Abstract
Background
Post‐transjugular intrahepatic portosystemic stent shunt (TIPSS) hepatic encephalopathy (HE) can occur in up to one third of patients. In 5%, this can be refractory to optimal medical treatment and may require shunt modification. The efficacy of shunt modification has been poorly studied.
Aims
To evaluate the efficacy of and natural history following TIPSS modification for treatment of refractory HE.
Methods
From a dedicated database, we selected and further studied patients who had TIPSS modification for refractory HE.
Results
Over a 14 year period, of 733 TIPSS insertions, 211(29%) patients developed HE post‐TIPSS. In 38 patients, shunt modification (reduction (n = 9) and occlusion (n = 29)) was performed for refractory HE. Indications for TIPSS were: variceal bleeding (n = 32), refractory ascites (n = 5), and other (n = 1). Child's grades A, B, and C were noted in 11%, 47%, and 42% of cases, respectively. HE improved in 58% of patients and remained unchanged or worsened in 42%, with similar results for occlusions and reductions. Following shunt modification, variceal bleeding recurred in three patients and ascites in three. Twenty five patients have died (liver related in 15) at a median duration of 10.2 months. Three patients died due to procedure related complications following shunt occlusions (mesenteric infarction (n = 2) and septicaemia (n = 1)). Median survival of patients whose HE did not improve following shunt modification was 79 days compared with 278 days in patients whose did (p<0.05). No variables independently predicted response to shunt modification.
Conclusions
TIPSS modification is a useful option for patients with refractory HE following TIPSS insertion. Due to the significant risk of iatrogenic complications with shunt occlusions, shunt reduction is a safer and preferred option.
Keywords: transjugular intrahepatic portosystemic stent shunt, TIPSS modification, TIPSS occlusion, hepatic encephalopathy
Transjugular intrahepatic portosystemic stent shunt (TIPSS) has been used for the management of the complications of portal hypertension for more than 15 years. As with surgically created shunts, hepatic encephalopathy (HE) is an important limitation following TIPSS insertion. The reported incidence of post‐TIPSS HE varies depending on the criteria used for diagnosis, patient characteristics, population studied, and intensity of follow up. New or worsened encephalopathy has been reported to range from 17% to 46%. The factors said to be predictive of development of post‐TIPSS HE are presence of pre‐TIPSS HE, shunt diameter, age, female sex, liver disease of non alcoholic aetiology, and severity of liver disease.1,2,3,4,5
Post‐TIPSS HE usually responds to standard treatments, including lactulose, elimination of precipitating factors, and in more refractory cases non‐absorbable antibiotics such as neomycin and/or protein restriction.6 In a minority of patients all of these measures are ineffective. This refractory HE post‐TIPSS is a challenging clinical problem, encountered by all units performing a large numbers of TIPSS. In such patients the available options are either liver transplantation or percutaneous shunt modification to either reduce the diameter or completely occlude the shunt.3 Shunt modification reduces the percentage volume of portosystemic shunting and this can result in improvement in post‐TIPSS HE. However, the potential benefits have to be balanced against the reported incidence of procedure related complications, and risk of recurrence of the primary indication for TIPSS. There is little in the literature regarding the efficacy and safety of, and outcomes following, shunt modification and there are no controlled studies.
We aimed to evaluate the efficacy of TIPSS modification for treatment of refractory post‐TIPSS HE and to describe the natural history and outcomes of such patients.
Methods
Patients
Clinical data on all patients who had a TIPSS inserted in our centre from 1991 until the present time were prospectively collected and entered into a dedicated database. We used this database and case notes to retrospectively obtain data on all patients who required shunt modification for refractory HE.
TIPSS insertion and modification
Index TIPSS placement was performed by a standard procedure described elsewhere.7,8 All patients were administered broad spectrum antibiotics before and for at least 48 hours after the procedure.
Patients were assessed for development of HE post‐TIPSS according to the West Haven criteria.6,9 Patients who developed new or worsened HE following TIPSS were treated for correctable precipitants, prescribed lactulose, and in some cases non‐absorbable antibiotics such as neomycin and/or restricted protein diet. Lactulose was not routinely administered to patients post‐TIPSS. Those patients in whom HE remained refractory to these measures were referred for shunt modification.
TIPSS modification for refractory HE was achieved by either shunt occlusion or shunt reduction.
Shunt occlusion
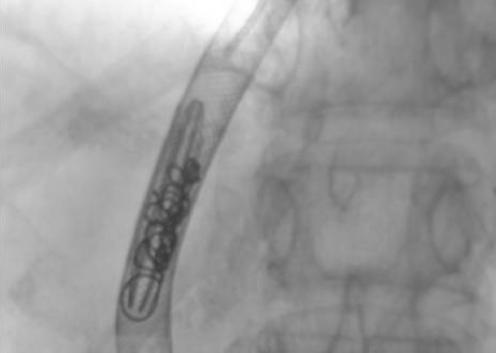
This was achieved by transjugular intrashunt placement of an IVC filter (LG Venatech filter; B Braun Medical, Boulogne Cedex, France) with or without coils (fig 1). The filter reduces the shunt lumen and prevents systemic embolisation of the coils. Care is taken during the procedure to prevent retrograde embolisation of coils into the portal vein. Following the procedure, the TIPSS gets completely occluded at varying intervals of time due to the development of thrombus within its lumen.
Figure 1 LG Venatech filter with stainless steel coils within the lumen of the transjugular intrahepatic portosystemic stent shunt.
Shunt reduction
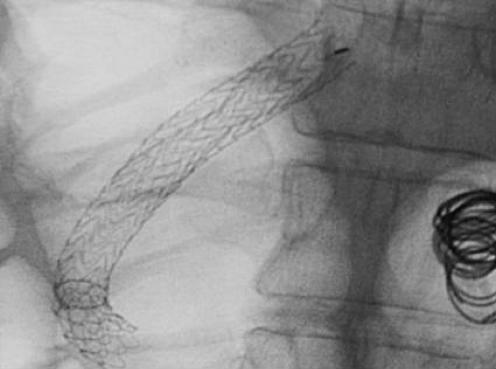
This was achieved by transjugular intrashunt placement of either reduction stents (Angiomed, Karlsruhe, Germany) (fig 2) or other devices. Although the intention is to reduce the diameter of the shunt without completely occluding the shunt, the latter is likely to occur over time due to the development of thrombus within the lumen.
Figure 2 Reduction stent within the lumen of the transjugular intrahepatic portosystemic stent shunt with coils in the gastric varices.
Definitions
Refractory HE
Refractory HE was defined as clinically significant HE which persists despite optimal medical management. HE was judged to be clinically significant if it interfered with the day to day activities of the patient(s) and/or led to repeated hospital admissions.
Early onset refractory HE
Early onset refractory HE refers to patients who developed refractory HE within six months of TIPSS insertion.
Late onset refractory HE
Late onset refractory HE refers to patients who developed refractory HE more than six months post‐TIPSS.
Responders
Patients were regarded as responders where there was a clinically significant improvement in HE following TIPSS modification. No routine ultrasound or angiographic TIPSS assessment was performed.
Non‐responders
Patients were regarded as non‐responders where there was no improvement in HE following TIPSS modification. In such patients, flow through the TIPSS was reassessed by Doppler ultrasonic examination. If persistent significant flow was found, a further attempt to modify the TIPSS lumen was made, usually by insertion of stainless steel coils.
Follow up
Doppler ultrasonography was performed within a week of TIPSS insertion with portography if indicated. Portography for assessment of shunt function was carried out routinely at six monthly intervals thereafter, or at other times if clinically indicated. Following shunt modification, improvement in HE was assessed clinically over a period of time. This was done either during the inpatient stay or in follow up outpatient visits at three or six monthly intervals. Data were collected on the patients until they were last seen, had a liver transplant, or died.
Statistical analysis
Descriptive statistics are provided as the mean (SD), median (range), or percentage for quantitative and qualitative variables, respectively. The Student's t test and χ2 test were used for statistical comparisons between the groups (responders and non‐responders, early and late onset HE). A p value < 0.05 was taken as significant. The actuarial probability of survival of the two groups (responders and non‐responders) was calculated via Kaplan‐Meier analysis, and the two curves were compared using log rank statistics. Cox regression analysis was used to determine the association between factors and response to shunt modification. Statistical analysis was performed using SSPS version 11.5 (SPSS Inc., Chicago, Illinois, USA).
Results
Baseline characteristics (table 1)
Table 1 Clinical characteristics of patients who underwent shunt modification (n = 38).
| Characteristic | |
|---|---|
| Age (y) | 63 (34–81) |
| Sex (M:F) | 26:12 |
| Bilirubin (µmol/dl) | 61.2 (47) |
| Albumin (g/dl) | 28.6 (5.9) |
| PT (s) | 14.3 (2.6) |
| Ascites (n) | 16 |
| HE (n) | 9 |
| Child Pugh score (mean (SD)) | 9.1 (1.7) |
| Child's grade (%) | |
| A | 11 |
| B | 47 |
| C | 42 |
| Aetiology of liver disease (n) | |
| Alcohol induced (ALD) | 22 |
| Primary biliary cirrhosis | 4 |
| Viral | 4 |
| Autoimmune | 2 |
| Granulomatous | 1 |
| Cryptogenic | 5 |
| Indication for TIPSS insertion (n) | |
| Variceal bleed | 32 |
| Refractory ascites | 5 |
| Other | 1 |
| Emergency/non‐emergency TIPSS | 16/22 |
| Pre‐TIPSS HE | 9 |
| Pre‐TIPSS HE (due to variceal bleed) | 5 |
| TIPSS to new/worsened HE (days) | 68 (0–1500) |
| Early HE post‐TIPSS (<6 months) | 24 |
| Pre‐TIPSS HE | 6 |
| Late new HE post‐TIPSS (>6 months) | 14 |
| Pre TIPSS HE | 3 |
Values are median (range), mean (SD), or number.
TIPSS, transjugular intrahepatic portosystemic stent shunt; HE, hepatic encephalopathy; PT, prothrombin time.
From July 1991 to September 2005, the TIPSS procedure was undertaken in 733 patients. A total of 211 (29%) patients developed HE post‐TIPSS. Thirty eight (18%) of these patients had refractory HE necessitating shunt modification (5% of all TIPSS). Median follow up following shunt modification was four months (1 day to 73 months).
In the majority of these patients, HE ranged from grade 2 to 3 and was thought to be related solely to the TIPSS. In 11 patients, additional precipitants for HE were identified and adequately treated (constipation (n = 5), infection (n = 4), hypokalaemia (n = 1) and gastrointestinal bleeding from a duodenal ulcer (n = 1)). HE was thought to be related to TIPSS if it persisted despite these measures.
Nine patients (24%) had HE prior to TIPSS. In five, this was related to variceal bleeding and it ranged in severity from grade 3 to 4. Twenty four (63%) patients developed early onset refractory HE. Fourteen patients developed late onset refractory HE.
TIPSS procedure and modification
At index TIPSS, uncovered stents were used in 31 and covered stents in seven patients (Viator Gore (WL Gore and Associates, Flagstaff, Arizona, USA) in six and Jostent (Jomed, Beringen, Switzerland) in one). Table 2 shows data regarding the index TIPSS and TIPSS modification. Following TIPSS creation, 48 modifications were performed at a median of 10 months (seven days to 72 months), with more than one procedure in nine patients. In six patients, TIPSS was modified within 30 days of TIPSS insertion. TIPSS occlusion was performed in 29 patients and TIPSS reduction in nine patients (reduction stents in six and other devices in three) depending on the shunt modification devices available.
Table 2 Data regarding index transjugular intrahepatic portosystemic stent shunt (TIPSS) and shunt modification.
| Characteristic | |
|---|---|
| Pre‐TIPSS PPG (mm Hg) | 23.3 (7.7) |
| Post‐TIPSS PPG (mm Hg) | 6.9 (3.2) |
| Mean reduction in PPG (mm Hg) | 16.2 (7.9) |
| Mean stent diameter (mm) | 10.8 (1.2) |
| Type of stent | |
| Uncovered | 31 |
| Covered | 7 |
| Shunt modification | |
| Reduction | 9* |
| Occlusion | 29 |
| Embolisation of varices | 13 |
*Reduction stent in six, other devices in three.
PPG, portal pressure gradient.
Values are mean (SD) or number.
Hepatic encephalopathy
Overall, HE improved in 15 patients following shunt occlusion and in six patients following shunt reduction procedure.
Following shunt occlusion (n = 29), HE improved in 13 patients after the initial procedure. Five patients who did not respond to the initial procedure had evidence of a patent shunt on ultrasonic examination and underwent a further procedure. The subsequent procedure resulted in resolution of HE in two further patients. Of the remaining 14 patients, two patients died within 48 hours of the procedure due to procedure related complications. Five other patients died and one had a liver transplant at a median interval of 17 days after TIPSS occlusion. In four patients, who were non‐liver transplant candidates, further intervention was not considered appropriate as the prognosis of these patients was considered to be extremely poor. Two patients died due to stroke before the improvement in HE could be assessed and were excluded from the analysis regarding response to shunt modification. These patients however were included in the survival analysis as non‐responders.
Following shunt reduction (n = 9), HE improved in three patients after the initial procedure. Of the six patients in whom HE did not respond to the initial procedure, a further procedure was performed in four patients (occlusion using coils in three and reduction stent in one). This led to an improvement in HE in three further patients. In the patient whose HE did not improve despite a second procedure, the persistently altered mental state was attributed to a combination of HE and organic brain damage due to alcohol and no further attempts at shunt reduction were made. A further shunt reduction was not attempted in two of the non‐responders to the initial procedure. One of these patients died within five days of the reduction procedure due to septicaemia and liver failure. For the other patient, a decision was made not to undertake any further procedures and the patient was put on a waiting list for liver transplant. The patient subsequently received a liver transplant five months after the shunt reduction procedure.
Overall, shunt modification resulted in resolution of HE in 21 (58%) patients within a year of the final procedure. HE recurred after initial improvement in two patients at 15 and 40 months following shunt modification and this was managed by medical treatment. HE did not improve or worsened in 15 (42%) patients.
Following Cox regression analysis, none of the pre‐shunt modification variables such as age, sex, Child Pugh score, aetiology of liver disease, indication for TIPSS, whether TIPSS was inserted for refractory bleeding (n = 17) or for prophylaxis of rebleeding (n = 15), presence of pre‐TIPSS HE, early versus late onset refractory HE, shunt diameter, type of shunt, or type of shunt modification independently predicted the response to shunt modification. Table 3 shows the characteristics of responders to shunt modification versus non‐responders.
Table 3 Characteristics of responders to shunt modification and non‐responders.
| Responders (n = 21) | Non‐responders (n = 15) | |
|---|---|---|
| Age (y) at shunt modification | 58 (34–81) | 64 (45–75) |
| Sex (M:F) | 18:3 | 7:8 |
| Child's class (A/B/C) | 2/10/9 | 2/6/7 |
| Aetiology | ||
| (ALD/non‐ALD) | 10/11 | 10/5 |
| Pre‐TIPSS HE | 5 | 4 |
| Pre‐TIPSS PPG (mm Hg) | 25.3 (7.3) | 21.5 (4.6) |
| Post‐TIPSS PPG (mm Hg) | 7.3 (3.2) | 6.9 (3.0) |
| Stent diameter (mm) | 11.1 (1.4) | 10.3 (0.5) |
| Uncovered/covered | 18/3 | 11/4 |
| TIPSS reduction | 6 | 3 |
| TIPSS occlusion | 15 | 12 |
TIPSS, transjugular intrahepatic portosystemic stent shunt; HE, hepatic encephalopathy; ALD, alcoholic liver disease; PPG, portal pressure gradient.
Values are median (range), mean (SD), or number.
Recurrence of primary indication
Variceal rebleeding
Of the 32 patients where the primary indication for TIPSS insertion was variceal bleeding, endoscopic treatment of varices was performed before shunt modification in 18 and after in two. Four patients with isolated gastric varices did not have endoscopic treatment. Embolisation of varices at the time of TIPSS modification was performed in 15 patients, six of whom had gastric varices.
Variceal bleeding recurred in three of 32 (9%) patients following shunt modification. In two Child's C patients (shunt reduction in one and shunt occlusion in one) this occurred within 24 hours of the TIPSS modification. In the first patient, variceal bleeding occurred despite apparent eradication of varices in a variceal band ligation (VBL) programme prior to shunt modification. Following successful control of bleeding with VBL, further investigation revealed that the patient had developed portal and superior mesenteric vein thrombosis following TIPSS occlusion and probably had developed varices due to that. The patient subsequently died due to intestinal infarction developing within 48 hours of TIPSS occlusion. In the second patient, who had not undergone endoscopic treatment for varices prior to shunt reduction, recurrent oesophageal variceal bleeding was controlled with VBL. However, despite subsequent regular VBL, the patient rebled from varices 38 days after TIPSS modification. This necessitated a repeat TIPSS insertion. The patient subsequently remained free of HE and variceal bleeding but died 17 months later of a chest infection and septicaemia. The third patient (Child's B; shunt reduction) developed refractory HE immediately post‐TIPSS. Gastric varices had been embolised at the time of TIPSS. A reduction stent inserted for refractory HE 12 days post‐TIPSS did not produce any improvement in HE. Subsequent shunt occlusion using coils led to initial resolution of HE but unfortunately the patient rebled from varices and died of liver failure on the day following the procedure.
Recurrent ascites
Following shunt modification, ascites recurred in three (occlusion in two and reduction in one) of the five patients (60%). In the first patient, the initial shunt reduction failed to resolve HE. Further shunt modification resolved the HE but led to recurrence of ascites. The patient subsequently succumbed to spontaneous bacterial peritonitis seven months after the TIPSS modification. The second patient required repeated hospital admissions for paracentesis of problematic ascites following shunt occlusion. The third patient developed intractable HE immediately after index TIPSS insertion necessitating shunt modification after seven days. Unfortunately, there was no improvement in HE and the ascites has worsened. The patient, who is not a transplant candidate, now has severe hepatorenal failure with an extremely poor prognosis. Of the two patients in whom ascites did not recur, one had a liver transplant four months after TIPSS modification and the other remained free of ascites and died of medical causes 75 months after shunt modification.
Survival
Of the 38 patients who had shunt modification, 25 have died (15 liver related, three due to procedure related complications) following shunt modification at a median duration of seven months (one day to 54 months). Eight patients died within a week of the procedure (liver related in four, mesenteric infarction in two, other causes in two). Following shunt modification, four patients (two responders and two non‐responders) have been transplanted for liver failure at a median duration of four months (two days to five months). One patient was transplanted within a week of final TIPSS modification. Of the patients who survived without transplant (n = 9), two were well when last seen, two require repeated hospital admissions for recurrent ascites, one has severe liver and renal failure, and four have various other medical problems (chronic renal failure (n = 1), recurrent lower respiratory tract infections (n = 1), temporal lobe disorder (n = 1), and haematological disorder (n = 1)) .
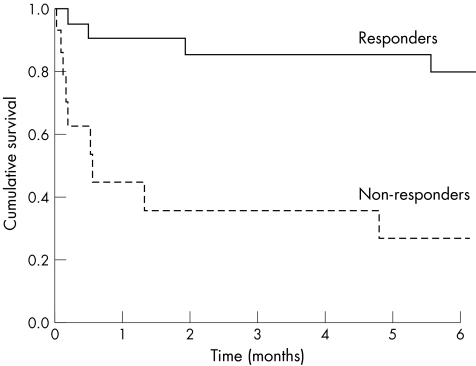
Median survival of non‐responders (n = 15) was 78 days compared with 278 days in responders (p<0.05) (fig 3). Cumulative survival of responders versus non‐responders at six weeks and six months were 91% versus 40% and 80% versus 27%, respectively. The majority of non‐responders either died (nine from liver failure, two from iatrogenic complications, one from medical causes) or required liver transplantation (n = 2). Only one of the non‐responders was alive at the time of study, but was very ill with terminal liver and renal failure.
Figure 3 Kaplan Meier curve showing survival of responders versus non‐responders (p = 0.0001).
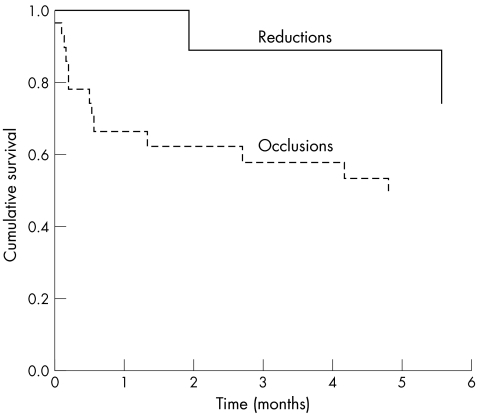
Median survival of patients following the shunt modification procedure did not appear to differ whether the patients had undergone a reduction or an occlusion procedure (62 days for reduction versus 71 days for occlusion) (fig 4, non‐significant by log rank statistic). Cumulative survival rates of patients who underwent occlusion versus reduction at six weeks and six months were 62% versus 100% and 49% versus 75%, respectively. However, the numbers involved are too small to make any valid conclusions.
Figure 4 Kaplan Meir curve showing survival of patients who underwent shunt reduction versus shunt occlusion (non‐significant by log rank statistic).
Median survival without transplantation in patients with early onset refractory HE following TIPSS insertion was 80 (1–2177) days compared with 399 (5–1921) days in patients with late onset refractory HE. Sixteen deaths (eight liver related) and three transplants occurred in the early HE group compared with nine deaths (liver related in seven and procedure related complications in two) and one transplant in the late onset HE group.
Complications
Three patients (all shunt occlusions) died due to procedure related complications within a week of shunt modification. These patients developed hypotension within hours of TIPSS occlusion. The first patient presented with oesophageal variceal bleeding, which had occurred despite apparently successful eradication of varices after VBL programme prior to TIPSS occlusion. Despite control of variceal bleeding with VBL, the patient continued to be hypotensive and developed metabolic acidosis. On computed tomography scan of the abdomen, the patient was found to have an occlusive thrombus extending from TIPSS into the superior mesenteric and portal vein with intestinal infarction. This led to the demise of the patient within 48 hours of the procedure. The second patient had changes suggestive of severe gut congestion with diffuse ooze from gut mucosa, on both gastroscopy and flexible sigmoidoscopy. Although no post mortem was performed, it is believed that this patient also died of mesenteric infarction as a consequence of mesenteric venous thrombosis developing after TIPSS occlusion. The third patient developed septicaemia and septic shock within hours of shunt modification, which set the chain of terminal events of liver failure and hepatorenal syndrome and death.
Discussion
Where medical treatment for refractory hepatic encephalopathy is unsuccessful, available options are either orthotopic liver transplantation for suitable candidates or percutaneous shunt modification of the existing TIPSS. In this series, the largest reported to date, we describe 38 patients (5% of all patients who had a TIPSS) who underwent shunt modification for refractory HE. This is in keeping with the incidence of post‐TIPSS refractory HE quoted in the literature.3,10 The majority of these patients were not transplant candidates, and shunt modification was the only remaining therapeutic option available. The only apparent factor which may have contributed to the higher incidence of refractory HE in this group may be the greater age of these patients. (median age 63 (cf mean age of 54.1 (0.6) years of patients undergoing TIPSS at our centre)).5 Other factors such as presence or absence of pre‐TIPSS HE (24% v 29%) or final PPG post‐TIPSS were not different in this cohort compared with other patients undergoing TIPSS at our centre (6.9 (3.2) v 7.2 (0.2))5 or that reported in the literature.11
Overall, shunt modification resulted in an improvement in HE in 58% of patients. There was a high incidence of procedure related complications following shunt occlusion.
Summarising the data from the published case series (table 4) and case reports,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 TIPSS modification for refractory HE has been performed in 33 patients (23 reduction and 14 occlusion procedures), with four patients undergoing more than one procedure. The underlying indication for TIPSS insertion was variceal bleeding in 25 and refractory ascites in eight. When we compared our data with the cumulative data from the literature, overall efficacy of shunt modification in our centre was lower (58% cf 71%). The reason for this is not clear but within the limits of a retrospective study our figures are likely to be more accurate on account of the larger sample size and duration of follow up. If the patients who died or had a transplant within a week of the TIPSS modification (n = 9) were excluded (that is, those patients in whom the response could not be ascertained due to these events), 70% of the patients responded to shunt modification, which is similar to the cumulative efficacy reported in the literature. We could not find any factors which independently predicted response to shunt modification. This is disappointing as such a parameter would be a very useful clinical tool. Perhaps a larger sample size may have helped. A noteworthy observation is that early onset refractory HE (within six months of TIPSS insertion) or failure of HE to improve following shunt modification were associated with a poor prognosis, with >80% mortality without a transplant in the latter group. The extremely poor prognosis of this subgroup suggests that alternative treatment strategies in this subgroup need to be developed.
Table 4 Results of four major published series on shunt modification.
| Author | Patients with HE | Indication for TIPSS | Type of shunt modification | Sustained improvement in HE | Recurrence of primary indication | Complications | |||
|---|---|---|---|---|---|---|---|---|---|
| VB | RA | Yes | No | VB | RA | ||||
| Kerlan21 | 5 | 4 | 1 | Occlusion | 4 | 1 | 1 | Balloon migration | |
| Rossle11 | 4 | 4 | Reduction | 4 | None | ||||
| Madoff22 | 8 | 5 | 3 | Occlusion, 2; reduction, 6 | 4 | 4 | 1 | 3 | No procedure related; 2 patients required further procedure (reduction, 1; occlusion, 1) |
| Saket27 | 5 | 4 | 1 | Reduction , 5 | 4 | 1 | No | None | None |
TIPSS, transjugular intrahepatic portosystemic stent shunt; HE, hepatic encephalopathy; VB, variceal bleed; RA, refractory ascites.
Variceal bleeding recurred in three (9.3%) patients following shunt modification, requiring repeat TIPSS insertion in two patients. Thus the risk of recurrence of variceal bleeding following shunt modification appears to be low and is similar to the risk of variceal rebleeding after index TIPSS insertion. This risk could be further minimised if varices were eradicated in a VBL programme before the procedure, as only one of the 18 patients in such a programme developed recurrent variceal bleeding. Intuitively, it may seem that once a patient has a functioning TIPSS in situ, it would not be possible to perform VBL. But we have found, on the basis of a previous randomised controlled study,28 that varices in such patients often appear collapsed and therefore require suction for their size to be appreciated and to allow VBL to be performed.
Following shunt modification, ascites recurred in three (60%) patients. It is of concern that one of these patients developed and died of spontaneous bacterial peritonitis. It seems that TIPSS modification, where ascites was the primary indication for TIPSS, has to be performed after very careful consideration due to the higher risk of recurrence of ascites, and should probably be avoided in transplant candidates. Recurrence of the primary indication (variceal bleed or ascites) did not appear to depend on the type of shunt modification performed. Our findings are similar to those published in the literature where variceal bleeding recurred in two (8%) and ascites recurred in five (62.5%) following TIPSS modification.12,13,14,15,16,17,18,19,20,21,22,25,26,27
An important finding in our study is the high incidence of procedure related complications following shunt occlusion. This led to three (9.3%) deaths, two of which were due to intestinal infarction (one suspected, one proven) due to development of mesenteric venous thrombosis following TIPSS occlusion. Indeed, extension of thrombus from occluded TIPSS into the main portal vein has also been reported in the literature.17 Paz‐Fumagalli and colleagues24 reported the death of a patient following intentional TIPSS occlusion using a Greenfield filter and coils. Their patient had become hypotensive immediately after the procedure and had developed progressive abdominal distension and metabolic acidosis prior to death. Although their patient did not have a post mortem examination and the death attributed to sudden severe haemodynamic alterations developing immediately after TIPSS occlusion, it is quite possible that their patient had suffered a fatal complication similar to our patients. Other complications reported in the literature, namely balloon rupture and balloon displacement, have mainly been reported with TIPSS occlusions. Based on the data available, shunt reduction rather than occlusion should be the preferred option of shunt modification. However, specifically designed reduction stents are not readily available, which limits the options available. Also, the response to shunt reduction is unpredictable and it is tempting to attribute a suboptimal clinical response to inadequate shunt reduction with a further attempt(s) at shunt modification increasing both the risk of procedural complications and the treatment costs in a group of patients with poor long term prognosis. Thus refractory HE post‐TIPSS is a challenging clinical problem for which we do not have an optimal solution.
A limitation of this study was its retrospective design. However, data on patients with TIPSS was prospectively and rigorously collected. For this study, carefully defined criteria for patient inclusion and outcomes were used, to minimise interpretive biases. Thus, as far as possible, efforts were taken to minimise any confounding variables. It is important to add that this study examined a very rare intervention and it would be difficult to conduct studies to prospectively evaluate shunt modification.
In conclusion, we have shown that TIPSS modification may be a useful option for patients with refractory HE following TIPSS insertion, particularly for those not considered transplant candidates. However, it is associated with a relatively frequent risk of complications, and of recurrence of the primary indication for TIPSS, particularly ascites. To minimise the risk of recurrence of variceal bleeding, varices should be treated endoscopically before shunt modification. Shunt reduction should be the preferred option of shunt modification to minimise procedure related complications. Poor response to shunt modification is associated with a worse outcome, and alternative strategies for this group need to be developed, especially where patients are not transplant candidates.
Acknowledgements
We would like to thank Dr NDC Finlayson, Dr AJ MacGilchrist, Dr KJ Simpson, Dr AJ Bathgate, and Dr JN Plevris whose patients were used in this study. We also wish to thank research nurses Sister Kim Macbeth, Sister Jane Holmes, and Sister Gwenyth Wilkie for their administrative assistance and help with data collection.
Abbreviations
TIPSS - transjugular intrahepatic portosystemic stent shunt
HE - hepatic encephalopathy
VBL - variceal band ligation
Footnotes
Conflict of interest: None declared.
References
- 1.Jalan R, Elton R A, Redhead D N.et al Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent‐shunt for variceal haemorrhage. J Hepatol 199523123–128. [DOI] [PubMed] [Google Scholar]
- 2.Nolte W, Wiltfang J, Schindler C.et al Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology 1998281215–1225. [DOI] [PubMed] [Google Scholar]
- 3.Rosado B, Kamath P S. Transjugular intrahepatic portosystemic shunts: an update. Liver Transpl 20039207–217. [DOI] [PubMed] [Google Scholar]
- 4.Somberg K A, Riegler J L, LaBerge J M.et al Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol 199590549–555. [PubMed] [Google Scholar]
- 5.Tripathi D, Helmy A, Macbeth K.et al Ten years' follow‐up of 472 patients following transjugular intrahepatic portosystemic stent‐shunt insertion at a single centre. Eur J Gastroenterol Hepatol 2004169–18. [DOI] [PubMed] [Google Scholar]
- 6.Atterbury C E, Maddrey W C, Conn H O. Neomycin‐sorbitol and lactulose in the treatment of acute portal‐systemic encephalopathy. A controlled, double‐blind clinical trial. Am J Dig Dis 197823398–406. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers N, Redhead D N, Simpson K J.et al Transjugular intrahepatic portosystemic stent shunt (TIPSS): early clinical experience. Clin Radiol 199246166–169. [DOI] [PubMed] [Google Scholar]
- 8.Richter G M, Roeren T, Brado M.et al Long‐term results after TIPSS with the Palmaz stent. Radiologe 199434178–182. [PubMed] [Google Scholar]
- 9.Ferenci P, Lockwood A, Mullen K.et al Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 200235716–721. [DOI] [PubMed] [Google Scholar]
- 10.Boyer T D, Haskal Z J. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 200541386–400. [DOI] [PubMed] [Google Scholar]
- 11.Rossle M, Deibert P, Haag K.et al Randomised trial of transjugular‐intrahepatic‐portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 19973491043–1049. [DOI] [PubMed] [Google Scholar]
- 12.Clarke G, Patel R, Tsao S.et al Treatment of refractory post‐transjugular portosystemic stent‐shunt encephalopathy: a novel case of stent luminal reduction. Eur J Gastroenterol Hepatol 2004161387–1390. [DOI] [PubMed] [Google Scholar]
- 13.Cox M W, Lin P H, Bush R L.et al Reversal of transjugular intrahepatic portosystemic shunt (TIPS)‐induced hepatic encephalopathy using a strictured self‐expanding covered stent. Cardiovasc Intervent Radiol 200326539–542. [DOI] [PubMed] [Google Scholar]
- 14.Forauer A R, McLean G K. Transjugular intrahepatic portosystemic shunt constraining stent for the treatment of refractory postprocedural encephalopathy: a simple design utilizing a Palmaz stent and Wallstent. J Vasc Interv Radiol 19989443–446. [DOI] [PubMed] [Google Scholar]
- 15.Gerbes A L, Waggershauser T, Holl J.et al Experiences with novel techniques for reduction of stent flow in transjugular intrahepatic portosystemic shunts. Z Gastroenterol 199836373–377. [PubMed] [Google Scholar]
- 16.Haskal Z J, Middlebrook M R. Creation of a stenotic stent to reduce flow through a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol 19945827–829. [DOI] [PubMed] [Google Scholar]
- 17.Haskal Z J, Cope C, Soulen M C.et al Intentional reversible thrombosis of transjugular intrahepatic portosystemic shunts. Radiology 1995195485–488. [DOI] [PubMed] [Google Scholar]
- 18.Hauenstein K H, Haag K, Ochs A.et al The reducing stent: treatment for transjugular intrahepatic portosystemic shunt‐induced refractory hepatic encephalopathy and liver failure. Radiology 1995194175–179. [DOI] [PubMed] [Google Scholar]
- 19.Karuppasamy K, Healey A E, Evans J C. Successful management of post‐TIPSS encephalopathy with balloon‐expandable covered stent: a controllable method to reduce shunt flow. Clin Radiol Extra 20056060–63. [Google Scholar]
- 20.Kaufman L, Itkin M, Furth E E.et al Detachable balloon‐modified reducing stent to treat hepatic insufficiency after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol 200314635–638. [DOI] [PubMed] [Google Scholar]
- 21.Kerlan R K, Jr, LaBerge J M, Baker E L.et al Successful reversal of hepatic encephalopathy with intentional occlusion of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 19956917–921. [DOI] [PubMed] [Google Scholar]
- 22.Madoff D C, Perez‐Young I V, Wallace M J.et al Management of TIPS‐related refractory hepatic encephalopathy with reduced Wallgraft endoprostheses. J Vasc Interv Radiol 200314369–374. [DOI] [PubMed] [Google Scholar]
- 23.Madoff D C, Wallace M J, Ahrar K.et al TIPS‐related hepatic encephalopathy: management options with novel endovascular techniques. Radiographics 20042421–36. [DOI] [PubMed] [Google Scholar]
- 24.Paz‐Fumagalli R, Crain M R, Mewissen M W.et al Fatal hemodynamic consequences of therapeutic closure of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol 19945831–834. [DOI] [PubMed] [Google Scholar]
- 25.Quaretti P, Michieletti E, Rossi S. Successful treatment of TIPS‐induced hepatic failure with an hourglass stent‐graft: a simple new technique for reducing shunt flow. J Vasc Interv Radiol 200112887–890. [DOI] [PubMed] [Google Scholar]
- 26.Rose S E, Katz M D. Intentional occlusion of a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol 199518109–111. [DOI] [PubMed] [Google Scholar]
- 27.Saket R R, Sze D Y, Razavi M K.et al TIPS reduction with use of stents or stent‐grafts. J Vasc Interv Radiol 200415745–751. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi D, Lui H F, Helmy A.et al Randomised controlled trial of long term portographic follow up versus variceal band ligation following transjugular intrahepatic portosystemic stent shunt for preventing oesophageal variceal rebleeding. Gut 200453431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]