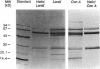
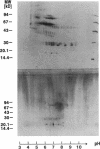
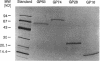
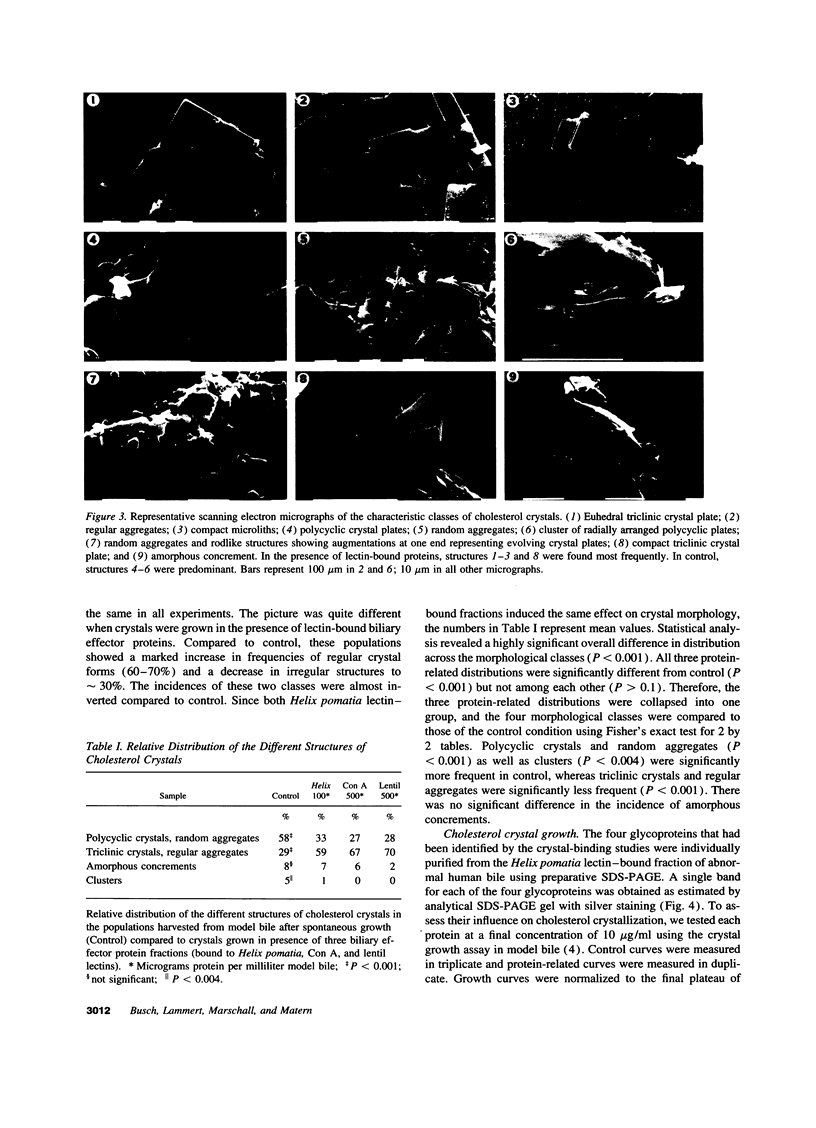
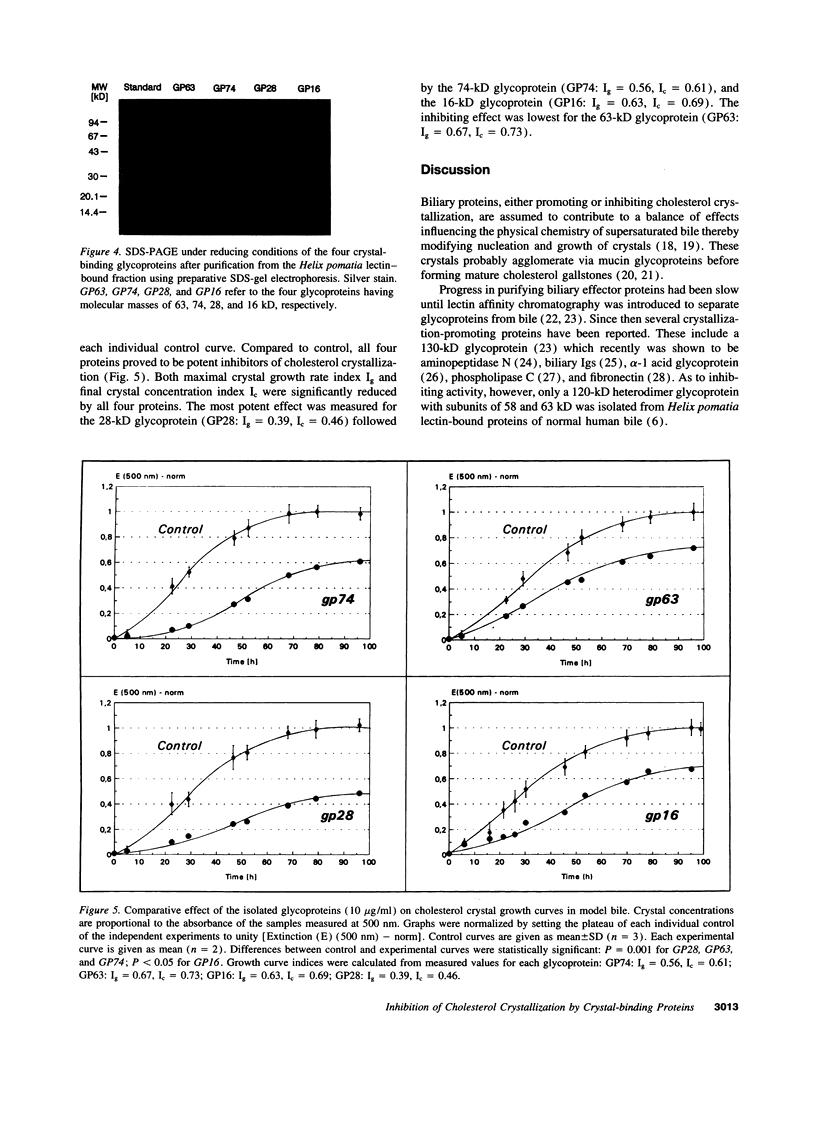
Abstract
Biliary proteins inhibiting or promoting cholesterol crystallization are assumed to play a major role in cholesterol gallstone pathogenesis. We now report a new group of biliary proteins that bind to cholesterol crystals, modify crystal morphology, and inhibit cholesterol crystallization. Various glycoprotein mixtures were extracted from abnormal human gallbladder bile using lectin affinity chromatography on concanavalin A, lentil, and Helix pomatia columns and were added to supersaturated model bile. Independent of the protein mixtures added, from the cholesterol crystals harvested, the same four GPs were isolated having molecular masses of 16, 28, 63, and 74 kD, respectively. Each protein was purified using preparative SDS-PAGE, and influence on cholesterol crystallization in model bile was tested at 10 microg/ml. Crystal growth was reduced by 76% (GP63), 65% (GP16), 55% (GP74), and 40% (GP28), respectively. Thus, these glycoproteins are the most potent biliary inhibitors of cholesterol crystallization known so far. Evidence that the inhibiting effect on cholesterol crystallization is mediated via protein-crystal interaction was further provided from scanning electron microscopy studies. Crystals grown in presence of inhibiting proteins showed significantly more ordered structures. Incidence of triclinic crystals and regular aggregates was shifted from 30 to 70% compared with controls. These observations may have important implications for understanding the role of biliary proteins in cholesterol crystallization and gallstone pathogenesis.
Full text
PDF

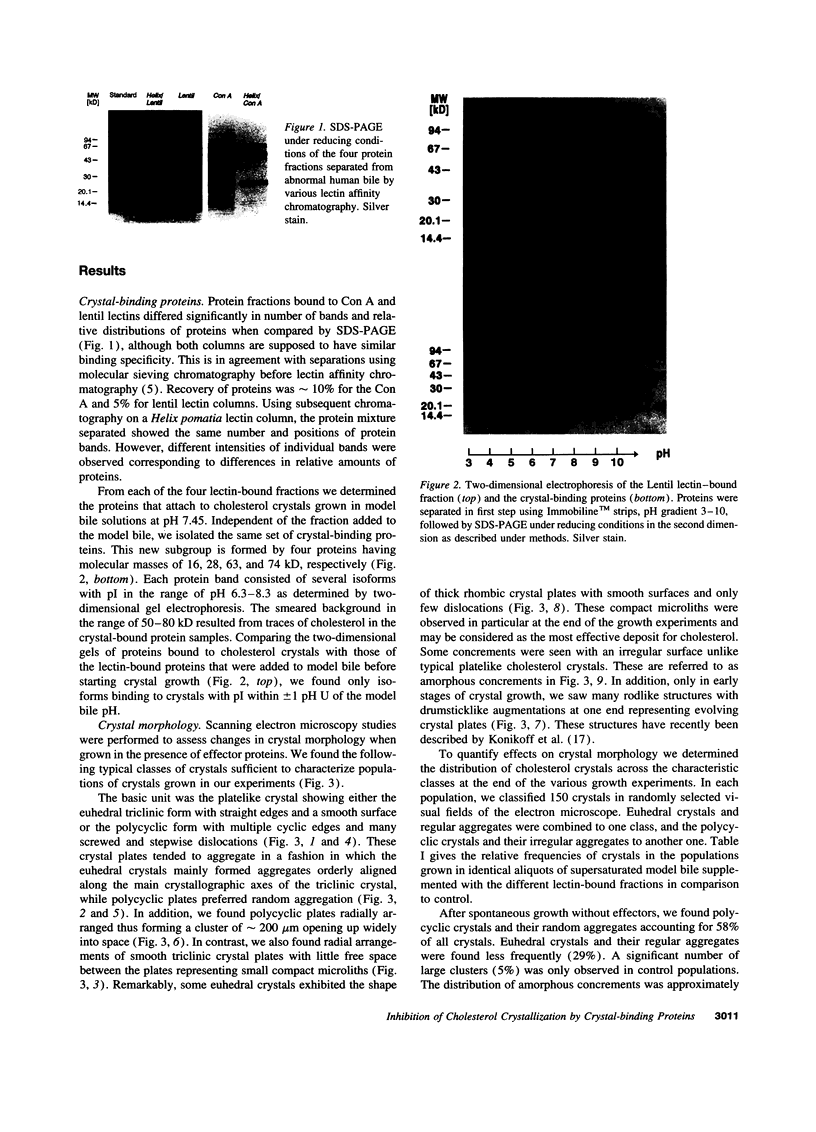


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abei M., Kawczak P., Nuutinen H., Langnas A., Svanvik J., Holzbach R. T. Isolation and characterization of a cholesterol crystallization promoter from human bile. Gastroenterology. 1993 Feb;104(2):539–548. doi: 10.1016/0016-5085(93)90424-b. [DOI] [PubMed] [Google Scholar]
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Been J. M., Bills P. M., Lewis D. Microstructure of gallstones. Gastroenterology. 1979 Mar;76(3):548–555. [PubMed] [Google Scholar]
- Bjellqvist B., Ek K., Righetti P. G., Gianazza E., Görg A., Westermeier R., Postel W. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J Biochem Biophys Methods. 1982 Sep;6(4):317–339. doi: 10.1016/0165-022x(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Busch N., Matern S. Current concepts in cholesterol gallstone pathogenesis. Eur J Clin Invest. 1991 Oct;21(5):453–460. doi: 10.1111/j.1365-2362.1991.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Busch N., Matiuck N., Sahlin S., Pillay S. P., Holzbach R. T. Inhibition and promotion of cholesterol crystallization by protein fractions from normal human gallbladder bile. J Lipid Res. 1991 Apr;32(4):695–702. [PubMed] [Google Scholar]
- Busch N., Tokumo H., Holzbach R. T. A sensitive method for determination of cholesterol growth using model solutions of supersaturated bile. J Lipid Res. 1990 Oct;31(10):1903–1909. [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C. Pathogenesis of gallstones. Am J Surg. 1993 Apr;165(4):410–419. doi: 10.1016/s0002-9610(05)80932-8. [DOI] [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiiwa K., Koga A., Yamasaki T., Shimada K., Noshiro H., Nakayama F. Fibronectin: a possible factor promoting cholesterol monohydrate crystallization in bile. Biochim Biophys Acta. 1991 Oct 15;1086(1):44–48. doi: 10.1016/0005-2760(91)90152-8. [DOI] [PubMed] [Google Scholar]
- Craven B. M. Crystal structure of cholesterol monohydrate. Nature. 1976 Apr 22;260(5553):727–729. doi: 10.1038/260727a0. [DOI] [PubMed] [Google Scholar]
- De Caro A., Multigner L., Lafont H., Lombardo D., Sarles H. The molecular characteristics of a human pancreatic acidic phosphoprotein that inhibits calcium carbonate crystal growth. Biochem J. 1984 Sep 15;222(3):669–677. doi: 10.1042/bj2220669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A. L. Antifreeze glycopeptides and peptides: interactions with ice and water. Methods Enzymol. 1986;127:293–303. doi: 10.1016/0076-6879(86)27024-x. [DOI] [PubMed] [Google Scholar]
- Feeney R. E., Burcham T. S., Yeh Y. Antifreeze glycoproteins from polar fish blood. Annu Rev Biophys Biophys Chem. 1986;15:59–78. doi: 10.1146/annurev.bb.15.060186.000423. [DOI] [PubMed] [Google Scholar]
- Gallinger S., Harvey P. R., Petrunka C. N., Ilson R. G., Strasberg S. M. Biliary proteins and the nucleation defect in cholesterol cholelithiasis. Gastroenterology. 1987 Apr;92(4):867–875. doi: 10.1016/0016-5085(87)90959-0. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Noordam C., Drapers J. A., Egbers P., Jansen P. L., Tytgat G. N. Isolation of a potent cholesterol nucleation-promoting activity from human gallbladder bile: role in the pathogenesis of gallstone disease. Hepatology. 1990 Apr;11(4):525–533. doi: 10.1002/hep.1840110402. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Stout J. P., Drapers J. A., Hoek F. J., Grijm R., Tytgat G. N. Cholesterol nucleation-influencing activity in T-tube bile. Hepatology. 1988 Mar-Apr;8(2):347–352. doi: 10.1002/hep.1840080226. [DOI] [PubMed] [Google Scholar]
- Harvey P. R., Upadhya G. A., Strasberg S. M. Immunoglobulins as nucleating proteins in the gallbladder bile of patients with cholesterol gallstones. J Biol Chem. 1991 Jul 25;266(21):13996–14003. [PubMed] [Google Scholar]
- Higuchi W. I., Liu C. L., Adachi Y., Mazer N. A., Lee P. H. Equilibrium dialysis studies on aqueous taurocholate-lecithin solutions: further validation of the method. Hepatology. 1990 Sep;12(3 Pt 2):45S–50S. [PubMed] [Google Scholar]
- Holzbach R. T., Busch N. Nucleation and growth of cholesterol crystals. Kinetic determinants in supersaturated native bile. Gastroenterol Clin North Am. 1991 Mar;20(1):67–84. [PubMed] [Google Scholar]
- Holzbach R. T., Kibe A., Thiel E., Howell J. H., Marsh M., Hermann R. E. Biliary proteins. Unique inhibitors of cholesterol crystal nucleation in human gallbladder bile. J Clin Invest. 1984 Jan;73(1):35–45. doi: 10.1172/JCI111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe A., Holzbach R. T., LaRusso N. F., Mao S. J. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science. 1984 Aug 3;225(4661):514–516. doi: 10.1126/science.6429856. [DOI] [PubMed] [Google Scholar]
- Konikoff F. M., Chung D. S., Donovan J. M., Small D. M., Carey M. C. Filamentous, helical, and tubular microstructures during cholesterol crystallization from bile. Evidence that cholesterol does not nucleate classic monohydrate plates. J Clin Invest. 1992 Sep;90(3):1155–1160. doi: 10.1172/JCI115935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Maher K., Nicholls J. F. Origin and fate of biliary sludge. Gastroenterology. 1988 Jan;94(1):170–176. doi: 10.1016/0016-5085(88)90626-9. [DOI] [PubMed] [Google Scholar]
- Montalto G., Bonicel J., Multigner L., Rovery M., Sarles H., De Caro A. Partial amino acid sequence of human pancreatic stone protein, a novel pancreatic secretory protein. Biochem J. 1986 Aug 15;238(1):227–232. doi: 10.1042/bj2380227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Abram V., Kézdy F. J., Kaiser E. T., Coe F. L. Purification and characterization of the principal inhibitor of calcium oxalate monohydrate crystal growth in human urine. J Biol Chem. 1983 Oct 25;258(20):12594–12600. [PubMed] [Google Scholar]
- Nakagawa Y., Ahmed M., Hall S. L., Deganello S., Coe F. L. Isolation from human calcium oxalate renal stones of nephrocalcin, a glycoprotein inhibitor of calcium oxalate crystal growth. Evidence that nephrocalcin from patients with calcium oxalate nephrolithiasis is deficient in gamma-carboxyglutamic acid. J Clin Invest. 1987 Jun;79(6):1782–1787. doi: 10.1172/JCI113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner G. D., Gong D., Afdhal N. H. Identification of a 130-kilodalton human biliary concanavalin A binding protein as aminopeptidase N. Gastroenterology. 1994 Mar;106(3):755–762. doi: 10.1016/0016-5085(94)90712-9. [DOI] [PubMed] [Google Scholar]
- Ohya T., Schwarzendrube J., Busch N., Gresky S., Chandler K., Takabayashi A., Igimi H., Egami K., Holzbach R. T. Isolation of a human biliary glycoprotein inhibitor of cholesterol crystallization. Gastroenterology. 1993 Feb;104(2):527–538. doi: 10.1016/0016-5085(93)90423-a. [DOI] [PubMed] [Google Scholar]
- Osuga T., Mitamura K., Miyagawa S., Sato N., Kintaka S., Portman O. W. A scanning electron microscopic study of gallstone development in man. Lab Invest. 1974 Dec;31(6):696–704. [PubMed] [Google Scholar]
- Osuga T., Portman O. W., Mitamura K., Alexander M. A morphologic study of gallstone development in the squirrel monkey. Lab Invest. 1974 Apr;30(4):486–493. [PubMed] [Google Scholar]
- Pattinson N. R., Willis K. E. Effect of phospholipase C on cholesterol solubilization in model bile. A concanavalin A-binding nucleation-promoting factor from human gallbladder bile. Gastroenterology. 1991 Nov;101(5):1339–1344. doi: 10.1016/0016-5085(91)90086-z. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Sabsay B., Veis A., Ostrow J. D., Rege R. V., Dawes L. G. Isolation of an acidic protein from cholesterol gallstones, which inhibits the precipitation of calcium carbonate in vitro. J Clin Invest. 1989 Dec;84(6):1990–1996. doi: 10.1172/JCI114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. G. Structural studies of the lipid components of bile. Hepatology. 1990 Sep;12(3 Pt 2):33S–38S. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Storey K. B., Storey J. M. Freeze tolerance in animals. Physiol Rev. 1988 Jan;68(1):27–84. doi: 10.1152/physrev.1988.68.1.27. [DOI] [PubMed] [Google Scholar]
- Takayama M., Itoh S., Nagasaki T., Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977 Aug 15;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Toor E. W., Evans D. F., Cussler E. L. Cholesterol monohydrate growth in model bile solutions. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6230–6234. doi: 10.1073/pnas.75.12.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978 Sep;19(7):924–928. [PubMed] [Google Scholar]
- Wolpers C., Hofmann A. F. Solitary versus multiple cholesterol gallbladder stones. Mechanisms of formation and growth. Clin Investig. 1993 Jun;71(6):423–434. doi: 10.1007/BF00180054. [DOI] [PubMed] [Google Scholar]
- Womack N. A. The development of gallstones. Surg Gynecol Obstet. 1971 Dec;133(6):937–945. [PubMed] [Google Scholar]
- van der Werf S. D., van Berge Henegouwen G. P., Palsma D. M., Ruben A. T. Motor function of the gallbladder and cholesterol saturation of duodenal bile. Neth J Med. 1987 Apr;30(3-4):160–171. [PubMed] [Google Scholar]