Abstract
The authors propose that light entering the eye interacts with retinal ganglion cell (RGC) axon mitochondria to generate reactive oxygen intermediates (ROI) and that when these neurons are in an energetically low state, their capacity to remove these damaging molecules is exceeded and their survival is compromised. They suggest that in the initial stages of glaucoma, RGCs exist at a low energy level because of a reduced blood flow at the optic nerve head and that in the mitochondrial optic neuropathies (MONs), this results from a primary, genetic defect in aerobic metabolism. In these states RGCs function at a reduced energy level and incident light on the retina becomes a risk factor. Preliminary laboratory studies support this proposition. Firstly, the authors have shown that light is detrimental to isolated mitochondria in an intensity dependent manner. Secondly, light triggers apoptosis of cultured, transformed RGCs and this effect is exacerbated when the cells are nutritionally deprived. Detailed studies are under way to strengthen the proposed theory. On the basis of this proposal, the authors suggest that patients with optic neuropathies such as glaucoma or at risk of developing a MON may benefit from the use of spectral filters and reducing the intensity of light entering the eye.
Keywords: glaucoma, mitochondrial optic neuropathies, light
In 2001 we summarised potential risk factors for retinal ganglion cell (RGC) death in glaucoma, concluding that their vulnerability was affected by reduced blood flow at the optic nerve head.1 We propose here that light transmitted to the retina is an additional risk factor both for glaucoma (fig 1) and the mitochondrial optic neuropathies (MONs).
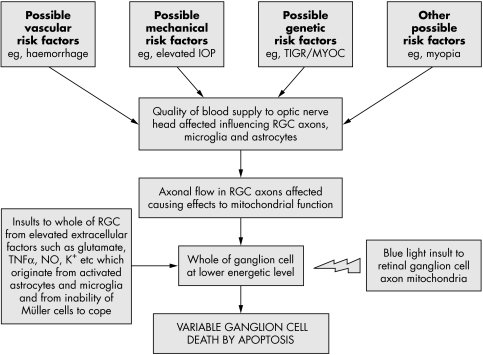
Figure 1 Theoretical events to explain RGC death in glaucoma. An initial insult is caused by an alteration in vascular dynamics in the optic nerve head by any of a number of risk factors (for example, vascular, genetic, or mechanical). Cells affected will include RGC axons and glial cells. As the disease develops, this forces the RGCs to function at a lower than normal energetic state. An increase in the accumulation of potential toxins (for example, glutamate, NO, TNF‐α) occurs in the extracellular space, because of abnormal glial cell function. These toxins, in addition to light acting on RGC axon mitochondria, represent an assortment of risk factors which threaten the survival of the retinal neurons. The present hypothesis describes how these combined influences may cause apoptotic RGC death. This will vary from cell to cell depending on their location, susceptibility, and axon morphology within the globe.
RGC axons within the globe are functionally specialised. These unmyelinated axons are richly provided with mitochondria, located in varicosities along their length.2 This satisfies the high energy requirement for nerve conduction in unmyelinated neurons,3,4 contrasting with the lower requirement in myelinated axons such as in the optic nerve, where transmission occurs more efficiently by saltatory conduction. Mitochondria are cytoplasmic structures that house the respiratory chain complexes I–V. Their chief role is in the provision of cellular energy in the form of ATP, by the process of oxidative phosphorylation.
Light transmission in the eye
The basis for this proposal is that light falling on the retina interacts with RGC mitochondria to generate reactive oxygen intermediates (ROI) and impair their viability. The cornea and lens absorb light at wavelengths below 295 nm and 400 nm, respectively,5,6 so that the retina is exposed to the visible component of the electromagnetic spectrum from 400–760 nm. Absorption of the shorter wavelengths by the lens rises exponentially with age, owing to the accumulation of yellow chromophores.7 Thus the greatest exposure of the retina to blue light occurs in the first two decades of life.8
It should be noted that the outer retina (retinal pigment epithelium and photoreceptors) is partially protected from blue light by the macular carotenoids, located in the photoreceptor axons and outer plexiform layer, absorbing with maxima around 450 nm.9 Mitochondrial chromophores of the nerve fibre layer of the inner retina may perform a similar function.
Light and retinal toxicity
It was recognised by Noell et al10 that light, particularly blue light, can induce retinal damage by a photochemical process leading to the generation of ROI. Blue light (450 nm–490 nm) is bioenergetically the most efficient source of retinal light damage. Such intermediates, which include singlet oxygen, superoxide, hydrogen peroxide, hydroxyl radicals, and nitric oxide, are highly toxic and give rise to cell damage by protein oxidation and lipid peroxidation. Cell defence mechanisms may be enzymic, such as superoxide dismutase, catalase, and glutathione peroxidase or involve antioxidant molecules such as vitamins A, E, and C.11 The turnover of cell components is also a potent protective mechanism.5 In a photochemical reaction, intermediates are formed by the interaction of light with photosensitisers, which in the retina may be present in retinal pigment epithelial (RPE) cells (for example, lipofuscin and possibly melanin)11 and photoreceptors (for example, rod and cone opsins and retinoids).12
Mitochondria offer an additional source of photosensitisers. Jung et al13 demonstrated the generation of singlet oxygen on exposure of soybean mitochondrial membranes to blue light, making the mitochondrial enzyme cytochrome oxidase a possible target. Exposure of RPE cells to blue light decreases their cytochrome oxidase content14 and generates ROI which can be neutralised by mitochondria specific antioxidants.15 In another study, light induced breakdown of the blood‐retinal barrier in albino rabbits was attributed to blue light absorption by cytochrome oxidase in the RPE.16 More recently Godley et al17 demonstrated the production of ROI on irradiating non‐pigmented RPE cells with blue light in vitro. Here it was argued that mitochondrial respiratory chain enzymes, the flavin and cytochrome c oxidases, absorbing light maximally at around 440–450 nm, acted as photosensitisers, chiefly with the generation of superoxide.17 This was associated with mitochondrial DNA damage.
Hypothesis
With this background we hypothesise that light is a risk factor for optic nerve damage in glaucoma and the MONs, in the following way:
RGC axons within the globe are laden with mitochondria.
The mitochondria of these axons are directly exposed to light photons.
Light, acting on mitochondrial photosensitisers such as cytochrome c oxidase and flavin containing oxidases is able to generate ROI.
In the healthy retina these intermediates are removed by the normal scavenging mechanisms of the mitochondria. However, when the RGCs are at a low bioenergetic state they have a diminished ability to scavenge ROI and are at increased risk of light induced injury.
This compromised energetic state is thought to affect selected ganglion cells during the evolution of glaucoma and the RGC neurons of the papillomacular bundle in patients with inherited MONs.
Light and the optic neuropathies
The chronic glaucomas, such as primary open angle glaucoma (POAG) and the inherited MONs, have features in common. In both glaucoma and MONs18 ganglion cell death is by apoptosis and leads to an ascending optic neuropathy. Pathologically enlarged optic cups may be encountered in both disorders.19 However, the MONs have a juvenile onset, while POAG is a disease of older age groups; differences in the patterns of ganglion cell loss also need explanation.
In POAG, RGC death is accompanied by a characteristic visual field loss, pathological nerve head cupping, and glaucomatous optic atrophy, which develop over several years. There is strong support for a vascular mechanism in which impairment of blood flow at the optic nerve head is caused either by raised intraocular pressure (IOP)1 or by intrinsic vascular factors.20,21 IOP is a major risk factor for field loss in glaucoma, whether raised (high tension glaucoma, HTG), or within the normal population pressure range (normal tension glaucoma, NTG), and there has been definite success in slowing progression by lowering IOP in both HTG and NTG.18
The vasogenic hypothesis of glaucoma1,20,21 implies that RGC axons are metabolically compromised by the impaired optic nerve head blood flow. The possible consequences are summarised in figure 1. The distribution of affected axons is determined by these initiating vascular factors. We suggest that once a bioenergetic defect is established, these axons are at additional risk of damage through the photochemical mechanism described above. The pattern and timing of ganglion cell loss will be determined by the timing and distribution of these initiating factors. In this case light damage is invoked as a mechanism which accelerates the progression of an established disease.
The inherited optic neuropathies are a group of disorders in which cell death occurs, chiefly confined to RGCs.22 In the inherited MONs, mitochondrial function is impaired by mutations in either mitochondrial or nuclear genes. An enigmatic feature of these disorders is that, despite the expression of these mutations in many organs of the body, the optic nerve is the major, and often the only clinically affected organ.23 This has raised questions as to factors that target the disease to the optic nerve.
Leber's hereditary optic neuropathy (LHON), is a maternally transmitted disorder, affecting males more frequently than females, because of mutations in mitochondrial genes encoding complex I subunit proteins.24 The disorder presents acutely or subacutely with a central or caecocentral field loss. Damage to the papillomacular nerve bundle is followed by the development of optic atrophy. A limited recovery of function may occur, which is influenced by the genotype.25 Histologically, a preferential loss of small axons has been reported.26
Mutations lead to an impairment of complex I driven ATP synthesis, affecting the key function of aerobic metabolism. Loss of complex I activity increases ROI production and leads to cell death by apoptosis27—for instance, by the release of cytochrome c into the cytosol.28 This mechanism has been clearly demonstrated in a cell model of mitochondrial function using hybrid tumour cells containing LHON mitochondria (cybrids).24,29 In such LHON cybrids, ATP synthesis from the tricarboxylic cycle is compromised by the complex I defect. When these cybrids are transferred from a glucose to a galactose medium, they are unable to sustain ATP production from glycolysis alone and a catastrophic fall in ATP production occurs. This leads to a rise in ROS production and occurrence of apoptotic cell death due to a mitochondrial, cytochrome c dependent mechanism. Cybrids containing normal mitochondria show only a reduction in growth rate.
There is evidence too for a deficiency of ATP production in another juvenile optic neuropathy, autosomal dominant optic atrophy (ADOA), which is caused by mutations in the gene OPA1.30,31 Onset is in the first decade of life and progression is slower than in LHON. Field loss is central, paracentral, or caecocentral.23 Mutations affect a complex I subunit protein encoded by nuclear DNA, which is a dynamin related GTPase involved in the maintenance of the mitochondrial network. Evidence for an impairment of calf muscle ATP regeneration after exercise was found in affected ADOA patients using phosphorus magnetic resonance spectroscopy.32 This emphasises the general nature of the functional mitochondrial defect, while disease is confined to the optic nerve.
Olichon et al33 showed that downregulation of OPAI in HeLa cells led to fragmentation of the mitochondrial network, cytochrome c release, and apoptosis. They suggested that a haplo‐insufficiency of OPAI in ADOA increases the susceptibility of RGCs to apoptogenic stimuli, including, it is of interest to note, “the daily exposure to ultraviolet light.”
As proposed for glaucoma, we hypothesise that in the presence of a bioenergetic defect, visible radiation incident upon the retina targets injury to the optic nerve in these inherited optic neuropathies. These mitochondrial neuropathies differ, however, from the adult glaucomas in that their bioenergetic defect is present from birth. As a result, retinas are exposed to the highest intensities of short wave (blue light) radiation in the earliest years. We think that this may explain the difference in the timing and pattern of neuronal loss between these disorders and glaucoma.
The hypothesis put forward here has implications for other forms of chronic glaucoma, particularly infantile and juvenile glaucoma, whose onsets coincide with periods of high retinal blue light exposure. Similarly, in Friedrich's ataxia, an autosomal disease associated with cerebellar ataxia and optic atrophy, there is a mutation in the gene for frataxin, which regulates mitochondrial iron levels.34 Also of relevance are conditions associated with optic atrophy which impair the blood supply to the optic nerve head, such as arteritic ischaemic anterior optic neuropathy,35 or to the inner retina, such as diabetic retinopathy.
Preliminary studies in support of the hypothesis (figs 2 and 3)
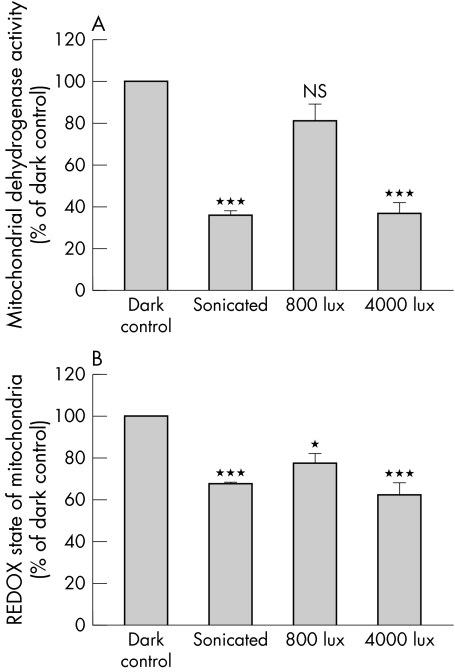
Figure 2 The influence of light on isolated mitochondria. Equivalent amounts of freshly isolated rat liver mitochondria in physiological medium were placed in the dark or light (800 or 4000 lux) for 12 hours. In some cases mitochondria were first sonicated to destroy their integrity. For the last hour of each treatment, 0.5 mg/ml of either WST‐1 or MTT were added to the mitochondrial suspensions. As shown in (A), mitochondrial dehydrogenase activity is markedly reduced by light exposure in an intensity dependent manner when compared with dark exposure. Also, sonication of mitochondria drastically reduced dehydrogenase activity with no difference between light and dark exposure. These results show that sonication disrupts mitochondrial function and that light exposure causes mitochondria to function less efficiently than in the dark. Similar conclusions are reached from analyses of the REDOX state of the mitochondria (B). When mitochondria are disrupted by sonication, similar results are found for dark or light conditions. In contrast, in the dark, intact mitochondria show a maximum capacity to reduce MTT while this is much reduced by light in an intensity dependent manner. ***p<0.01, *p<0.05, comparing preparations to dark control by Bonferroni post‐test analysis (n = 6 preparations for each test).
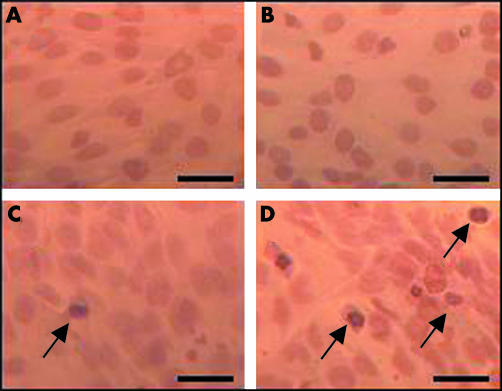
Figure 3 The effect of light and serum deprivation on cultured RGC‐5 rat RGCs. Cells were passaged onto borosilicate glass coverslips and then exposed to (A) normal serum conditions in the dark for 48 hours, (B) serum deprivation in the dark for 48 hours, (C) normal serum plus filtered light of intensity 800 lux (wavelength 400–760 nm) for 48 hours, and (D) serum deprivation and 800 lux lighting for 48 hours. Cells were then fixed and stained by the TUNEL procedure as an index of apoptosis and counterstained with haematoxylin. It can be seen that TUNEL positive cells are particularly plentiful (up to 20%) in cultures exposed to light and serum deprivation (D) and occurred in low amounts in cultures exposed to serum deprivation in the dark (B) and normal serum in the light (C). However, in normal serum in the dark no TUNEL positive cells were detected (A). Scale bar, 20 μm.
We have data to show that light in the range 400–760 nm, reduces the functional status of isolated mitochondria in an intensity dependent manner. In these studies mitochondria were isolated from the liver and incubated overnight, in the dark or in light at two different intensities. Thereafter, the samples were analysed for an indication of the REDOX potential (by MTT assay) and for mitochondrial dehydrogenase activity (using the WST‐1 assay). MTT is reduced to an insoluble, blue formazan product because of acceptance of electrons from cellular reducing equivalents such as NADH, NADPH, or succinate, thus providing an assay for the REDOX state of a sample.36 The REDOX potential is a measure of the oxidative status of a cell—a fall in the potential implies that the balance is in favour of oxidation, as might arise through the action of ROI. WST‐1 (4‐[3‐(4‐iodophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazolio]‐1,3‐benzene disulfonate) is a tetrazolium dye containing an electron coupling agent that is cleaved by mitochondrial dehydrogenases to a formazan dye with an absorbance at 490 nm (Roche, USA).37 Mitochondrial dehydrogenase activity is directly related to mitochondrial energy production—a fall in activity implies reduced production.
Using the MTT assay, we found that the REDOX potential of the mitochondria was reduced by light exposure in an intensity dependent manner (fig 1B) suggesting that light generates an oxidative environment within the mitochondria—with a fall in reduced cofactors necessary for mitochondrial respiratory activity. We have also shown that light causes an intensity dependent decrease in mitochondrial dehydrogenase activity (fig 2A), implying that mitochondrial function is inhibited by light. These studies support the principle that light (optical radiation above 400 nm) entering the globe can interact with mitochondria to generate ROI and influence their metabolic state.
That a light insult can affect RGC viability is shown in figure 3. In this preliminary study on a transformed ganglion cell line (RGC‐5), light (400–760 nm) was found to cause a greater amount of cells to appear apoptotic by labelling for the TdT‐dUTP linked nick end labelling technique (TUNEL)38 when compared with a dark exposure over the same time period. Most importantly, this light effect was enhanced when the cultured cells were nutritionally deprived by reducing the serum content of the medium.
Conclusions
We have summarised the evidence suggesting that RGC death can be brought about by light. We conceive this to be a two step process: (1) a deficient production of mitochondrial ATP in RGCs impairs their ability to scavenge ROI; and (2) a failure to scavenge ROI generated by light in intraretinal RGC axons leads to RGC apoptosis and death. In this way, a unique feature of the human retina, the presence of unmyelinated, intraretinal RGC axons, rich in mitochondria, is suggested to be the means by which light, the raison d'etre of the visual process, targets damage to the RGCs, to cause optic atrophy and blindness.
Should light prove to be a risk factor in such diseases then reducing the intensity and modulating the wavelength of light entering the eye may be beneficial. There is an extensive literature indicating how this might be achieved to prevent various disorders, by the provision of protective headgear, including wraparound, light filtering spectacles and by behavioural and other modifications of environmental exposure.39,40 In devising protective spectacles it will be important to recognise the action spectrum of the target tissue, in this case the RGC mitochondria.
Acknowledgements
GL is grateful to the Public Benefit Foundation Alexander S Onassis for support.
Abbreviations
ADOA - autosomal dominant optic atrophy
HTG - high tension glaucoma
IOP - intraocular pressure
LHON - Leber's hereditary optic neuropathy
MON - mitochondrial optic neuropathy
NTG - normal tension glaucoma
POAG - primary open angle glaucoma
RGC - retinal ganglion cell
ROI - reactive oxygen intermediates
RPE - retinal pigment epithelium
TUNEL - TdT‐dUTP linked nick end labelling technique
References
- 1.Osborne N N, Melena J, Chidlow G.et al A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol 2001851252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Dong J, Cull G.et al Varicosities of intraRGC axons in human and nonhuman primates. Invest Ophthalmol Vis Sci 2003442–9. [DOI] [PubMed] [Google Scholar]
- 3.Bristow E A, Griffiths P G, Andrews R M.et al The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol 2002120791–796. [DOI] [PubMed] [Google Scholar]
- 4.Barron M J, Griffiths P, Turnbull D M.et al The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br J Ophthalmol 200488286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall J. Radiation and the ageing eye. Ophthalmic and Physiological Optics 19855241–263. [PubMed] [Google Scholar]
- 6.Sliney D H. How light reaches the eye and its components. Int J Toxicol 200221501–509. [DOI] [PubMed] [Google Scholar]
- 7.Said F S, Weale R A. The variation with age of the spectral transmissivity of the living human crystalline lens. Gerontologia 19593213–231. [DOI] [PubMed] [Google Scholar]
- 8.Lerman S. An experimental and clinical evaluation of lens transparency and aging. J Gerontol 198338293–301. [DOI] [PubMed] [Google Scholar]
- 9.Krinsky N I, Landrum J T, Bone R A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 200323171–201. [DOI] [PubMed] [Google Scholar]
- 10.Noell W K, Walker V S, Kang B S.et al Retinal damage by light in rats. Investigative Ophthalmology 19665450–473. [PubMed] [Google Scholar]
- 11.Margrain T H, Boulton M, Marshall J.et al Do blue light filters confer protection against age‐related macular degeneration? Prog Retin Eye Res 200423523–531. [DOI] [PubMed] [Google Scholar]
- 12.Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B 200164144–161. [DOI] [PubMed] [Google Scholar]
- 13.Jung J S, Kim H J, Cho M. Action spectra for the generation of singlet oxygen from mitochondrial membranes from soybean (Glycine max) hypocotyls. Photochem Photobiol 199051561–566. [Google Scholar]
- 14.Chen E, Soderberg P G, Qian W.et al Inhibition of cytochrome oxidase by blue light (404 nm). A factor that causes retinal injury? Invest Ophthal Vis Sci 199233919 [Google Scholar]
- 15.King A, Gottlieb E, Brooks D G.et al Mitochondria‐derived reactive oxygen species mediate blue light‐induced death of retinal pigment epithelial cells. Photochem Photobiol 200479470–475. [DOI] [PubMed] [Google Scholar]
- 16.Putting B J, Van Best J A, Vrensen G F.et al Blue‐light‐induced dysfunction of the blood‐retinal barrier at the pigment epithelium in albino versus pigmented rabbits. Exp Eye Res 19945831–40. [DOI] [PubMed] [Google Scholar]
- 17.Godley B F, Shamsi F A, Liang F Q.et al Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem 200528021061–21066. [DOI] [PubMed] [Google Scholar]
- 18.Collaborative Normal‐Tension Glaucoma Study Group X. The effectiveness of intraocular pressure reduction in the treatment of normal‐tension glaucoma. Am J Ophthalmol 1998126498–505. [DOI] [PubMed] [Google Scholar]
- 19.Fournier A V, Damji K F, Epstein D L.et al Disc excavation in dominant optic atrophy: differentiation from normal tension glaucoma. Ophthalmology 20011081595–1602. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh S S. The role of age and cardiovascular disease in glaucomatous optic neuropathy. Surv Ophthalmol 199943(Suppl 1)S27–S42. [DOI] [PubMed] [Google Scholar]
- 21.Flammer J, Orgul S, Costa V P.et al The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 200221359–393. [DOI] [PubMed] [Google Scholar]
- 22.Votruba M. Molecular genetic basis of primary inherited optic neuropathies. Eye 2004181126–1132. [DOI] [PubMed] [Google Scholar]
- 23.Newman N J, Biousse V. Hereditary optic neuropathies. Eye 2004181144–1160. [DOI] [PubMed] [Google Scholar]
- 24.Carelli V, Ross‐Cisneros F N, Sadun A A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 20042353–89. [DOI] [PubMed] [Google Scholar]
- 25.Riordan‐Eva P, Sanders M D, Govan G G.et al The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain 1995118(Pt 2)319–337. [DOI] [PubMed] [Google Scholar]
- 26.Saadati H G, Hsu H Y, Heller K B.et al A histopathologic and morphometric differentiation of nerves in optic nerve hypoplasia and Leber hereditary optic neuropathy. Arch Ophthalmol 1998116911–916. [DOI] [PubMed] [Google Scholar]
- 27.Kokoszka J E, Coskun P, Esposito L A.et al Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age‐related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA 2001982278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun 2003304433–435. [DOI] [PubMed] [Google Scholar]
- 29.Carelli V, Ross‐Cisneros F N, Sadun A A. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int 200240573–584. [DOI] [PubMed] [Google Scholar]
- 30.Alexander C, Votruba M, Pesch U E.et al OPA1, encoding a dynamin‐related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 200026211–215. [DOI] [PubMed] [Google Scholar]
- 31.Delettre C, Lenaers G, Griffoin J M.et al Nuclear gene OPA1, encoding a mitochondrial dynamin‐related protein, is mutated in dominant optic atrophy. Nat Genet 200026207–210. [DOI] [PubMed] [Google Scholar]
- 32.Lodi R, Tonon C, Valentino M L.et al Deficit of in vivo mitochondrial ATP production in OPA1‐related dominant optic atrophy. Ann Neurol 200456719–723. [DOI] [PubMed] [Google Scholar]
- 33.Olichon A, Baricault L, Gas N.et al Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 20032787743–7746. [DOI] [PubMed] [Google Scholar]
- 34.Lynch D R, Farmer J. Practical approaches to neurogenetic disease. J Neuroophthalmol 200222297–304. [DOI] [PubMed] [Google Scholar]
- 35.Orgul S, Gass A, Flammer J. Optic disc cupping in arteritic anterior ischemic optic neuropathy. Ophthalmologica 1994208336–338. [DOI] [PubMed] [Google Scholar]
- 36.Wood J P, Chidlow G, Graham M, Osborne N N. Energy substrate requirements of rat retinal pigmented epithelial cells in culture: relative importance of glucose, amino acids, and monocarboxylates. Invest Ophthalmol Vis Sci 2004451272–1280. [DOI] [PubMed] [Google Scholar]
- 37.Toimela T, Tahti H. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch Toxicol 200478565–574. [DOI] [PubMed] [Google Scholar]
- 38.Gavrieli Y, Sherman Y, Ben‐Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992119493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sliney D H. Eye protective techniques for bright light. Ophthalmology 198390937–944. [DOI] [PubMed] [Google Scholar]
- 40.McCarty C A, Taylor H R. Protecting eyes from sun damage. Med J Aust 1997166671. [DOI] [PubMed] [Google Scholar]