Abstract
Aim
To elucidate the role of CCR2/MCP‐1 in corneal inflammation.
Methods
A cauterisation induced corneal inflammation model was used. The corneas were cauterised with silver nitrate in CCR2 knockout (KO) mice, MCP‐1 KO mice, and control mice. Clinical signs such as corneal oedema and opacity were examined 96 hours after cauterisation and the phenotypes of the cells infiltrating the cornea were analysed by flow cytometry. Corneal inflammation in neutrophil depleted mice was also analysed.
Results
After cauterisation both CCR2 KO and MCP‐1 KO mice showed the same levels of corneal oedema and opacity as control mice. Flow cytometry revealed that in control mice most of the infiltrating cells were neutrophils and macrophages, whereas in both CCR2 KO mice and MCP‐1 KO mice, the number of macrophages infiltrating the cornea were markedly reduced. However, prominent infiltrates of neutrophils were still observed in the cornea in CCR2 KO mice and MCP‐1 KO mice. The depletion of neutrophils significantly reduced the oedema and opacity induced in the cornea by cauterisation.
Conclusion
The CCR2 and MCP‐1 molecules are not essential for cauterisation induced corneal inflammation. Neutrophils, rather than migrated macrophages, are the final effector cells involved in inducing inflammation in this model.
Keywords: immunocytochemistry, knockout animals, neutrophils, macrophages, mice
Excessive inflammation induces an irreversible opacity in the cornea.1 In general, corneal inflammation was initiated by so called “innate immunity,” especially neutrophils and macrophages.2 They are thought to be the possible cellular targets to analyse corneal inflammation. Corneal expression of pro‐inflammatory cytokines and chemokines in response to injury or infection leads to the recruitment of cells of the innate immune system.3 Several reports have recently demonstrated that bone marrow derived antigen presenting cells (APC) existed in and trafficked through even the non‐treated cornea.4,5 These cells, along with resident corneal APCs, migrate to regional lymph nodes to induce specific immune reactions.6
The roles of chemokines in corneal inflammation have been a focus of interest.7 Corneal infection was shown to induce CC and CXC chemokines in the stroma and in numerous bone marrow derived inflammatory cells such as neutrophils,8 lymphocytes, and monocytes/macrophages.9
CCR2 is mainly expressed by cells of the macrophage lineage10 and is the receptor for monocyte chemoattractant protein‐1 (MCP‐1).11 Despite having normal numbers of circulating leucocytes and resident macrophages, both CCR2 KO and MCP‐1 knockout (KO) mice fail to recruit macrophages to inflammatory sites.12,13,14 To investigate the role of macrophage attracting chemokines in cauterised induced corneal inflammation, we examined the phenotypes of cells infiltrating this tissue in both CCR2 KO and MCP‐1 KO mice.
Materials and methods
Mice
Female 8 week old C57BL/6 mice were purchased from Japan SLC, Shizuoka, Japan, and kept under specific pathogen free conditions at Kyushu University. CCR2 KO (−/−) mice were generated by homologous recombination as previously described.15 CCR2 KO mice and CCR2 WT (+/+) littermate controls were generated from crosses between heterozygous (CCR2 +/−; 50/50 C57BL/6×129/Sv) mice. MCP‐1 KO mice were kindly provided by Dr Barrett J Rollins (Children's Hospital, Harvard Medical School, MA, USA)14 and back crossed for more than eight generations on a C57BL/6 background. We thus used C57BL/6 mice as controls in experiments with MCP‐1 KO mice. All animals were treated in accordance with the ARVO statement for the use of animals in ophthalmic and vision research.
Induction and evaluation of corneal inflammation
Corneal inflammation was induced as described previously.16 The right cornea of each mouse was cauterised with plastic sticks containing 0.4 g/ml silver nitrate, 1 mm in diameter at the centre of the cornea. After 96 hours, corneal oedema and opacity were evaluated by stereoscopic microscopy, using a slit lamp in a double blind fashion. Lesions were scored for corneal oedema and opacity on scales of increasing severity from 0 to +4 as described before.2 Experiments were repeated four times with comparable results. Following clinical evaluation, mice were sacrificed and their right eyes removed. Three eyes were pooled and used for flow cytometry studies and remaining eyes were used for histological evaluation.
Isolation of corneal infiltrating cells
Inflammatory cells were isolated from corneas according to the procedure used for hepatic lymphocytes,17 with some modifications.2 Three corneas were pooled to obtain enough viable cells for flow cytometry. The corneas were teased away with scissors and shaken at 37°C for 40 minutes with 0.5 mg/ml collagenase type D (Boehringer Mannheim, Germany). The supernatants were collected and viable cells were counted using trypan blue dye exclusion. A total of 18 corneas were examined as six pools of three individual eyes.
Antibodies and reagents
The following reagents were used for flow cytometry: phycoerythrin (PE) conjugated anti‐CD11b mAb (clone M1/70.15); and fluorescein isothiocyanate (FITC) conjugated anti‐F4/80 mAb (clone A3‐1) from Caltag Lab Inc (South San Francisco, CA); FITC conjugated anti‐Gr‐1 mAb (clone RB6‐8C5); and propidium iodide staining solution from PharMingen (San Diego, CA, USA); FITC conjugated anti‐mouse neutrophil mAb from Cedarlane Lab (Ontario, Canada). The antibodies used for labelling sections were anti‐F4/80 mAb (clone A3‐1; Serotec, Oxford, UK) and Cy5 conjugated goat anti‐rat IgG Ab (Zymed Lab, San Francisco, CA, USA).
The anti‐Gr‐1 mAb for in vivo neutrophil depletion was prepared in our laboratory. Five week old female SCID mice were given intraperitoneal injections of pristane purchased from Sigma (St. Louis, MO, USA). Ten days later the mice received intraperitoneal inoculations of hybridoma cells (RB6‐8C5), which were kindly provided by Dr F Sendo (Yamagata University, Yamagata, Japan). Two weeks after the inoculation, anti‐mouse Gr‐1 mAb was purified from ascites of SCID mice using ammonium sulfate treatment.18
Flow cytometry
Intraocular infiltrating cells were adjusted to the designated concentrations for two colour labelling.2 Macrophages within the intraocular infiltrate were identified as cells double labelled with PE‐anti‐CD11b mAb and FITC conjugated anti‐F4/80 mAb.19,20 The cells stained with both PE‐anti‐CD11b mAb and FITC‐anti‐Gr‐1 mAb/FITC‐anti‐neutrophil mAb were identified as neutrophils. Analysis was performed on an EPICS XL flow cytometer (Beckman Coulter, Mannheim, Germany), using FlowJo software (Tree Star, San Carlos, CA, USA). Propidium iodide staining was used to discriminate dead cells. The number of macrophages and neutrophils infiltrating the cornea was calculated from the percentage of each population measured by flow cytometry and the total number of viable cells counted by trypan blue dye exclusion. The gate within which the F4/80 and Gr‐1 positive cells were counted was set using an isotype matched control antibody (FITC conjugated rat IgG2b; Caltag Lab Inc, South San Francisco, CA, USA). It was reported that keratocytes did not express either CD11b or F4/80.21 CD11b‐F4/80 (double negative) cells, possibly containing keratocytes, were constantly less than 2–3% of total isolated viable cells.
Depletion of neutrophils
To deplete neutrophils, C57/BL6 mice were injected twice intraperitoneally with 400 μg anti‐Gr‐1 mAb 24 hours before and 24 hours after cauterisation. The effect of anti‐Gr‐1 mAb administration on circulating neutrophils was assessed by counting the total number of peripheral white blood cells, stained with May‐Grunwald Giemsa and we have confirmed completely depletion of neutrophils.18 The depletion of neutrophils was also confirmed in the splenocytes by flow cytometry, 2 days after treatment, and 95% of neutrophil mAb positive cells were constantly depleted.
Fluorescent microscopy and image analysis
For microscopic analysis, eyes were enucleated and prepared for freezing and cryostat sectioning by embedding in OCT medium (Tissue‐Tek, Sakura Finetechnical Co Ltd, Tokyo, Japan). Cryostat sections were cut (8–10 μm), and fixed in acetone for 2 minutes. Sections were incubated for 45 minutes with either F4/80 antibody or a control IgG at a 1:100 dilution. As a secondary antibody, Cy5 labelled goat anti‐rat IgG at a dilution of 1:100 was incubated with sections for 20 minutes. Cell nuclei were stained with propidium iodide at a 1:1250 dilution. Sections were observed with an epifluorescence microscope (BX50, Olympus, Tokyo, Japan).
Statistics
Significant differences in the grade of corneal inflammation were assessed using the Student's t test. A value of p⩽0.05 was considered to be significant.
Results
Both CCR2 and MCP‐1KO mice show equivalent level of corneal inflammation
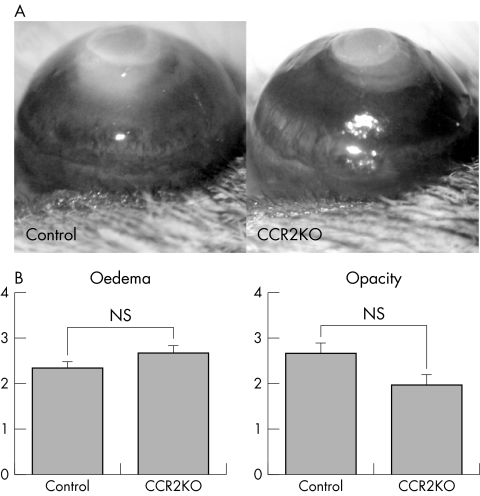
Initially, we examined corneal inflammation in CCR2 KO mice. Ninety six hours after cauterisation, both control and CCR2 KO mice showed prominent oedema and central opacity (fig 1A). The degree of either oedema or opacity of CCR2 KO mice was equivalent compared to the control mice (fig 1B).
Figure 1 Corneal inflammation in CCR2 KO mice. (A) Photographs of cauterised corneas after 96 hours. (B) The clinical evaluation of corneal inflammation. Wild type (WT) mice (n = 20), CCR2 KO mice (n = 20); NS, not significant.
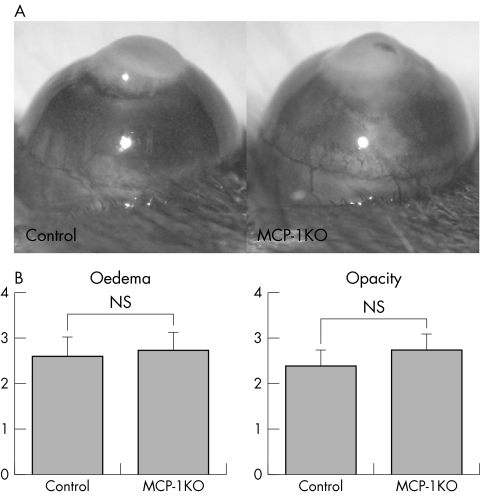
Since MCP‐1 is a ligand for CCR2, we confirmed the above CCR2 KO mice result by investigating corneal inflammation in MCP‐1 KO mice. In MCP‐1 KO mice, the degree of corneal oedema and opacity was not significantly less than in control mice (fig 2A, 2B).
Figure 2 Corneal inflammation in MCP‐1 KO mice. (A) Photographs of cauterised corneas after 96 hours. (B) The clinical evaluation of corneal inflammation. WT mice (n = 20), MCP‐1 KO mice (n = 20); NS, not significant.
Both CCR2 and MCP‐1 KO mice reduced macrophage infiltration but not neutrophils infiltration
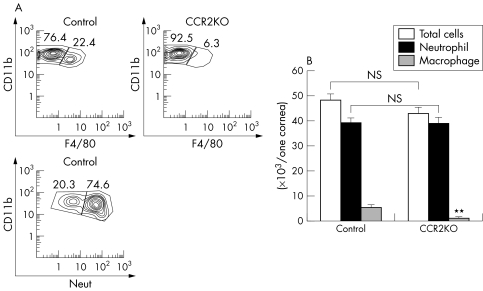
The total number of cells infiltrating each cornea was calculated. As shown in figure 3B, the total number of infiltrating cells was not significantly lower in CCR2 KO mice than in control mice. In both groups, the majority of infiltrating cells were neutrophils and the number of neutrophils in the CCR2 KO mice was not significantly different from that in control mice. However, the number of infiltrating macrophages was significantly lower in CCR2 KO corneas (3.0% (SD 1.2%), n = 6) compared to controls (11.3% (SD 2.1%), n = 6, three eyes were pooled to obtain enough cells for FACs; we thus used a total of 18 eyes) (fig 3A, 3B).
Figure 3 (A) The phenotype of cells infiltrating corneas in CCR2 KO mice. (B) The analysis of corneal cell infiltrates in CCR2 KO mice. **p<0.01; NS, not significant.
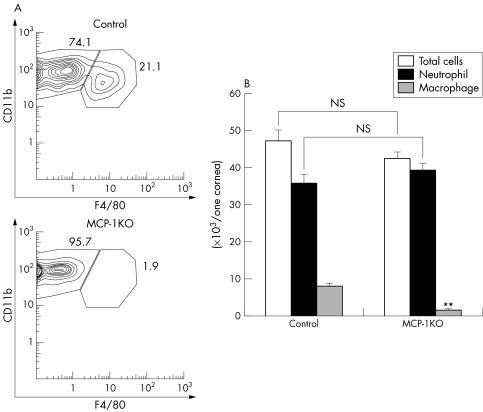
Similarly, in MCP‐1 KO mice the number of macrophages infiltrating the cornea was significantly lower (3.5% (SD 0.7%), n = 6) than in control mice (16.7% (SD 1.6%), n = 6) (fig 4A), and the majority of infiltrating cells were neutrophils (fig 4B). Thus, in both CCR2 KO and MCP‐1 KO mice, macrophage migration into the inflamed corneas was reduced.
Figure 4 The phenotype of cells infiltrating corneas in MCP‐1 KO mice. (A) The analysis of corneal cell infiltrates in MCP‐1 KO mice. **p<0.01; NS, not significant.
Neutrophil depleted mice significantly reduced corneal inflammation
Since the major infiltrating cells seen in this model were neutrophils and the grade of the clinical effects seen were unchanged in the absence of CCR2 or MCP‐1, we next examined corneal inflammation in neutrophil depleted mice. We compared corneal inflammation between neutrophil depleted and control mice. As shown in figure 5, the extent of corneal inflammation, measured by oedema and opacity, was significantly reduced after the depletion of neutrophils. Corneal cell infiltrates in neutrophil depleted cells were reduced by half compared to the control (fig 5C). Furthermore, neovascularisation was significantly inhibited in the neutrophil depleted mice (data not shown). This suggested that neutrophils had a crucial role in corneal inflammation in this study.
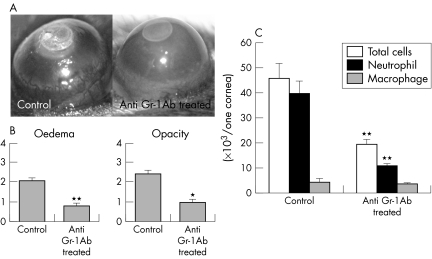
Figure 5 Corneal inflammation in mice depleted of neutrophils using anti‐Gr‐1 antibody. (A) Photographs of the cauterised corneas after 96 hours. (B) The clinical evaluation of corneal inflammation. C57/BL6 mice (n = 20), anti‐Gr‐1 antibody treated C57/BL6 mice (n = 20). *p<0.05; **p<0.01. (C) The analysis of corneal cell infiltrates 96 hours after cauterisation in untreated WT mice and WT mice treated with anti‐Gr‐1 antibody. **p<0.01.
The existence of resident F4/80 positive cells in non‐treated cornea
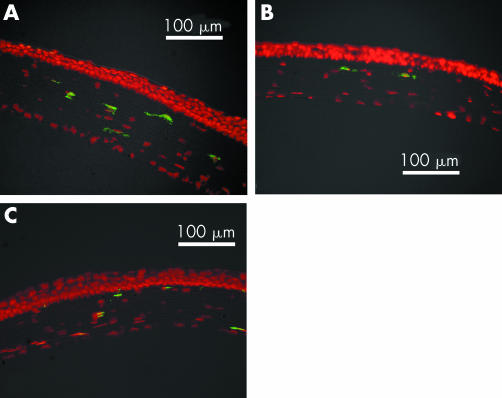
The previous results showed that corneal infiltration by macrophages was inhibited in CCR2 KO and MCP‐1 KO mice in inflammatory conditions. However, a small number of F4/80 positive cells, whose presence in the cornea must be independent of CCR2/MCP‐1 induced migration, were observed in these mice (fig 3B, 4B). To determine whether the non‐treated corneal stroma of the eye in CCR2 KO and MCP‐1 KO mice contained F4/80 positive cells, we performed fluorescent immunohistology. As shown in figure 6A, F4/80 positive cells were observed in the normal corneal stroma as previously reported.4 We found that the corneal stroma in CCR2 KO and MCP‐1 KO mice also contained cells expressing the F4/80 marker (fig 6B, C).
Figure 6 Immunofluorescent staining of resident macrophages in C57BL6 mice (A), CCR2 KO mice (B), and MCP‐1 KO mice (C). Each section was stained with anti‐F4/80 mAb (green) and propidium iodide (red). Original magnification ×200.
Discussion
We have demonstrated that CCR2/MCP‐1 dependent, corneal infiltrating macrophages had little effect on cauterisation induced corneal inflammation. In contrast, we found that neutrophils are critical to the formation of corneal opacity and oedema in this model. Laurie et al also identified a crucial role for neutrophils in a helminth mediated model of keratitis using CXCR2 KO mice.22
At the beginning of this study, it was hypothesised that infiltrating macrophages must be crucial in corneal inflammation because macrophages produce various pro‐inflammatory cytokines and chemokines at inflammatory sites,23 and because depletion of macrophages was known to prolong the survival of corneal allografts.24 Moreover, it was known that corneal angiogenesis induced by cauterisation was reduced in anti‐MCP‐1 mAb treated mice25 and in CCR2 KO mice.26 The data observed in this study were therefore unexpected.
Although corneal angiogenesis must be an important step in the development of corneal oedema and opacity, they might be separable processes. Our experiments focused on corneal oedema and opacity, which are directly associated with the loss of visual acuity in patients, rather than corneal angiogenesis. We currently postulate that macrophages are essential in the development of corneal angiogenesis but, in contrast, neutrophils are essential in the development of corneal oedema and opacity.
Although we have shown that CCR2 and MCP‐1 are not essential for corneal inflammation, it does not follow that macrophages are not required for this process. CCR2/MCP‐1 deficiency only impaired the recruitment of macrophages to the inflammatory site, having no effect on resident macrophages, and these MCP‐1/CCR2 independent cells may still have a role in corneal inflammation. We thus decided to confirm the existence of resident macrophages in the cornea of KO mice. Indeed, even in the absence of CCR2 or MCP‐1, we observed some F4/80+ cells in untreated corneas (fig 6). The MCP‐1/CCR2 independent resident macrophages could be important to induce local inflammation.
Our current hypothesis is that resident macrophages respond to cauterisation, leading to the recruitment of neutrophils and corneal inflammation. Many investigators have recently reported that bone marrow derived cells of the macrophage lineage were present even in untreated corneas, free from capillaries.3,4,5 We found that F4/80+ cells existed even in the CCR2 and MCP‐1 KO mice (fig 6), which suggests that the normal tissue distribution of these cells is independent of CCR2/MCP‐1. These resident macrophages have the potential to respond to injuries and contribute to the initiation of a local inflammatory response.
Other explanations for our observations can be suggested, which involve other cells resident in the cornea, in particular keratocytes. These cells have been shown to produce pro‐inflammatory cytokines and chemokines, such as IL‐8,27 and express class II molecules.21 Our data showed that neutrophils were the final effector cells mediating cauterisation induced corneal inflammation. However, the transfer of neutrophils directly into the corneal stroma has been reported not to induce an inflammatory response.28 We thus propose that neutrophils need to be activated by contact with keratocytes and/or resident macrophages, to induce corneal inflammation. Moreover, it was shown that corneal epithelial cells and tears in the inflammatory conditions contained pro‐inflammatory cytokines.29 Epithelial cells and tears can also activate local accumulated neutrophils. Once activated, neutrophils easily release a variety of pro‐inflammatory factors that can cause tissue damage.
Recently, the therapeutic potential of neutralising chemokines in vivo has focused on various inflammatory disorders.30,31 Our data imply that anti‐chemokine therapy using a MCP‐1 blocking reagent may not always give good results when applied to the eye. Blocking neutrophil infiltration might be an alternative strategy to control corneal inflammation and prevent the development of opacity.
Acknowledgements
This work was supported by grants from the Ministry of Education, Science, Sports and Culture, Japan (C2 No 16591757: K‐H Sonoda). We especially thank Dr Barrett J Rollins for providing the MCP‐1 KO mice. The authors also thank Ms Mari Imamura and Ms Michiyo Takahara for their excellent technical support.
Abbreviations
APC - antigen presenting cells
CCR2 - C‐C chemokine receptor 2
FITC - fluorescein isothiocyanate
KO - knockout
MCP‐1 - monocyte chemoattractant protein‐1
PE - phycoerythrin
WT - wild type
References
- 1.Matsuda H, Smelser G K. Epithelium and stroma in alkali‐burned corneas. Arch Ophthalmol 197389396–401. [DOI] [PubMed] [Google Scholar]
- 2.Sonoda K H, Sakamoto T, Yoshikawa H.et al Inhibition of corneal inflammation by the topical use of Ras farnesyltransferase inhibitors: selective inhibition of macrophage localization. Invest Ophthalmol Vis Sci 1998392245–2251. [PubMed] [Google Scholar]
- 3.Hamrah P, Liu Y, Zhang Q.et al Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol 20031211132–1140. [DOI] [PubMed] [Google Scholar]
- 4.Brissette‐Storkus C S, Reynolds S M, Lepisto A J.et al Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci 2002432264–2271. [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T, Ishikawa F, Sonoda K H.et al Characterization and distribution of bone marrow‐derived cells in mouse cornea. Invest Ophthalmol Vis Sci 200546497–503. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Hamrah P, Zhang Q.et al Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II‐positive dendritic cells derived from MHC class II‐negative grafts. J Exp Med 2002195259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spandau U H, Toksoy A, Verhaart S.et al High expression of chemokines Gro‐alpha (CXCL‐1), IL‐8 (CXCL‐8), and MCP‐1 (CCL‐2) in inflamed human corneas in vivo. Arch Ophthalmol 2003121825–831. [DOI] [PubMed] [Google Scholar]
- 8.Foster C S, Zelt R P, Mai‐Phan T.et al Immunosuppression and selective inflammatory cell depletion. Studies on a guinea pig model of corneal ulceration after ocular alkali burning. Arch Ophthalmol 19821001820–1824. [DOI] [PubMed] [Google Scholar]
- 9.Cook W J, Kramer M F, Walker R M.et al Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol J 200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C‐C chemokines JE and FIC. J Biol Chem 199627111603–11607. [DOI] [PubMed] [Google Scholar]
- 11.Moser B, Wolf M, Walz A.et al Chemokines: multiple levels of leukocyte migration control. Trends Immunol 20042575–84. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara T, Warr G, Loy J.et al Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 19971861757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumi C, Sonoda K H, Egashira K.et al The critical role of ocular‐infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol 20037425–32. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Rutledge B J, Gu L.et al Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1‐deficient mice. J Exp Med 1998187601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boring L, Gosling J, Chensue S W.et al Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C‐C chemokine receptor 2 knockout mice. J Clin Invest 19971002552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon B M, Voest E E, Chen C C.et al A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 1996371625–1632. [PubMed] [Google Scholar]
- 17.Hiromatsu K, Matsuzaki G, Tauchi Y.et al Sequential analysis of T cells in the liver during murine listerial infection. J Immunol 1992149568–573. [PubMed] [Google Scholar]
- 18.Watanabe K, Noda K, Hamano S.et al The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol Res 200086188–193. [DOI] [PubMed] [Google Scholar]
- 19.Leenen P J, de Bruijn M F, Voerman J S.et al Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods 19941745–19. [DOI] [PubMed] [Google Scholar]
- 20.Haidl I D, Jefferies W A. The macrophage cell surface glycoprotein F4/80 is a highly glycosylated proteoglycan. Eur J Immunol 1996261139–1146. [DOI] [PubMed] [Google Scholar]
- 21.Seo S K, Gebhardt B M, Lim H Y.et al Murine keratocytes function as antigen‐presenting cells. Eur J Immunol 2001313318–3328. [DOI] [PubMed] [Google Scholar]
- 22.Hall L R, Diaconu E, Patel R.et al CXC chemokine receptor 2 but not C‐C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth‐mediated keratitis (river blindness). J Immunol 20011664035–4041. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Sica A, Sozzani S.et al The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 200425677–686. [DOI] [PubMed] [Google Scholar]
- 24.Slegers T P, van der Gaag R, van Rooijen N.et al Effect of local macrophage depletion on cellular immunity and tolerance evoked by corneal allografts. Curr Eye Res 20032673–79. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Yoshida A, Matsui H.et al Involvement of macrophage chemotactic protein‐1 and interleukin‐1beta during inflammatory but not basic fibroblast growth factor‐dependent neovascularization in the mouse cornea. Lab Invest 200383927–938. [DOI] [PubMed] [Google Scholar]
- 26.Ambati B K, Joussen A M, Kuziel W A.et al Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea 200322465–467. [DOI] [PubMed] [Google Scholar]
- 27.Oakes J E, Monteiro C A, Cubitt C L.et al Induction of interleukin‐8 gene expression is associated with herpes simplex virus infection of human corneal keratocytes but not human corneal epithelial cells. J Virol 1993674777–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore J W, 3rd, Sholley M M. omparison of the neovascular effects of stimulated macrophages and neutrophils in autologous rabbit corneas. Am J Pathol 198512087–98. [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada J, Dana M R, Sotozono C.et al Local suppression of IL‐1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res 200376161–167. [DOI] [PubMed] [Google Scholar]
- 30.Dawson T C, Beck M A, Kuziel W A.et al Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol 20001561951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang D R, Wang J, Kivisakk P.et al Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen‐specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 2001193713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]