Cirrhosis represents the third cause of mortality among Mexican people of productive age.1 Several drugs have been tested in the clinical scenario2,3 although conclusive evidence concerning drug efficacy has proven elusive.
PFD is an orally bioavailable pyridone derivative (5‐methyl‐1‐phenyl‐2‐(1H)‐pyridone) that affects a variety of profibrogenic cytokines and its mechanism of action mostly resides in its anti‐inflammatory and antifibrotic activity.4,5,6 Here we present data obtained from a pilot clinical trial evaluating the safety and efficacy of PFD in 15 patients with established advanced liver disease caused by hepatitis C virus chronic infection. This is the first report showing improvements in liver histology (that is, necrosis, inflammation, steatosis, fibrosis, and cell regeneration) 12 months after PFD therapy. Colour Doppler ultrasound guided liver biopsies were obtained at baseline and after 12 months of PFD treatment and evaluated for stage of fibrosis and grade of activity according to the modified histological activity index (HAI) of Knodell and Ishak fibrosis stage. Two pathologists who were blinded to the sequence and clinical and biochemical characteristics of the patients evaluated the biopsies.
Fifteen patients who gave written informed consent and had no history of alcohol intake were included in the final analysis based on the size of the liver biopsy satisfying international criteria. None of these patients had taken antiviral therapy previously. Mean age was 57 years (range 48–70) and there were five males. PFD was well tolerated at the dose used in this study (1200 mg/day), and only 15% of patients developed photosensitivity, rash and itching, and gastrointestinal symptoms such as nausea, abdominal discomfort, and diarrhoea. After 2–3 months of PFD therapy, adverse reactions disappeared.
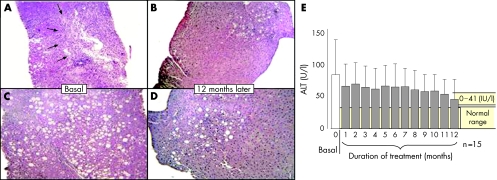
Histological differences were noted in the liver biopsies at the end of therapy. In 53.3% of patients a 2 point or greater reduction in the HAI necroinflammatory score was noted. Steatosis decreased in 60% of patients, remained unchanged in 26.7%, and worsened in 13.3%. Liver cell regeneration was detected in 70% of patients with different degrees of anti‐proliferating cell nuclear antigen immunostaining. Fibrosis was reduced in 30% of patients by the end of 12 months of treatment (table 1). Representative photomicrographs of liver biopsies from two different patients are shown in fig 1 where steatosis and chronic hepatitis with portal tract inflammation, piecemeal necrosis, and necroinflammatory foci per lobule were clearly lessened after 12 months of treatment.
Table 1 Treatment outcomes determined by fibrosis stage.
| Patient No | Genotype | Ishak staging score | |
|---|---|---|---|
| Baseline | 12 months | ||
| 1 | 1b | 4 | 1 |
| 2 | 1b | 2 | 1 |
| 3 | 1b | 6 | 6 |
| 4 | ND | 4 | 2 |
| 5 | 1b | 4 | 4 |
| 6 | 1b | 4 | 1 |
| 7 | 3a | 6 | 6 |
| 8 | 1b | 6 | 2 |
| 9 | 1a–1b | 1 | 1 |
| 10 | 1b | 6 | 6 |
| 11 | 1b | 2 | 2 |
| 12 | 1 | 5 | 5 |
| 13 | 2a–2c | 4 | 4 |
| 14 | 2a–2c | 6 | 6 |
| 15 | 1a | 5 | 5 |
HCV genotyping was conducted by specific viral DNA sequencing. Fibrosis stage was estimated using the Ishak‐Kamal index at baseline (0) and 12 months after PFD treatment.
ND, not determined.
Figure 1 Liver tissues (5 μm) were stained with haematoxylin‐eosin. (A) Patient 02/011 (hepatitis C virus (HCV) genotype 1b). Chronic hepatitis with portal tract inflammation, piecemeal necrosis, and necroinflammatory foci per lobule are clearly seen before PFD treatment. (B) Same patient as in (A), 12 months after PFD treatment. Decreased necroinflammatory activity is noticeable. (C) Patient 02/008 (HCV genotype 3a) shows marked steatosis in the first biopsy. (D) Same patient as in (B), 12 months later. Liver steatosis has decreased markedly. (E) Gradual decrease in alanine aminotransferase (ALT) levels during the course of PFD treatment (mean (SD) of 15 patients).
HCV RNA levels were measured at six months; nine patients had a decrease in viral load, two patients remained unchanged, and four patients displayed an increase in viral load compared with baseline. No patient had a sustained virological response. Median (range) values for changes in alanine aminotransferase (ALT) levels over time are given in fig 1E. A tendency to normal values was evident; 4/15 (27%) HCV patients had normalisation of ALT, 7/15 (47%) had decreased ALT values, one showed no change (7%), and three patients showed a modest increase in ALT (20%). Ultrasonographic measurements by colour Doppler imaging indicated no significant differences between spleen size before and after PFD treatment. None the less, hepatic echogenicity decreased significantly. There was no significant difference in portal vein diameter after PFD treatment but a noticeable increase in portal vein flow velocity was observed 12 months after PFD (p<0.05).
An SF‐36 survey, self‐administered by patients, demonstrated an improvement in quality of life.7
Real Time PCR was used to detect gene expression of key molecules involved in collagen turnover. mRNAs coding for profibrogenic molecules such as Col α1, transforming growth factor β, and tissue inhibitor of metalloproteinase 1 were markedly downregulated at the end of treatment.
Although promising, these results need to be verified and extended, in the context of a placebo controlled, double blind clinical trial.
Acknowledgements
This work was supported by grants from Marnac, Inc., Cell Therapy and Technology, and Intermune, Inc. We are indebted to Ing Rogelio Troyo for his invaluable assistance in the analysis of HRQOL assays.
Supplementary Material
Footnotes
Conflict of interest: None declared.
References
- 1. http://www.inegi.gob.mx/prod_serv/contenidos/espanol/bvinegi/productos/continu1 as/vitales/demograficas/2003/cua‐demo16.pdf (last accessed 17 August 17 2005)
- 2.Friedman S L, Maher J J, Bissell D M. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology 2000321403–1408. [DOI] [PubMed] [Google Scholar]
- 3.Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol 200542S22–S36. [DOI] [PubMed] [Google Scholar]
- 4.Garcia L, Hernandez I, Sandoval A.et al Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol 200237797–805. [DOI] [PubMed] [Google Scholar]
- 5.Di Sario A, Bendia E, Macarri G.et al The anti‐fibrotic effect of pirfenidone in rat liver fibrosis is mediated by downregulation of procollagen alpha1(I), TIMP‐1 and MMP‐2. Dig Liver Dis 200436744–751. [DOI] [PubMed] [Google Scholar]
- 6.Armendariz‐Borunda J, Islas‐Carbajal M C, Meza E.et al A pilot study of a novel anti‐inflammatory and anti‐fibrotic agent, pirfenidone, in patients with liver cirrosis. Hepatology 200338(suppl 1)308A [Google Scholar]
- 7.Bonkovsky H L, Woolley J M. Reduction of health‐related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology 199929264–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.