Abstract
Aim
To assess the effect of triamcinolone acetonide and preservative vehicle formulations on human retinal pigment epithelium (ARPE19) cells over a range of concentrations.
Methods
Triamcinolone acetonide, in its trade and preservative free formulations, along with the preservative vehicle were added to ARPE19 cell cultures in various concentrations (0.01–1.0 mg/ml). Cell viability was assessed using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay at day 5 after exposure. Functionality of the cultured ARPE19 cell line was confirmed by exposure to a previously characterised toxic agent, tamoxifen.
Results
The ARPE19 cell line behaved as predicted with exposure to tamoxifen. All formulations caused significant reductions in ARPE19 cell viability at the highest concentrations (1.0 mg/ml for triamcinolone preparations and undiluted vehicle). Cell viability was reduced to the greatest degree in trade formulation triamcinolone, less so by the vehicle, and least by preservative free triamcinolone. At lower concentrations no significant effect on cell viability was observed, although cell viability was found to be inversely proportional to increasing concentration of all tested reagents
Conclusions
Both the trade and preservative free formulations of triamcinolone acetonide as well as the vehicle result in cell loss at in vitro concentrations of 1 mg/ml. Although this represents theoretical vitreous concentrations achieved with current widespread therapeutic use it probably does not indicate the actual exposure of cells in their biological milieu. That cell viability was reduced most in the trade formulation suggests a possible potentiated inhibitory toxic effect of triamcinolone acetonide and vehicle at higher concentrations.
Keywords: triamcinolone acetonide, preservative vehicle formulations
Triamcinolone acetonide (9α‐fluoro‐16α‐hydroxyprednisolone) is a synthetic crystalline corticosteroid with potent anti‐inflammatory properties. Various trade formulations are approved for the therapy of inflammatory conditions throughout the body and include tablets, oral syrups, and dermatological preparations. An injectable suspension, known in the United States by the trade name Kenalog (Bristol‐Myers‐Squibb, NY, USA), is approved for intra‐articular and intramuscular use but not for use in or around the eye. None the less, triamcinolone acetonide has been used with efficacy for many decades to treat various ocular inflammatory conditions by periocular injection. More recently, it has been administered directly into the eye as a visual aid in intraocular surgery and as an effective therapeutic agent in various retinal vascular and inflammatory diseases that were, for the better part, previously untreatable.1,2,3,4 In short order it has become the most widely administered intravitreal corticosteroid agent. Unlike other corticosteroids which also exhibit potent anti‐inflammatory properties, triamcinolone acetonide has the added advantage of being constituted in a sustained release crystalline form that acts as a depot when injected into the vitreous humour.
The now widespread use of intraocular Kenalog has raised concerns regarding its potential toxicity. Along with the typical side effects resulting from ocular needle penetration reports of sterile inflammatory reactions have been noted.5 The most commonly reported intravitreal dose of triamcinolone acetonide is 4 mg but total dose and preparation varies throughout the literature.6 Current recommendations for the injection of intravitreal triamcinolone include strict adherence to sterile technique and postoperative monitoring of infection and intraocular pressure elevation. The trade formulation, generally administered well away from the eye, contains preservatives and suspending agents including benzyl alcohol, commonly referred to as the vehicle, which may be potentially toxic to intraocular tissue when injected intravitreally.7 The neurosurgical literature warns against the use of trade formulations of steroids in intrathecal therapy as the benzyl alcohol in the suspending vehicle has demonstrated toxicity to neural tissue.8 Intrathecal injection with direct exposure to neural tissue is analogous in good degree to intraocular neural tissue exposure by intravitreal injection.
Both in vitro and in vivo studies have produced contradictory results regarding a precise safety profile.9,10,11,12 It remains unclear whether preservative free formulations of triamcinolone would be less toxic to the eye. Currently, the RPE (ATCC cell line, ARPE‐19) is the only normal human retinal cell line that can be maintained for laboratory study.13 The cell line exhibits morphological and functional characteristics similar to those of RPE cells in vivo and has thus become an important tool for studies of ocular toxicity. The present study evaluates the in vitro toxicity of the various formulations of triamcinolone acetonide along with the preservative vehicle on human RPE cells.
Materials and methods
The human retinal pigment epithelial cell line, ARPE19, was obtained from the American Type Culture Collection (Manassas, VA, USA). It was maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium containing 1.2 g/l sodium bicarbonate, 2.5 mM l‐glutamine, 15 mM HEPES and 0.5 mM sodium pyruvate, supplemented with 10% fetal bovine serum and 1% antibiotic‐antimycotic solution. Cell suspensions with a cell volume of 5000 cells/ml were seeded onto tissue culture plates. After overnight incubation at 37°C in an environment containing 95% O2 and 5% CO2 the cells were washed gently with phosphate buffered saline, observed for attachment to their substrate, and fresh culture medium was reintroduced. All cultures were subsequently maintained in fresh media at 48 hour intervals and cultivated at 37°C in a humidified environment containing 5% CO2.
Preservative free triamcinolone acetonide was obtained from Leider's Pharmacy (San Jose, CA, USA). The preservative free formulation had been constituted per pharmacy by mixing appropriate amounts of US Pharmacopeia grade triamcinolone acetonide powder with sodium carboxymethylcellulose (0.75%), polysorbate 80 (0.04%), and sterile sodium chloride. The pH was adjusted to a range of 5.0–7.5 with sodium hydroxide or hydrochloric acid. The solution was homogenised and autoclaved.
Trade triamcinolone acetonide (Kenalog, Bristol‐Myers‐Squibb, New York, USA) and preservative free triamcinolone acetonide (Leider's Pharmacy, San Jose, CA USA) formulations were serially diluted to the appropriate concentrations (0.01 mg/ml, 0.1 mg/ml, and 1.0 mg/ml). Concentrations studied reflected dilutional factors of current intravitreal dosing regimens. All vials had been stored and kept in opaque wrapping at all times to prevent light exposure and destabilisation of the reagents. The vehicle was constituted according to specifications used in the trade formulation, specifically benzyl alcohol (0.9%), sodium carboxymethlycellulose (0.75%), and polysorbate 80 (0.04%). The vehicle was diluted to the same concentrations studied for the triamcinolone formulations.
The behaviour of the RPE cell line was confirmed by observing predicted responses when exposed to a previously studied toxic reagent, tamoxifen (AstraZeneca Pharmaceuticals LP Wilmington, DE, USA).14 Trade and preservative free trimacinolone acetonide at the corresponding concentrations (0.01 mg/ml, 0.1 mg/ml, and 1.0 mg/ml) as well as the vehicle (undiluted, 1:0, diluted by a factor of 10, 1:10, and by a factor of 100, 1:100) were then added to the RPE cells (day 0). Cells were cultured in quadruplicate in the presence of the three test compounds and in the absence of test compounds (control).
After 120 hours (day 5), cells were washed and the extent of cell growth was assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay (CellTiter‐96 Non‐Radioactive Cell Proliferation Assay; Promega Corporation, Madison, WI, USA). A volume of 15 µl of MTT was added to each well and mixed. Plates were incubated for 4 hours at 37°C in a humidified, 5% CO2 atmosphere after which 100 µl of solubilisation/stop solution were added to each well. Formazan levels, corresponding to the number of viable cells, were quantified using a microplate reader (MTX Lab Systems, Vienna, VA, USA) at a wavelength of 570 nm. Results were expressed as mean units of absorbance of MTT at 570 nm. As each group originated from a single pool of cells, the absorbance reflected the proportion of viable cells of the same population over time and in relation to the reagent concentrations tested. The control and treated group values were compared and statistical significance was achieved for p<0.05.
Results
The cell culture line behaved as predicted with exposure to various tamoxifen concentrations (mean absorbance: control, 0.535; 25.0 µM, 0.082; 2.5 µM, 0.404; 0.25 µM, 0.381). Only the highest concentrations of tamoxifen (25.0 µM) produced a statistically significant reduction (p = 0.04) in the number of ARPE19 cells at 5 days, at a concentration range that has demonstrated cellular toxicity in the literature.14,15
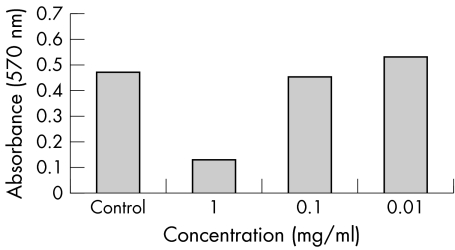
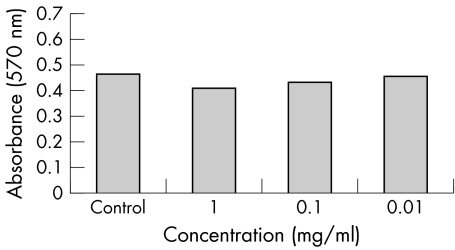
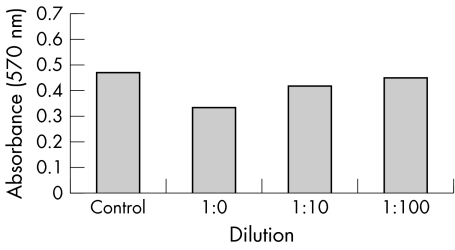
Both triamcinolone formulations along with the vehicle were found to cause significant reductions in number of ARPE19 cells only at the highest range of concentrations (1.0 mg/ml for the triamcinolone acetonide formulations and the undiluted vehicle) (figs 1–3). The reduction was of greatest magnitude in the trade formulation triamcinolone group (mean absorbance 0.132, p<0.01), followed by the vehicle group (mean absorbance 0.334, p<0.01) followed by the preservative free triamcinolone group (mean absorbance 0.412, p = 0.03). At the other dilutions the degree of cell reduction in all groups was found to be directly proportional to increasing reagent concentration but not statistically significant.
Figure 1 ARPE‐19 cell viability, reflected as MTT absorbance at 570 nm, after 5 days' exposure to various concentrations of trade formulation triamcinolone acetonide.
Figure 2 ARPE‐19 cell viability, reflected as MTT absorbance at 570 nm, after 5 days' exposure to various concentrations of preservative free triamcinolone acetonide.
Figure 3 ARPE‐19 cell viability, reflected as MTT absorbance at 570 nm, after 5 days' exposure to various dilutions of preservative vehicle.
Phase contrast microscopy demonstrated clumping of triamcinolone crystals with the trade formulation versus more uniform dispersion with the preservative free formulation at therapeutic concentrations (fig 4). This effect was also present but less pronounced at lower concentrations of triamcinolone (fig 5). RPE cells were notably absent near the larger crystal clumps of the trade formulation and more uniformly distributed among the smaller preservative free triamcinolone crystals.
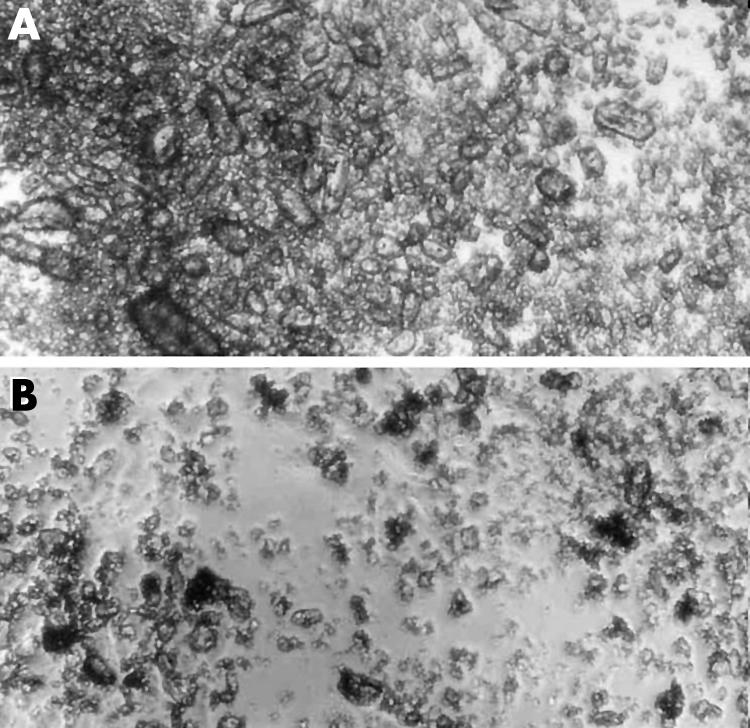
Figure 4 Triamcinolone acetonide, 1.0 mg/ml, in cell culture media. Phase microscopy 100× magnification. Trade formulation triamcinolone (A) demonstrates marked clumping of large crystals versus more uniformly dispersed smaller crystals in the preservative free formulation (B).
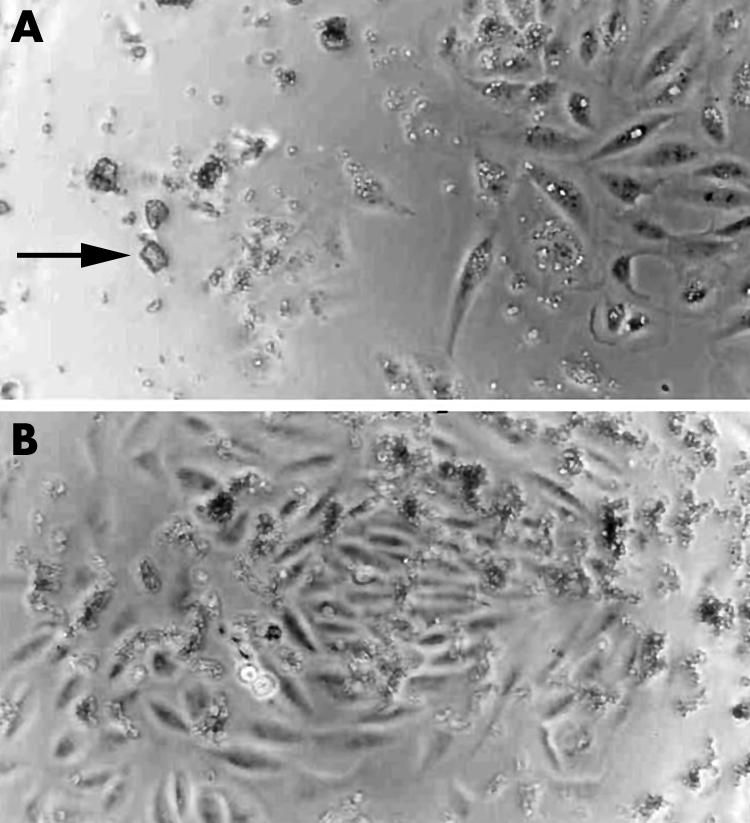
Figure 5 Triamcinolone acetonide, 0.10 mg/ml, in cell culture media. Phase microscopy 100× magnification (A). Retinal pigment epithelial cells are noticeably absent near the larger crystal clumps of the trade formulation (arrow). (B) A more uniform dispersion of triamcinolone crystals is noted in the preservative free formulation. Retinal pigment epithelial cells are distributed evenly and in the vicinity of the smaller crystals.
Discussion
Despite the now extensive use of intravitreal triamcinolone acetonide, mainly in its trade formulation with preservative vehicle, published data on toxicity for intraocular use remain limited. Clinical reports demonstrate that patients experience rapid and dramatic improvement in visual acuity in otherwise refractory conditions such as macular oedema, venous occlusive disease, and exudative macular degeneration.1,2,3,4 For this reason adverse effects such as sterile endophthalmitis, elevated ocular pressure, and the risk of intraocular injection are considered, but more often than not tolerated by patients who perceive benefit. Such favourable outcomes have generated interest on the part of the United States' National Eye Institute and other multicentre collaboratives in the clinical study of this beneficial drug but, to date, there remains no approved formulation for injection into the eye.
A more comprehensive understanding of the physiological effects upon exposure of various formulations of triamcinolone to human retinal pigment epithelial cells, which in recent years have served as a model for ocular toxicity, may contribute to the establishment of an accurate safety profile. It is not clear whether the current trade formulation of the drug, formulated for intra‐articular and intramuscular injection, or the suspending preservative vehicle alone is toxic to the eye and responsible for the cases of sterile endophthalmitis observed.
In this study, we observed that both the trade and preservative free triamcinolone formulations along with the preservative vehicle found in the trade formulation cause significant cell loss at therapeutic vitreous concentrations of the drug. The effect is most pronounced with the trade formulation, less so by the vehicle, and least by the preservative free formulation. A gradient effect inversely related to cell viability was noted in the various concentrations of the vehicle and triamcinolone formulations. Recently, Yeung, et al published their findings in RPE cell culture studies which demonstrated a similar gradient effect at similar concentrations of the trade formulation of triamcinolone. In their study the preservative free formulation was not studied but the trade formulation was found to have significant cell loss at all study concentrations.6 Significant cell loss was not noted, however, with exposure to benzyl alcohol, although the preservative vehicle in its complete form was not studied.
Why triamcinolone alone should display cell loss at higher concentrations is not clear but dexamethasone, an analogous but more potent corticosteroid, has a well demonstrated antiproliferative effect at higher concentrations on RPE cells.16 The vehicle, whose main active component is benzyl alcohol and is known to be toxic to biological cells, also appears to be inhibitory at injected vitreous concentrations. The trade formulation, which essentially combines preservative free triamcinolone with vehicle, appears to demonstrate a potentiated inhibitory effect on cell viability. This may very well be the result of localised toxic effects from poor solubility of the formulation as observed in our phase contrast microscopy images and as demonstrated by others in vivo.17 It is possible that some component of the vehicle, perhaps the alcohol ligand and associated polarity of the benzyl alcohol molecule, interacts with the triamcinolone molecule affecting its solubility and thereby leading to the clumping of crystals observed. Notably, RPE cells were absent near the larger less soluble trade formulation crystals.
Although significant RPE cell loss is suggested here at therapeutic concentrations of both the preservative free and trade formulations of triamcinolone it should not be extrapolated that in vivo cell loss actually occurs. In vitro concentrations are often higher than those observed in vivo, especially with regard to the RPE where in theory the triamcinolone particles must pass through the internal limiting membrane and the neurosensory retina undiluted to exert the effect noted here. Additionally, cells in their normal biological milieu benefit from cell rescue mechanisms and dynamic biological conditions that cannot be replicated in laboratory models. Hence, it is highly unlikely that RPE cells in the eyes of our patients are subject to the toxic concentrations levels suggested in this study. None the less, the preservative free formulation of triamcinolone may be preferable for intraocular administration. In situations where the RPE may be exposed directly to triamcinolone as in macular holes, retinal breaks, or in subretinal administration of triamcinolone caution might be observed with use of any of the triamcinolone formulations.
Other mechanisms may be responsible for the sterile inflammatory reactions observed with intraocular administration of triamcinolone. Host susceptibility, instability of the medication after exposure to light, reuse of medication vials, or even conditions related to packaging of the medicine may be to blame. Although RPE cells were studied here, different cells in the retina under biological conditions may respond differently to triamcinolone exposure. As additional research interest is expressed in the study of triamcinolone we will probably achieve a better understanding of the safety profile of this remarkably beneficial ophthalmic drug, thereby elucidating many of our early concerns.
Acknowledgements
We would like to thank Maryanne Hunt, PhD, in the statistics department at Rollins College for her assistance in statistical models of the data.
Abbreviations
MTT - 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
RPE - retinal pigment epithelium
References
- 1.Jonas J B, Sofker A, Hayler J.et al Intravitreal crystalline triamcinolone acetonide as an additional tool in pars plana vitrectomy for complicated proliferative vitreoretinopathy? Acta Ophthalmol Scand 200381663–665. [DOI] [PubMed] [Google Scholar]
- 2.Danis R P, Ciulla T A, Pratt L M.et al Intravitreal triamcinolone acetonide in exudative age‐related macular degeneration. Retina 200020244–250. [PubMed] [Google Scholar]
- 3.Jonas J B, Akkoyun I, Kreissig I.et al Diffuse diabetic macular oedema treated by intravitreal triamcinolone acetonide: a comparative, non‐randomised study. Br J Ophthalmol. 2005 Mar 89321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas J B, Akkoyun I, Kamppeter B.et al Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye 20051965–71. [DOI] [PubMed] [Google Scholar]
- 5.Nelson M L, Tennant M T, Sivalingam A.et al Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 200323686–691. [DOI] [PubMed] [Google Scholar]
- 6.Yeung C K, Chan K P, Chiang S W.et al The toxic and stress responses of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci 2003445293–5300. [DOI] [PubMed] [Google Scholar]
- 7.Hida T, Chandler D, Arena J E.et al Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol 1986101190–195. [DOI] [PubMed] [Google Scholar]
- 8.Hetherington N J, Dooley M J. Potential for patient harm from intrathecal administration of preserved solutions. Med J Aust 2000173141–143. [DOI] [PubMed] [Google Scholar]
- 9.McCuen BW I I, Bessler M, Tano Y.et al The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol 198191785–788. [DOI] [PubMed] [Google Scholar]
- 10.Perry H T, Cohn B T, Nauheim J S. Accidental intraocular injection with Dermojet syringe. Arch Dermatol 19771131131 letter. [PubMed] [Google Scholar]
- 11.Modarres M, Parvaresh M M, Peyman G A. Accidental sub‐retinal injection of triamcinolone acetonide. Ophthalmic Surg Lasers 199829935–938. [PubMed] [Google Scholar]
- 12.Kivilcim M, Peyman G A, El‐Dessouky E S.et al Retinal toxicity of triamcinolone acetonide in silicone‐filled eyes. Ophthalmic Surg Lasers 200031474–478. [PubMed] [Google Scholar]
- 13.Dunn K C, Aotaki‐Keen A E, Putkey F R.et al ARPE19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 199662155–169. [DOI] [PubMed] [Google Scholar]
- 14.Mannerstrom M, Zorn‐Kruppa M, Diehl H.et al Evaluation of the cytotoxicity of selected systemic and intravitreally dosed drugs in the cultures of human retinal pigment epithelial cell line and of pig primary retinal pigment epithelial cells. Toxicol In Vitro 200216193–200. [DOI] [PubMed] [Google Scholar]
- 15.Mannerstrom M, Maenpaa H, Toimela T.et al The phagocytosis of rod outer segments is inhibited by selected drugs in retinal pigment epithelial cell cultures. Pharmacol Toxicol 20018827–33. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Kao Y.et al A comparative study of effects of antiproliferative drugs on human retinal pigment epithelial cells in vitro. J Ocular Pharmacol Ther 200218251–263. [DOI] [PubMed] [Google Scholar]
- 17.Bakri S, Shah A.et al Intravitreal preservative‐free triamcinolone acetonide for the treatment of macular oedema. Eye 20041–3. [DOI] [PubMed]