Optical coherence tomography (OCT) is an optical imaging technique that uses infrared light reflectance and produces high resolution microstructural cross sectional images of tissues in vivo.1,2,3 The OCT probe can be inserted inside a standard transparent endoscopic retrograde cholangiopancreatography (ERCP) catheter. To date, only the epithelium of the main pancreatic duct (MPD) has been examined by OCT in humans in three studies: one post mortem4 and two ex vivo.5,6 The aim of the present prospective pilot study was to assess the feasibility of intraductal OCT in vivo during an ERCP procedure, its ability to identify changes in MPD wall structure in vivo, and its ability to differentiate non‐neoplastic from neoplastic tissue in the presence of MPD strictures.
Fifteen consecutive patients with documented or suspected MPD strictures at a previous computed tomography scan or magnetic resonance cholangio‐pancreatography (MRCP) were investigated by endoscopic ultrasonography (EUS) and ERCP; the two procedures were done at the same time under propofol sedation. Mean age of the patients was 61.9 years (range 38–78); there were 11 men and four women. Fine needle aspiration biopsy (FNAB) was planned during EUS when pancreatic neoplasia was suspected; intraductal OCT followed by brush cytology was scheduled during ERCP in all cases when the MPD stricture was confirmed. OCT findings were compared with EUS, cytology (FNAB and/or intraductal brushing), and histopathological findings in those undergoing surgical pancreatic resection. All patients gave informed consent to the endoscopic procedures and the institutional ethics committee approved OCT use in humans.
A near focus OCT probe (Lightlab Imaging, Westford, Massachusetts, USA) was used with a penetration depth of about 1 mm, resolution of approximately 10 μm, and outer diameter of 1.2 mm. An MPD stricture was confirmed by EUS and/or ERCP in 12 of 15 patients. The three patients in whom the stricture was not confirmed were excluded from the study. EUS findings suggested a neoplastic lesion in seven cases, chronic pancreatitis with segmental MPD stricture in three, neuroendocrine tumour compressing the MPD in one, and normal tissue in the remaining case. EUS guided FNAB was performed in eight patients with findings suggesting neoplasia or neuroendocrine tumour. Three patients with neoplastic findings were judged unfit for surgery at EUS. In 10 cases, MPD segments not affected by the stricture showed normal morphology at ERCP, with mild upstream dilatation (4–6 mm); in two cases with EUS findings suggesting chronic pancreatitis, minimal ductal changes were also documented at ERCP.
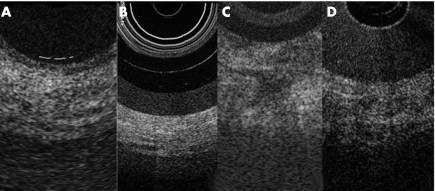
Both intraductal OCT and brush cytology were performed in 11 patients; in one patient with adenocarcinoma the stricture was too tight to pass the ERCP catheter upstream of the lesion. FNAB findings confirmed the diagnosis of adenocarcinoma and neuroendocrine tissue in all cases. Brush cytology was concordant with FNAB findings in 4/6 patients with pancreatic adenocarcinoma (66.7%) and negative for neoplastic cells in the five cases in which EUS findings did not suggest ductal adenocarcinoma. Five patients were operated on and pancreatic adenocarcinoma and neuroendocrine tumour were confirmed in the surgical specimens. OCT imaging showed a recognisable three layer structure in cases with a normal MPD and chronic pancreatitis whereas in all cases with ductal adenocarcinoma the layer structure was totally unrecognisable (fig 1). Concordance of OCT and brush cytology with the final diagnosis in patients with non‐neoplastic strictures was 100% for both techniques; in the presence of a neoplastic MPD stricture, values were 100% and 66.7%, respectively.
Figure 1 Magnification of an optical coherence tomography (OCT) image from a segmental stricture of the main pancreatic duct with non‐neoplastic and neoplastic stricture, with the OCT probe outside (A, B) and inside (C, D) the endoscopic retrograde cholangiopancreatography catheter.
In conclusion this pilot study showed that OCT is feasible during ERCP, could distinguish a non‐neoplastic from a neoplastic MPD wall with a diagnostic accuracy superior to brush cytology, but appeared to be unable to discriminate between a normal structure and chronic pancreatitis ductal changes.
Footnotes
Conflict of interest: None declared.
References
- 1.Huang D, Swanson E A, Lin C P.et al Optical coherence tomography. Science 1991 2541178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tearney G J, Brezinski M E, Bouma B E.et al In vivo endoscopic optical biopsy with optical coherence tomography. Science 19972762037–2039. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto J G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol 2003211361–1366. [DOI] [PubMed] [Google Scholar]
- 4.Tearney G J, Brezinski M E, Southern J F.et al Optical biopsy in human pancreatobiliary tissue using optical coherence tomography. Dig Dis Sci 1998431193–1199. [DOI] [PubMed] [Google Scholar]
- 5.Testoni P A, Mariani A, Mangiavillano B.et al Main pancreatic duct, common bile duct and sphincter of Oddi structure visualized by optical coherence tomography: an ex‐vivo study compared with histology. Dig Liver Dis 200638409–414. [DOI] [PubMed] [Google Scholar]
- 6.Testoni P A, Mangiavillano B, Albarello L.et al Optical coherence tomography to detect epithelial lesions of the main pancreatic duct: an ex vivo study. Am J Gastroenterol 20051002777–2783. [DOI] [PubMed] [Google Scholar]