Abstract
Aims
To determine if asymptomatic carriers from a previously identified large pedigree of the Leber's hereditary optic neuropathy (LHON) 11778 mtDNA mutation have colour vision deficits.
Methods
As part of a comprehensive analysis of over 200 members of a large Brazilian LHON pedigree spanning seven generations, colour vision tests were obtained from 91 members. Colour vision was tested one eye at a time using the Farnsworth‐Munsell 100 (FM‐100) hue colour vision test. The test was administered under uniform conditions, taking into account: ambient light levels, daylight colour temperature of 6700 kelvin, and neutral uniform background. Tests were scored using the FM‐100 MS‐Excel computer scoring program. Defects were determined and categorised as tritan, deutan, or protan. Categorisation of each dyschromatopsia was based on review of demonstrated axis computer generated plots and age adjusted error scores which coincided with Verriest 95% confidence intervals. Only the axis with the greatest magnitude error score was used to classify the defect. 55 of the 91 test subjects were LHON mtDNA 11778 J haplotype mutation carriers, proved by mtDNA analysis. The remaining 36 subjects were age matched non‐blood relatives (off pedigree), who served as controls.
Results
27 of 55 carriers (49.10%) were shown to have colour vision defects in one or both eyes. 13 of the 27 (48%) abnormal tests in the carrier group were tritan defects and the remaining 14 (52%) were deutan defects. Nine of the 27 (33%) abnormals in the carrier group were identified as having bilateral defects. Six of these were deutan, and the remaining three were tritan dyschromatopsias. Only six of the 36 (16.66%) age matched controls were found to have any type of dyschromatopsia. Five (83.3%) of these were deutan defects. The remaining one was a tritan defect. The difference between the two groups using a χ2 test with one degree of freedom was statistically significant with a p value less that 0.001.
Conclusions
Until now, LHON has always been characterised by a sudden, devastating vision loss. Asymptomatic carriers, those without vision loss, were considered unaffected by the disease. It now appears that asymptomatic carriers of the LHON mutation are affected by colour vision defects and may manifest other subtle, yet chronic, changes.
Keywords: optic nerve disease, hereditary eye disease, colour vision defects, mitochondrial DNA, colour perception
Leber's hereditary optic neuropathy (LHON) is characterised by acute and profound loss of visual acuity, severe dyschromatopsia, central or caecocentral scotomas, and deficiencies in high spatial frequency contrast sensitivity.1,2 Visual acuity is often in the 20/400 to counting fingers range, and fundus examination reveals selective loss of the smaller fibres of the papillomacular bundle, with optic atrophy that is more severe on the temporal side.1,3,4 Visual loss tends to occur in one eye initially, which may be followed by visual loss in the other eye within weeks to months. It is unknown why this visual loss tends to occur in previously unaffected males, in the second and third decades of life. Long term follow up usually reveals symmetric disease.
The pathogenesis of LHON is understood to be related to the mtDNA point mutations 11778, 3460, and 14484. These three point mutations are respectively located in the genes of NADH dehydrogenase subunits 4, 1, and 6.5,6,7,8 of complex 1 of the respiratory chain. These mutations may either decrease the net ATP synthesis for a given cell, or induce a chronic overproduction of reactive oxygen species (ROS) in the mitochondria. The resultant oxidative stress may trigger apoptosis for the cell. Because the energy expenditure of an axon is related to its surface area, small fibre axons are thought to be particularly susceptible to damage resulting from these mutations. The fibres of the papillomacular bundle have a small ratio of volume to surface area, and are therefore expected to be most sensitive to deficits in energy metabolism.9 LHON has the tendency to be an “all or none” disease. Those affected tend to be severely affected, and those asymptomatic carriers of the above mtDNA mutations have little to no identifiable deficiencies in visual performance. Although unaffected carriers appear to be completely free of signs of any disease, there may exist subtle signs of optic neuropathy that escape detection by standard clinical examination. Any such putative subtle dysfunction would most likely be a specialised function of the fibres most vulnerable to the pathophysiology of LHON (that is, the papillomacular bundle). Because colour vision is a specialised function of the papillomacular bundle and because dyschromatopsia is one of the earliest signs of LHON, we hypothesised that subtle dyschromatopsia may be a sign of occult subclinical optic neuropathy in carriers of the LHON 11778 mutation. Using the Farnsworth‐Munsell 100 hue colour vision test, we conducted a comparison of colour vision performance between normal subjects (age matched, off pedigree controls) and asymptomatic carriers of the 11778 mtDNA mutation.
Materials and methods
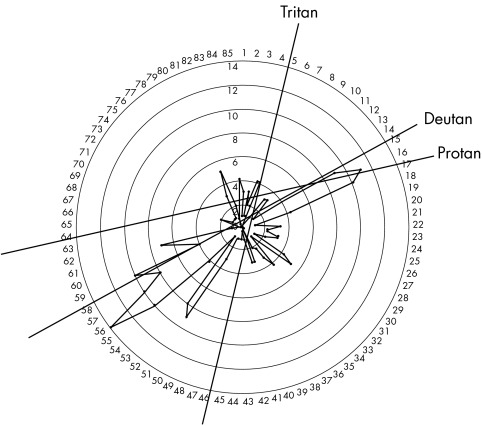
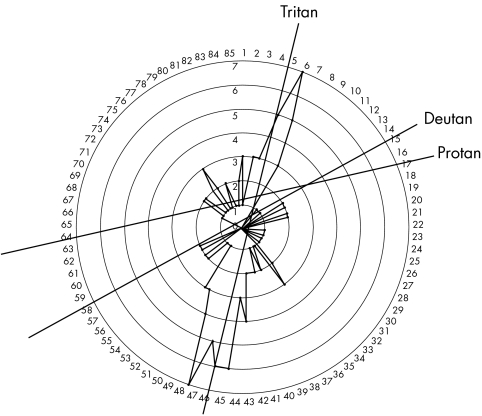
As part of a Federal University of Sao Paulo institutional review board approved protocol for the comprehensive analysis of over 200 members of a large Brazilian LHON pedigree spanning seven generations, colour vision tests were obtained from 91 members. All patients signed informed consent forms prior to submission to research protocol. Colour vision was tested one eye at a time using the Farnsworth‐Munsell 100 (FM‐100) hue colour vision test. The test was administered under uniform conditions, taking into account ambient light levels, daylight colour temperature of 6700 kelvin, and neutral uniform background. Tests were scored using the FM‐100 MS‐excel computer scoring program, which normalised test scores. Defects were determined and categorised as tritan, deutan, or protan. Categorisation of each dyschromatopsia was based on review of demonstrated axis computer generated plots and age adjusted error scores which coincided with Verriest10 95% confidence intervals. Only the axis with the greatest magnitude error score was used to classify the defect (figs 1 and 2). Fifty five of the 91 test subjects were LHON mtDNA 11778 J haplotype mutation carriers, proved by mtDNA analysis. The remaining 36 subjects were age matched non‐blood relatives, off pedigree controls.
Figure 1 Representative deutan defect shown by asymptomatic carrier group.
Figure 2 Representative tritan defect seen in asymptomatic carrier group.
Results
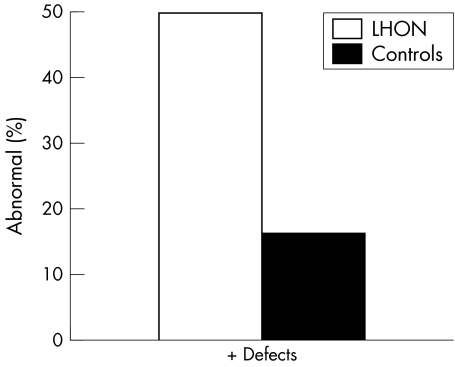
In all, 25 of 55 carriers (45.40%) were shown to have colour vision defects in one or both eyes; 11 of the 27 (40%) abnormal tests in the carrier group were tritan defects and the remaining 14 (56%) were deutan defects (see fig 3).
Figure 3 Percentage of LHON carriers with abnormalities versus controls.
Figure 3 depicts percentage of LHON carriers with abnormalities versus controls
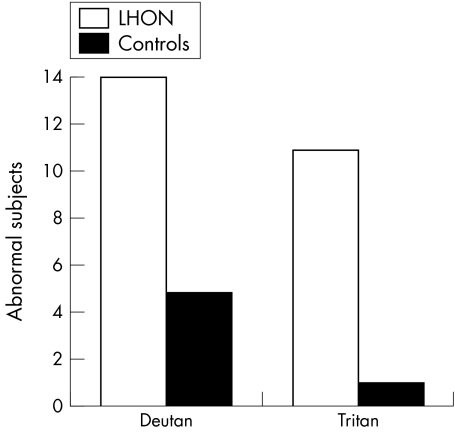
Nine of the 25 (36%) abnormals in the carrier group were identified as having bilateral defects. Six of these were deutan, and the remaining three were tritan dyschromatopsias. Only six of the 36 (16.66%) age matched controls were found to have any type of dyschromatopsia. Five (83.3%) of these were deutan defects. The remaining one was a tritan defect (see fig 2).
Figure 4 depicts numbers of abnormal subjects broken down by defect type. There is an overwhelming number of abnormal subjects in the LHON carrier group compared to controls.
Figure 4 Numbers of abnormal subjects broken down by defect type.
The difference between the two groups using a χ2 test with one degree of freedom was statistically significant with a p value less that 0.001.
Discussion
In general, carriers are either blinded by the disease or are asymptomatic. This dichotomy seems to be true regardless of whether the individual is homoplasmic or heteroplasmic for the mutation. Although heteroplasmic populations exhibit a lower incidence of LHON, those who are affected are subject to the full spectrum of the disease.11 Speculations on the pathophysiology relating mitochondrial mutations to inefficient energy metabolism and oxidative stress in the development of LHON do not account for the lack of any clinical findings in asymptomatic carriers.
Mitochondrial dysfunction resulting from the 11778, 3460, and 14484 mutations have a role in the development of LHON. All three mutations affect the site of interaction of complex I with its natural quinone (CoQ) substrate.12,13 In a cybrid cellular model these mtDNA mutations induced a variable impairment of mitochondrial respiratory function.14 Consequentially there may develop a chronic increase of reactive oxygen species (ROS). Some recent publications have implicated the importance of ROS in LHON.15,16,17 Environmental (toxin exposure, diet, and exercise) and genetic factors have also been proposed as important in the pathogenesis of LHON.18 None of these theories can explain why asymptomatic carriers, who should also undergo some degree of the same pathological metabolic dysfunction, have absolutely no clinical signs or symptoms of visual impairment.
This study identifies dyschromatopsia in asymptomatic carriers of the 11778 LHON mutation. Verriest19 defined three types of acquired dyschromatopsias based on the FM‐100. Type I (protan‐like) defects are red/green defects with little or no loss of blue/yellow discrimination, frequently with moderate reduction in central acuity. Type II (deutan‐like) defects are red/green defects and significant but less severe blue/yellow defects. Type III (tritan‐like) defects are mild to moderate blue/yellow defects, lesser or absent red/green defects, and normal or mildly reduced visual acuity. Each congenital cone defect demonstrates a well defined axis of colour confusion on FM‐100 plots.
In acquired dyschromatopsias, colour errors tend to be more diffusely distributed, making FM‐100 polar plots more difficult to interpret; leading to difficulties in determining whether different optic nerve defects result in characteristic dyschromatopsias. One previously well accepted generalisation was Köllner's rule which stated that patients with macular disease tend to have blue‐yellow defects, and patients with optic nerve disease tend to have red‐green defects.20 There are several exceptions to this rule. For example, the ONTT dyschromatopsia data reveals that optic neuritis patients initially present with a preponderance of blue‐yellow defects, and months later demonstrate a preponderance of red‐green defects.21 The results of our investigation also seem to violate Köllner's rule, with the presence of blue‐yellow defects in many asymptomatic carriers of LHON.
LHON selectively and severely degrades the small calibre fibres of the papillomacular bundle in affected individuals.22 If asymptomatic carriers of the mtDNA 11778 mutation exhibit any neural defect at all, it would probably be a subtle dysfunction corresponding to the same small calibre papillomacular fibres. These fibres subserve the foveal and perifoveal regions, with a greater area obviously devoted to the perifoveal region. The fovea itself is rich in red‐green cones while the larger perifoveal region is rich in blue cones. Therefore, loss of the papillomacular fibres subserving the perifoveal region would be expected to decrease blue‐yellow discrimination and produce a tritanopsia, violating Köllner's rule. Patients with retrobulbar optic neuritis exhibiting perifoveal visual field defects also exhibited a preponderance of tritanopsia while RBN patients with foveal field defects exhibited a preponderance of red‐green defects.23 We suggest the observed tritan defects in our carrier population may serve as a sensitive marker for subclinical, papillomacular fibre dysfunction and eagerly await further analysis though the Cambridge Colour test.
It is also important to note the preponderance for deutan‐like defects in the carrier group relative to the off pedigree controls. This finding also is consistent with the small calibre fibre dysfunction model. The fovea is considered to be primarily tritanopic, with sensitivity to red/green light constant throughout the central 2° of the visual field.24,25,26 The central fovea has a markedly reduced sensitivity to blue light, which increases in the perifoveal regions.25,26 Therefore, impairment of afferent channels serving the foveal region would prevent transmission of signals from red/green cones, whereas impairment of afferent channels from the perifoveal region would affect signals from blue cones. This red/green defect and significant but less severe blue/yellow defect is consistent with Verriest's type II deutan‐like dyschromatopsia and can explain the clinical findings of deutan dyschromatopsias in the carrier group.
The observed dyschromatopsias of the carriers are remarkable in that they are atypical of those commonly seen in other optic neuropathies, and may reflect a selective degeneration of primarily perifoveal as well as foveal fibres This study also serves to suggest that asymptomatic carriers of the mtDNA 11778 mutation may exhibit other subclinical, as of yet unidentified optic nerve dysfunctions.
Abbreviations
LHON - Leber's hereditary optic neuropathy
ROS - reactive oxygen species
Footnotes
Competing interests: none declared
References
- 1.Sadun A A. Acquired mitochondrial impairment as a cause of optic nerve disease. Trans Am Ophthalmol Soc 199846881–923. [PMC free article] [PubMed] [Google Scholar]
- 2.Sobel R S, Yanuzzi L A. Optic nerve toxicity: a classification. In: Singerman LJ, Jampol LM, eds. Retinal and choroidal manifestations of disease. Baltimore: Williams & Wilkins, 1991226–250.
- 3.Newman N J. Leber's hereditary optic neuropathy: new genetic considerations. Arch Neurol 199350540–548. [DOI] [PubMed] [Google Scholar]
- 4.Nikoskelainen E K. Clinical picture of LHON. Clin Neurosci 19942115–120. [Google Scholar]
- 5.Wallace D C, Singh G, Lott M T.et al Mitochondral DNA mutation associated with Leber's hereditary optic neuropathy. Science 19882421427–1430. [DOI] [PubMed] [Google Scholar]
- 6.Huoponen K, Vilkki J, Aula P.et al A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet 1991481147–1153. [PMC free article] [PubMed] [Google Scholar]
- 7.Howell N, Bindoff L A, McCullough D A.et al Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet 199149939–950. [PMC free article] [PubMed] [Google Scholar]
- 8.Johns D R, Neufeld M J. An ND‐6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Commun 19921871551–1557. [DOI] [PubMed] [Google Scholar]
- 9.Sadun A A, Win P H, Ross‐Cisneros F N.et al Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophth Soc 200098223–235. [PMC free article] [PubMed] [Google Scholar]
- 10.Verriest G, Van Laethem J, Uvijls A. A New assessment of the ranges of the Farnsworth‐Munsell 100‐hue test scores. Am J Ophthalmol 198293635–642. [DOI] [PubMed] [Google Scholar]
- 11.Smith K H, Johns D R, Heher K L.et al Heteroplasmy in Leber's hereditary optic neuropathy. Arch Ophthalmol 19931111486–1490. [DOI] [PubMed] [Google Scholar]
- 12.Carelli V, Ghelli A, Ratta M.et al Leber's hereditary optic neuropathy: biochemical effect of the 11778/ND4 and 3460/ND1 mutations and correlation with the mitochondrial genotype. Neurology 1997481623–1632. [DOI] [PubMed] [Google Scholar]
- 13.Carelli V, Ghelli A, Bucchi L.et al Biochemical features of mtDNA 14484 (ND6/M64V) point mutation associated with Leber's hereditary optic neuropath. Ann Neurol 199945320–328. [PubMed] [Google Scholar]
- 14.Brown M D, Trounce I A, Jun A S.et al Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778 or 14484 Leber's hereditary optic neuropathy. J Biol Chem 200027539831–39836. [DOI] [PubMed] [Google Scholar]
- 15.Wong A, Cortopassi G. mtDNA mutations confer cellular sensitivity to oxidant stress that is partially rescued by the calcium depletion and cyclosporin A. Biochem Biophys Res Commun 1997239139–145. [DOI] [PubMed] [Google Scholar]
- 16.Barrientos A, Moraes C T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem 199927416188–16197. [DOI] [PubMed] [Google Scholar]
- 17.Klivenyi P, Karg E, Rozsa C.et al α‐Tocopherol/lipid ratio in blood is decreased in patients with Leber's hereditary optic neuropathy and asymptomatic carriers of the 11778 mtDNA mutation. J Neurol Neurosurg Psychiatry 200170359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadun A A, Carelli V, Belfort R.et al A very large Brazilian pedigree with 11778 Leber's hereditary optic neuropathy. Trans Am Ophthalmol Soc 2002100169–177. [PMC free article] [PubMed] [Google Scholar]
- 19.Verriest G. Further studies on acquired deficiency of color discrimination. J Opt Soc Am 196353185–195. [DOI] [PubMed] [Google Scholar]
- 20.Köllner H.Die Störungen des Farbensinnes: Ihre klinische Bedeutung and ihre Diagnose. Berlin: Karger, 1912
- 21.Katz B. The dyschromatopsia of optic neuritis: a descriptive analysis of data from the optic neuritis treatment trial. Trans Am Ophthalmol Soc 199593685–708. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadun A A, Win P H, Carelli V.et al Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 200098223–232. [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman S E, Hart W M, Gordon M O.et al The dyschromatopsia of optic neuritis is determined in part by the foveal/perifoveal distribution of visual field damage. Invest Ophthalmol Vis Soc 1990311895–1902. [PubMed] [Google Scholar]
- 24.De Monasterio F M, McCrave E P, Newlander J K.et al Density profile of blue‐sensitive cones along the horizontal meridian of macaque retina. Invest Ophthalmol Vis Sci 198526289–302. [PubMed] [Google Scholar]
- 25.Williams D R, MacLeod D I, Hayhoe M M. Foveal tritanopia. Vis Res 1981211341–1356. [DOI] [PubMed] [Google Scholar]
- 26.Williams D R, MacLeod D I, Hayhoe M M. Punctate sensitivity of the blue‐sensitive mechanism. Vis Res 1981211357–1375. [DOI] [PubMed] [Google Scholar]