosis was quantified using a digital imaging system. As shown in fig 2, bile duct ligation was associated with extensive bridging fibrosis (portal to portal and portal to central linkage with fibrotic bands) which was significantly reduced by naltrexone treatment in BDL animals (figs 2, 3B).
Figure 2 Hepatic fibrosis was reduced in naltrexone treated rats compared with untreated bile duct ligated (BDL) rats. Twenty eight days after the surgical procedure, liver tissue was obtained from BDL and sham operated (CTR) saline treated and naltrexone treated rats. Collagen fibres were stained with Sirius red. Bile duct ligation was associated with extensive bridging fibrosis (portal to portal and portal to central linkage with fibrotic bands). The degree of hepatic fibrosis was significantly reduced in naltrexone treated BDL animals.
Figure 3 Quantification of the degree of liver fibrosis assessed by measuring hydroxyproline content of the liver (A) and digital image analysis of the Sirius red stained slides (B). ***p<0.001 compared with control (CTR) groups; ††p<0.01, †††p<0.001 compared with bile duct ligated (BDL)/saline group.
Morphological examination of the liver specimens (using haematoxylin‐eosin and Masson's Trichrome staining) demonstrated marked periductular proliferation after 28 days of bile duct ligation (0.00 (0.00) to 3.81 (0.18) in sham/saline and BDL/saline, respectively; p<0.001) (fig 1). However, no significant difference in ductular proliferation score was observed after opioid receptor blockade in BDL rats (3.81 (0.18) v 3.90 (0.21), in BDL/saline and BDL/naltrexone, respectively). To assess the infiltration of leucocytes in more detail, leucocytes were stained for the CD45 antigen (fig 4). Portal tract expansion was accompanied by mild to moderate inflammatory cell infiltration in both saline and naltrexone treated cirrhotic rats, and leucocyte infiltration was unaffected by chronic opioid receptor in BDL rats (fig 4, table 2).
Figure 4 Immunohistochemical staining for CD45 in liver tissues obtained from bile duct ligated (BDL) and sham operated (CTR) rats treated with saline or naltrexone.
In parallel with the observed improvement in liver histology, fibrosis was also quantified by measurement of hepatic hydroxyproline levels (fig 3A). There was a significant increase in hepatic hydroxyproline levels in rats with biliary cirrhosis (BDL/saline group) compared with sham/saline controls (452 (23) v 132 (13) µg/g wet liver, respectively; p<0.001) and administration of naltrexone prevented the increase in hepatic hydroxyproline content in BDL rats (195 (25) v 452 (23) µg/g in BDL/naltrexone and BDL/saline groups, respectively).
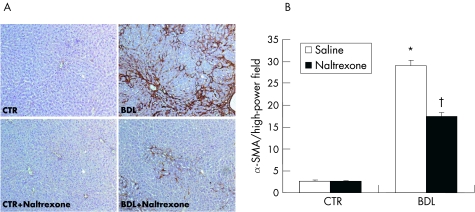
To study the underlying cellular events responsible for decreased liver fibrosis in naltrexone treated BDL rats, we performed α‐SMA staining in liver samples. There was a significant decrease in hepatic α‐SMA staining in naltrexone treated BDL animals compared with BDL/saline controls (fig 5). Counting of α‐SMA stained cells showed that opioid receptor blockade resulted in a 40% reduction in the numbers of activated HSC/liver myofibroblasts in BDL rats (fig 5).
Figure 5 Immunohistochemical staining for α smooth muscle actin (α ‐SMA) in liver tissues obtained from bile duct ligated (BDL) and sham operated (CTR) rats treated with saline or naltrexone. (A) Photomicrographs showed elevated numbers of wound healing hepatic myofibroblasts in BDL livers which were attenuated by naltrexone treatment. (B) Number of α‐SMA positive cells were counted in 15 high power fields and expressed as the average number of α‐SMA positive cells/high power field. *p<0.05 compared with control groups; †p<0.05 compared with BDL/saline group.
Effect of naltrexone treatment on the hepatic redox state
To determine whether long term naltrexone therapy had any effect on the redox state of liver tissue, hepatic concentrations of GSH, GSSG, and S‐nitrosothiols were measured in rats with and without biliary cirrhosis and with and without long term opioid blockade. Hepatic GSH levels were significantly decreased in BDL/saline rats (4.3 (0.2) µmol/g) compared with sham controls (6.1 (0.2) µmol/g) but were increased significantly following treatment with naltrexone to 5.4 (0.3) µmol/g (p<0.05). The same pattern was observed for the GSH/GSSG ratio in the liver (fig 6). The redox state of the liver was also reflected by concentrations of tissue S‐nitrosothiols, which are formed by reaction of NO as a nitrosonium ion with cysteine residues in proteins, and broken down by low molecular weight thiols such as GSH. Hepatic levels of S‐nitrosothiols were markedly higher in BDL rats compared with controls (147 (28) v 56 (23) pmol/g wet of liver; p<0.01) and chronic naltrexone administration decreased hepatic levels of S‐nitrosothiols (66 (20) pmol/g in BDL/naltrexone group; p<0.05) (fig 6).
Figure 6 Hepatic levels of reduced glutathione (GSH), oxidised glutathione (GSSG), GSH/GSSG ratio, and S‐nitrosothiols in control (CTR) and bile duct ligated (BDL) rats given naltrexone or saline. **p<0.01 compared with control groups; †p<0.05, ††p<0.01 compared with BDL/saline group.
Effects of naltrexone on nitric oxide synthesis
As the effect of naltrexone on tissue S‐nitrosothiols could be secondary to a direct or indirect effect of naltrexone on NO synthesis,25 plasma concentrations of nitrite/nitrate were also measured. Naltrexone treatment had no effect on plasma nitrite/nitrate levels in either sham controls or rats with biliary cirrhosis (table 1), suggesting that its effects are not mediated via altered NO synthesis but rather by altering the redox state of the liver.
Effect of naltrexone on activity of hepatic MMP‐2
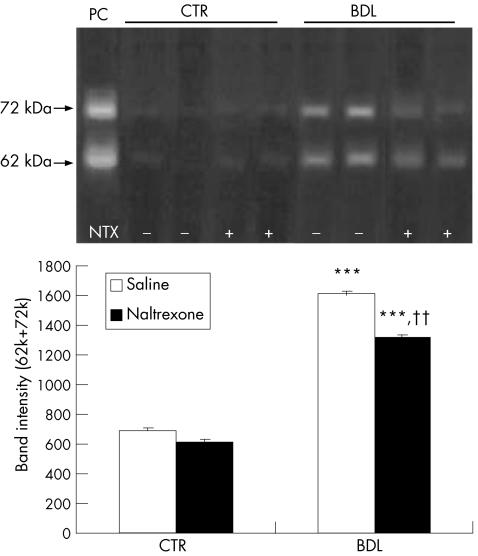
The development of fibrosis represents a balance between collagen deposition and degradation. Therefore, MMP‐2 activity in liver homogenates was measured by gelatine zymography. As shown in fig 7, two prominent bands appeared after zymography and corresponded to the proenzyme (72 kDa, pro‐MMP‐2) and the active form (62 kDa) of MMP‐2.13 The intensity of both bands increased significantly in BDL rats (p<0.001) and were decreased by treatment with naltrexone (p<0.01), suggesting that naltrexone decreases the level or activity of MMP‐2 in the liver.
Figure 7 Matrix metalloproteinase 2 activity of liver homogenates evaluated after sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and gelatin zymography. Quantitative analysis showed that the intensity of both 72 kDa and 62 kDa bands increased significantly in the bile duct ligated (BDL)/saline group which was attenuated after opioid receptor blockade. ***p<0.001 compared with control (CTR) groups; ††p<0.01 compared with BDL/saline group. PC, positive control (HT1080 cell extracts).
Activated HSCs express δ1 opioid receptor in vitro
Fibrosis is also characterised by activation of HSCs which transdifferentiate from a quiescent to a proliferative myofibroblast‐like phenotype.8 To investigate the potential for opioids to modulate HSC function, we investigated expression of opioid receptors in both quiescent and activated rat HSCs. Using RT‐PCR, we could not show μ and κ receptor expression in either quiescent or activated HSCs. However, we detected expression of δ1 opioid receptor mRNA in activated HSCs (fig 8). Delta opioid receptor has two subtypes but at present we cannot study expression of δ2 receptor as it has yet to be cloned.
Figure 8 Polymerase chain reaction of opioid receptor mRNA expression in quiescent (Q‐HSC) and activated (A‐HSC) rat hepatic stellate cells. mRNA extracted from rat brain was used as a positive control.
Effect of δ1 and δ2 opioid agonists on HSC proliferation
As the above studies demonstrated that activated HSCs express δ1 receptors, we examined the effects of δ1 and δ2 opioid receptor agonists on proliferation of activated HSCs in both serum free and serum supplemented conditions. As shown in table 3, none of the δ agonists had any significant effect on culture growth. In contrast, addition of serum and PDGF stimulated cell proliferation significantly (table 3). Incubation of HSCs with each of the δ receptor agonists in the presence of serum did not potentiate the effect of serum on cell proliferation in vitro.
Table 3 Culture growth of activated human hepatic stellate cells was determined using [3H] thymidine incorporation.
| Compound | Pharmacological activity | Proliferation index |
|---|---|---|
| Serum free media (SF) | – | 100 (00) |
| SF+DPDPE | δ1 receptor agonist | 89 (9) |
| SF+deltorphin II | δ2 receptor agonist | 127 (49) |
| SF+PDGF‐BB | Growth factor | 398 (88)* |
| Fetal calf serum (SP) | Growth factor | 1524 (1193)* |
| SP+DPDPE | Growth factor/δ1 receptor agonist | 1065 (863)* |
| SP+deltorphin II | Growth factor/δ2 receptor agonist | 1316 (913)* |
| SP+PDGF‐BB | Growth factor/growth factor | 1828 (1432)* |
Following 24 hours of serum starvation, cells were cultured for an additional 16 hours in serum supplemented (16%) or serum free media in the presence of DPDPE or deltorphin II (selective δ1 and δ2 receptor agonists, respectively) or recombinant human platelet derived growth factor (PDGF‐BB) prior to addition of [methyl‐3H] thymidine. Cells were incubated for a further 24 hours before washing with Hanks balanced salt solution and fixing.
Data are presented as mean (SEM) of three double experiments (n = 3).
*p<0.05 compared with serum free group.
Effect of δ1 and δ2 opioid receptor agonists on TIMP‐1 and procollagen I expression in hepatic stellate cells
To further explore the functional role of δ receptor activation on HSC function, we measured expression levels of TIMP‐1 and procollagen I transcripts in HSCs after treatment with δ receptor agonists with or without serum supplementation in vitro. Serum effectively increased expression of both TIMP‐1 and procollagen I, as demonstrated in fig 9C and D. Deltorphine II, a δ2 receptor agonist, increased expression of TIMP‐1 transcript in cells under serum free conditions (p<0.05) (fig 9A). We were unable to demonstrate a stimulatory effect for the δ1 agonist in HSCs cultured in serum free medium. However, both δ1 and δ2 agonists were able to synergise with serum to stimulate further increases in TIMP‐1 expression (fig 9C).
Figure 9 Effect of δ1 and δ2 opioid receptor agonists (DPDPE and deltorphin II, respectively) on expression of tissue inhibitor of metalloproteinase 1 (TIMP‐1) (A, C) and procollagen I (B, D) transcripts in hepatic stellate cells (HSCs) in serum free (A, B) or serum supplemented (C, D) media were assessed by quantitative Taqman reverse transcription‐polymerase chain reaction. Following 24 hours of serum starvation, cultured activated HSCs were incubated for another 12 hours with each of the drugs with or without addition of fetal calf serum (16%). The relative level of transcriptional difference between treated and untreated serum free cells was calculated and expressed as mean (SEM). SF, serum free group (without δ receptor agonist); SP, serum positive group (without δ receptor agonists). n = 3 for A, B and n = 5 for C, D experiments. *p<0.05, **p<0.01 compared with serum‐free control group; †p<0.05 compared with serum positive group.
Delta 1 (δ1) receptor activation by DPDPE increased expression of procollagen I in HSCs under serum free condition (p<0.05). Incubation of cells with each of the δ agonists in serum supplemented media did not potentiate the effect of serum on procollagen I expression (p>0.05).
Discussion
The current study demonstrates for the first time that administration of an opioid antagonist prevents the development of hepatic fibrosis in a rat model of biliary cirrhosis. This was confirmed both by liver histology and as well as quantitative measurement of hepatic hydroxyproline content, a marker of collagen deposition in the liver. There is already good evidence that endogenous opioids contribute to some of the manifestations of liver disease, such as hepatic encephalopathy26 and cholestatic pruritus.27 However, there have been no studies on the effect of opioids on fibrosis in the liver, or any other system, as far as we are aware.
Endogenous opioids act through three classes of receptors: μ, δ, and κ. All three opioid receptor types are antagonised by naltrexone. Expression of μ and δ opioid receptors has been shown in rat liver tissues16 but the pattern of distribution of these receptors in different parenchymal liver cells remains unclear. In a recent study, Jaume and colleagues28 were unable to show expression of any of these opioid receptors in mouse hepatocytes but they did observe that opioid receptor blockade improved resistance of mice to Fas induced hepatitis via a mechanism that did not involve a decrease in proinflammatory activity of neutrophils. Absence of opioid receptors in hepatocytes together with lack of morphine effect in Fas induced apoptosis of primary cultured hepatocytes ruled out a direct effect of opioids on hepatocytes.28
A key cellular cellular element in liver fibrosis is the HSC which, in response to injury, undergoes activation to a profibrogenic myofibroblast‐like cell. As a first step in our study, different subtypes of opioid receptors were characterised in primary cultures of HSCs .We observed that activated HSCs expressed δ1 opioid receptor mRNA in vitro (fig 7). δ1 receptor is the only δ receptor gene cloned to date, and at present we do not have information about expression of the δ2 subtype in either activated or quiescent HSCs. Such temporal expression of δ1 opioid receptor suggests a role for this receptor during HSC activation in liver injury. Some previous reports have demonstrated that activation of opioid receptors can affect proliferation of neuronal and non‐neronal cells both in vivo and in vitro.29,30 Agonist activation of δ opioid receptor has been shown to potentiate fetal calf serum or tyrosine kinase receptor mediated cell proliferation in a cell line specific manner in vitro.31 However, in the present study, 16 hours of incubation of activated HSCs with specific δ receptor agonists (with or without serum supplementation) did not affect cellular proliferation.
Hepatic TIMP‐1 expression correlates with the histological changes of liver fibrosis and significantly increases in both the liver and serum during development of liver fibrosis.13,32,33 TIMP‐1 has been shown to inhibit apoptosis in HSCs as well as several other types of cells, and this effect is sometimes independent of MMP inhibition.34 In this study, we showed that opioids modulate expression of TIMP‐1 in activated HSCs by δ receptor activation. Potentiation of TIMP‐1 expression by δ opioid agonists in the presence of serum may indicate that opioids are able to synergise with other soluble profibrogenic factors to enhance certain phenotypic characteristics of the activated HSC. This may be critical in rats with biliary cirrhosis in which there is increased production of endogenous opioids in the liver. Treatment of cultured HSCs with a δ1 agonist under serum free conditions stimulated procollagen I expression. However, neither the δ1 or δ2 receptor agonists influenced procollagen I expression in HSC cultured in the presence of serum. Taken together with the TIMP‐1 data, these results clearly demonstrate the potential for δ opioid receptor modulation of HSC expression of both TIMP‐1 and procollagen I, although there appear to be subtle differences in the mode of stimulation of the two genes that warrants further investigations. Critically, the ability of δ opioid receptors to stimulate TIMP‐1 and procollagen I expression helps to explain, at least in part, the antifibrogenic effect of opioid receptor blockade in the BDL model. However, we do not feel it can account for the magnitude of the changes observed.
Many factors are involved in the activation of HSCs into fibrogenic myofibroblasts, including oxidative stress. The underlying basis of oxidative stress in cholestatic liver disease is complex, involving generation of reactive oxygen and nitrogen species, possibly mediated by endotoxin, bile acids, and leucocyte infiltration.35,36 In recent years there has been much focus on the role of oxidative stress as many pathological processes which cause oxidative stress in the liver lead to hepatic fibrosis. Moreover, the activity of important proinflammatory factors such as nuclear factor κB are under redox control.37 Redox regulation has also been perceived as a simple on‐off switch in some key regulatory proteins, such as MMPs and nuclear factor κB, via S‐nitrosation of their thiol groups.9,38 The control of protein function by S‐nitrosation is counterregulated by denitrosation, a process which may involve low molecular weight thiols such as GSH which leads to decomposition of the S‐NO bond.39 Previous studies have shown that administration of opioids such as morphine causes decreased hepatic GSH synthesis through an undefined mechanism.5,6 Thus Roberts and colleagues5 have shown that intraperitoneal administration of 100 mg/kg morphine in mice resulted in an approximate 25% decrease in hepatic GSH. In their study, the same magnitude of GSH depletion was observed following intracerebroventricular injection of 100 µg of morphine, but no effect was observed when 100 µg of morphine was administered intravenously.5 In the current study, we observed that naltrexone prevented or reversed the decrease in hepatic GSH levels following bile duct ligation in rats, and this is the first study to demonstrate that activation of the opioid system may be involved in the modulation of the liver redox state in liver disease. Although the magnitude of the change in hepatic GSH or GSH/GSSG level may superficially seem quite small, subtle changes in cellular GSH can have a major impact on the redox potential (Eh) of the intracellular milieu and affect redox sensitive process. In the Nernst equation for calculation of the redox state (Eh = Eo + RT/2F ln [(GSSG)/(GSH)2]), the redox state is a second order function of GSH concentration.40 This means that a subtle change in concentration of GSH even without a change in GSH/GSSG ratio could alter the cellular redox state. We also observed that concentrations of S‐nitrosothiols were markedly increased in the liver of cirrhotic rats and that chronic opioid receptor blockade led to a significant decrease in hepatic S‐nitrosothiol concentrations. These observations suggest an important effect for opioid receptor blockade on the redox state of the liver (fig 5). The observation that naltrexone also attenuated liver injury, as assessed by liver enzyme alterations, is consistent with the observation that hepatic GSH is important in limiting liver injury in a number of disease models.41,42,43 While one might speculate that the decreased fibrosis observed is simply secondary to decreased liver injury, the improvement in liver function was relatively minor, and there was no effect on hepatic inflammation.
Hepatic MMP‐2 is predominantly secreted by activated HSC in the injured liver and is responsible for degrading type IV collagen, which is a major constituent of the basement membrane‐like matrix of the perisinusoidal space of Disse.8 Replacement of perisinusoidal type IV collagen with type I/III collagen is promoted by HSC derived MMP‐2 and is a key matrix remodelling event in the fibrogenic process.8 The activity of MMPs can be modulated by several endogenous factors, including the cellular redox state and S‐nitrosation,9,10,11 which is modulated in part by the interaction between intracellular GSH and the free thiol groups of proteins. Thus previous studies have shown that GSH markedly inhibits MMP‐2 activity in transformed fibroblast cells10 and in a landmark study, Okamoto and colleagues11 discovered a unique GSH dependent mechanism which directly regulates MMP activation via S‐glutathiolation of pro‐MMP. Partial suppression of MMP‐2 activity following opioid receptor blockade in BDL rats may therefore be explained by redox regulation of MMP activity or attenuation of oxidative stress induced activation of HSC after liver injury.
Injured livers were infiltrated by leucocytes, as assessed by immunohistochemical staining for CD45. However, this inflammatory response was not significantly affected by naltrexone. This shows that the hepatoprotective effect of naltrexone is independent of leucocyte infiltration and corroborates with the report of Jaume et al who showed that granulocyte depleted mice were not protected against the enhancing apoptotic effect of morphine.28 The current study might also have implications for the pathogenesis of liver disease in intravenous drug addicts, many of whom are positive for hepatitis C virus (HCV). The current data suggest that regular injection of opioids, such as heroin, together with poor nutrition, often evident in intravenous drug users, may lead to decreased hepatic GSH levels, and together with interaction of opiates on HCV replication44 may enhance or accelerate the rate of disease progression in HCV carriers. To determine whether this type of interaction is important clinically will require long term epidemiological studies but may lead to important changes in our therapeutic approach in intravenous drug users, such as administration of drugs (for example, N‐acetylcysteine or lipoic acid) which increase liver GSH and which could slow disease progression.
In the present study, we observed that treatment with naltrexone markedly decreased the development of hepatic fibrosis, and this was associated with changes in the redox state of the liver tissue which may or may not have a direct effect on HSC activation and proliferation. We also demonstrated that there is δ1 opioid receptor expression in activated HSCs in vitro. Agonist activation of δ1 and δ2 opioid receptors enhanced TIMP‐1 and procollagen I expression with no significant effect on HSC proliferation in vitro. These studies suggest that overactivation of the endogenous opioid system contributes to modulation of liver profibrogenic mechanisms in the BDL model of hepatic fibrosis, possibly through a direct effect on liver HSC function in addition to modulation of the liver redox state. This study may have direct implications for the treatment of primary biliary cirrhosis in which opioid antagonists have also been used for the treatment of pruritus.
Acknowledgements
The authors wish to thank Drs Karen Munro and Silvia Ippolito for their support in this study. This work was supported partially by the European Association for the Study of the Liver (EASL) and the British Council Exploratory Scientific Research Visits (ESRV) scheme. M R Ebrahimkhani is an EASL Sheila Sherlock post‐doctoral research fellow.
Abbreviations
BDL - bile duct ligated
MMP - matrix metalloproteinase
α‐SMA - α smooth muscle actin
GSH - glutathione
GSSG - oxidised glutathione
HSC - hepatic stellate cell
RT‐PCR - reverse transcriptase‐polymerase chain reaction
TIMP - tissue inhibitor of metalloproteinase
SDS - sodium dodecyl sulphate
NO - nitric oxide
ALP - alkaline phosphatase
ALT - alanine aminotransferase
PDGF - platelet derived growth factor
ct - cycle threshold
HCV - hepatitis C virus
Footnotes
Conflict of interest: None declared.
This work was presented at EASL 2004, Berlin, and was awarded the best poster prize.
References
- 1.Thornton J R, Losowsky M S. Opioid peptides and primary biliary cirrhosis. BMJ 19882971501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskari S A, Mani A R, Ejtemaei‐Mehr S.et al Do endogenous opioids contribute to the bradycardia of rats with obstructive cholestasis? Fundam Clin Pharmacol 200216273–279. [DOI] [PubMed] [Google Scholar]
- 3.Kiani S, Ebrahimkhani M R, Shariftabrizi A.et al Opioid system blockade decreases collagenase activity and improves liver injury in a rat model of cholestasis. J Gastroenterol Hepatol (in press) [DOI] [PubMed]
- 4.Ebrahimkhani M R, Payabvash S, Mani A R.et al Cholestasis and systemic oxidative stress: role of endogenous opioids. J Hepatol 20044051S [Google Scholar]
- 5.Roberts S M, Skoulis N P, James R C. A centrally‐mediated effect of morphine to diminish hepatocellular glutathione. Biochem Pharmacol 1987363001–3005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y T, Zheng Q S, Pan J.et al Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol 20049553–58. [DOI] [PubMed] [Google Scholar]
- 7.Campbell S J, Hughes P M, Iredale J P.et al CINC‐1 is an acute‐phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J 2003171168–1170. [DOI] [PubMed] [Google Scholar]
- 8.Benyon R C, Arthur M J. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis 200121373–384. [DOI] [PubMed] [Google Scholar]
- 9.Gu Z, Kaul M, Yan B.et al S‐nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 20022971186–1190. [DOI] [PubMed] [Google Scholar]
- 10.Tyagi S C, Kumar S, Borders S. Reduction‐oxidation (redox) state regulation of extracellular matrix metalloproteinases and tissue inhibitors in cardiac normal and transformed fibroblast cells. J Cell Biochem 199661139–151. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Akaike T, Sawa T.et al Activation of matrix metalloproteinases by peroxynitrite‐induced protein S‐glutathiolation via disulfide S‐oxide formation. J Biol Chem 200127629596–29602. [DOI] [PubMed] [Google Scholar]
- 12.Jamall I S, Finelli V N, Hee S S. A simple method to determine nanogram levels of 4‐hydroxyproline in biological tissues. Anal Biochem . 1981;11270–75. [DOI] [PubMed]
- 13.Kossakowska A E, Edwards D R, Lee S S.et al Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol 19981531895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore K P, Mani A R. Measurement of protein nitration and S‐nitrosothiol formation in biology and medicine. Methods Enzymol 2002359256–268. [DOI] [PubMed] [Google Scholar]
- 15.Fernando B, Marley R, Holt S.et al N‐acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology 199828689–694. [DOI] [PubMed] [Google Scholar]
- 16.Wittert G, Hope P, Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem Biophys Res Commun 1996218877–881. [DOI] [PubMed] [Google Scholar]
- 17.Trim J E, Samra S K, Arthur M J.et al Upstream tissue inhibitor of metalloproteinases‐1 (TIMP‐1) element‐1, a novel and essential regulatory DNA motif in the human TIMP‐1 gene promoter, directly interacts with a 30‐kDa nuclear protein. J Biol Chem 20002752257–2263. [DOI] [PubMed] [Google Scholar]
- 18.Iuvone T, Capasso A, D'Acquisto F.et al Opioids inhibit the induction of nitric oxide synthesis in J774 macrophages. Biochem Biophys Res Commun 1995212975–980. [DOI] [PubMed] [Google Scholar]
- 19.Kampa M, Hatzoglou A, Notas G.et al Opioids are non‐competitive inhibitors of NOS in T47D human breast cancer cells. Cell Death Differ 20018943–952. [DOI] [PubMed] [Google Scholar]
- 20.Scheuer P J. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 199113372–374. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi H, Rust C, Roberts P J.et al Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 1999117669–677. [DOI] [PubMed] [Google Scholar]
- 22.Oakley F, Meso M, Iredale J P.et al Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 2005128108–120. [DOI] [PubMed] [Google Scholar]
- 23.Mason D Y, Gatter K C. The role of immunocytochemistry in diagnostic pathology. J Clin Pathol 1987401042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishak K, Baptista A, Bianchi L.et al Histological grading and staging of chronic hepatitis. J Hepatol 199522696–699. [DOI] [PubMed] [Google Scholar]
- 25.Stefano G B, Hartman A, Bilfinger T V.et al Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem 199527030290–30293. [DOI] [PubMed] [Google Scholar]
- 26.Yurdaydin C, Karavelioglu D, Onaran O.et al Opioid receptor ligands in human hepatic encephalopathy. J Hepatol 199829796–801. [DOI] [PubMed] [Google Scholar]
- 27.Bergasa N V, Talbot T L, Alling D W.et al A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992102544–549. [DOI] [PubMed] [Google Scholar]
- 28.Jaume M, Jacquet S, Cavailles P.et al Opioid receptor blockade reduces Fas‐induced hepatitis in mice. Hepatology 2004401136–1143. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal D, Glasel J A. Differential effects of opioid and adrenergic agonists on proliferation in a cultured cell line. Cell Prolif 199932215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie F M, Chen Y, Winzer‐Serhan U H. Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can J Physiol Pharmacol 199876284–293. [PubMed] [Google Scholar]
- 31.Law P Y, McGinn T M, Campbell K M.et al Agonist activation of δ‐opioid receptor but not μ‐opioid receptor potentiates fetal calf serum or tyrosine kinase receptor‐mediated cell proliferation in a cell‐line‐specific manner. Mol Pharmacol 199751152–160. [DOI] [PubMed] [Google Scholar]
- 32.Benyon R C, Iredale J P, Goddard S.et al Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996110821–831. [DOI] [PubMed] [Google Scholar]
- 33.Kasahara A, Hayashi N, Mochizuki K.et al Circulating matrix metalloproteinase‐2 and tissue inhibitor of metalloproteinase‐1 as serum markers of fibrosis in patients with chronic hepatitis C. Relationship to interferon response. J Hepatol 199726574–583. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiji H, Kuriyama S, Yoshii J.et al Tissue inhibitor of metalloproteinases‐1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology 200236850–860. [DOI] [PubMed] [Google Scholar]
- 35.Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 200047710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ottesen L H, Harry D, Frost M.et al Increased formation of S‐nitrothiols and nitrotyrosine in cirrhotic rats during endotoxemia. Free Radic Biol Med 200131790–798. [DOI] [PubMed] [Google Scholar]
- 37.Toledano M B, Leonard W J. Modulation of transcription factor NF‐kappa B binding activity by oxidation‐reduction in vitro. Proc Natl Acad Sci U S A 1991884328–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall H E, Hess D T, Stamler J S. S‐nitrosylation: physiological regulation of NF‐kappaB. Proc Natl Acad Sci U S A 20041018841–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hess D T, Matsumoto A, Kim S O.et al Protein S‐nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 20056150–166. [DOI] [PubMed] [Google Scholar]
- 40.Jones D P, Carlson J L, Mody V C.et al Redox state of glutathione in human plasma. Free Radic Biol Med 200028625–635. [DOI] [PubMed] [Google Scholar]
- 41.Burk R F. Glutathione‐dependent protection by rat liver microsomal protein against lipid peroxidation. Biochim Biophys Acta 198375721–28. [DOI] [PubMed] [Google Scholar]
- 42.Jaeschke H, Smith C V, Mitchell J R. Reactive oxygen species during ischemia‐reflow injury in isolated perfused rat liver. J Clin Invest 1988811240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maellaro E, Casini A F, Del Bello B.et al Lipid peroxidation and antioxidant systems in the liver injury produced by glutathione depleting agents. Biochem Pharmacol 1990391513–1521. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Zhang T, Douglas S D.et al Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol 20031631167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]