Abstract
Aim
To evaluate the effect of oral pilocarpine treatment on conjunctival epithelium of patients with Sjögren's syndrome (SS).
Methods
15 primary SS patients were included in this prospective, single masked, comparative study. Patients underwent oral pilocarpine treatment for 2 months and were studied before (T0) and after 1 month (T1), 2 months (T2), and 15 days after treatment suspension (T3). Systemic and ocular symptoms, tear film break up time (BUT), corneal fluorescein vital staining, Schirmer I test, tear basal secretion test, and conjunctival imprinting were performed. Student's t test and Mann‐Whitney U test were used for statistics.
Results
The conjunctival imprinting showed an increase of goblet cells number at T1 (1.6 (1.2) v 0.6 (0.7) at T0, p = 0.025) improving at T2 (5.1 (1.7); p<0.001 v T0 and T1). At T3 the number of goblet cells significantly decreased (1.9 (1.1); p<0.001 v T2). An improvement of dry mouth started at T1 and returned towards baseline values at T3. For ocular symptoms, burning and foreign body sensation were improved at T1 while ocular dryness improved at T2. BUT showed a statistically significant improvement at T2.
Conclusions
Oral pilocarpine induced an increase in goblet cells number and an amelioration of conjunctival epithelium not dependent on tear secretion.
Keywords: pilocarpine, conjunctiva, epithelium, goblet cells
Sjögren's syndrome (SS) is an autoimmune disease characterised by exocrine glands lymphocytic infiltration with consequent dysfunction of lacrimal and salivary glands, resulting in a sicca syndrome.1,2 Also the ocular surface is directly involved in the process with conjunctival lymphocytic infiltrates and apoptosis of epithelial cells.3,4
SS may occur in association with other autoimmune diseases (secondary SS) as rheumatoid arthritis, systemic lupus erythematosus, scleroderma, or in their absence (primary SS).5 Primary SS has extra glandular organ involvement including lung (interstitial pneumonia), kidney (interstitial nephritis), peripheral and central nervous system manifestations, vasculitis of skin and other organs, and increased frequency of lymphoma.6
The initial lymphocytic infiltrates have properties of Th‐1 type cells based on their secretion of IFN‐gamma, while, later in the course of inflammation, Th‐2 (IL‐4) and B cells are detected.
Even during the earliest stages of inflammation cytokines and inflammatory mediators are not only released by the lymphocytes but are also present on epithelial cells.4,7,8,9
Several cytokines were identified in tears of SS patients such as interleukin (IL)‐1, IL‐6, IL‐8, tumour necrosis factor (TNF)‐α, and transforming growth factor (TGF)‐β. It was suggested that these cytokines may have a role in the pathogenesis of the ocular surface alteration and could be used as markers of the disease.4,10
It was shown that cytokines such as IL‐1 or TNF‐α can dramatically decrease the ability of cholinergic nerves to release acetylcholine (Ach) in response to stimulation of their receptors (mAChR).11
An additional way in wich lymphocytes may inhibit secretory response in SS is the production of antibodies directed against the M3 mAChR.12
A neural regulation of goblet cells activity was demonstrated by the presence of parasympathetic and sympathetic nerves adjacent to human and mouse goblet cells; furthermore, muscarinic and adrenergic receptors were found on goblet cells.13,14
Lacrimal gland fluid production is largely under muscarinic receptor control and in fact the M3 receptor subtype was identified in the lacrimal gland tissue.15 Subsets of patients with primary or secondary SS produce functional salivary IgA autoantibodies that interact with the M3 mAChRs and interfere with the parasympathetic neurotransmitters at the acinar cell membranes of salivary glands.1 The possibility that IgG anti‐mAChR might have a role in the pathogenesis of dry eye in SS was proposed.16
The M3 mAChR upregulation in the SS biopsy suggests a decreased release of ACh from the residual nerve, with a compensatory increase in the expression of M3 AChR. The increased number of receptors could be a therapeutic target for analogues of ACh such as pilocarpine.17
Pilocarpine is a natural alkaloid first used for the treatment of dry mouth induced by radiation and for treatment of dry eye in SS.18 Recently oral pilocarpine was shown to be effective in improving symptoms and signs of dry eye in SS patients.19 All three muscarinic receptors subtypes (RM1; RM2; RM3) were detected on goblet cells in human conjunctiva.14
Goblet cells can release their secretory granules as a reflex response mediated by the activation of either parasympathetic or sympathetic nerves that surround them. The neural regulation of goblet cell secretion is essential to provide protection of the ocular surface. Goblet cells synthesise and secrete mucins which are essential for maintaining the health of the ocular surface.20,21 A modified goblet cell mucin secretion can result in injury to both the cornea and conjunctiva.
The aim of this work was to evaluate the effects of systemic pilocarpine treatment on the ocular surface and symptoms of discomfort in SS patients.
Patients and methods
In this prospective, single masked, comparative study, 15 women (age range 41–73 years), affected by primary SS, diagnosed according to the classification criteria proposed by the American‐European Consensus Group,22 were randomly chosen, according to a list of random numbers, and enrolled among patients referring to the ocular surface diseases unit of the ophthalmology section of the department of surgical specialties at the University of Messina, Italy. This research was conducted in accordance with the tenets of the Declaration of Helsinki.
Inclusion criteria
Patients able and willing to participate in the study signed an informed consent. The patients included in the study should present symptoms of ocular discomfort and a stable disease and general therapy for at least 1 month before the beginning of the study. Patients were allowed to continue their usual general therapy and topical treatment with tear substitutes.
Exclusion criteria
Unstable general disease or changes in systemic therapy within 1 month before the beginning of the study. Concurrent disease such as diabetes, contact lens wear, use of drugs such as β blockers, calcium antagonists, benzodiazepines, antidepressants, antihistaminics, hormones, any ocular therapy other than tear substitutes.
Treatment protocol
Patients were given with pilocarpine hydrochloride 5 mg tablets (Salagen, Novartis Farma, Hettlingen, Switzerland). For the first month increasing doses from one to four tablets daily, increasing each week, were administered. In the second month the therapeutic regimen of four tablets daily was followed. Treatment was stopped at the end of the second month.
Patients were studied before (T0) and after 1 month (T1) and 2 months (T2) of treatment and then at follow up after 15 days of treatment suspension (T3).
Symptom questionnaire
A symptom questionnaire was administered to the patients by operators who were not involved in the clinical testing of the patients. Visual analogue rating scales (VARS) for systemic symptoms (xerostomia, vaginal dryness, and skin dryness) and ocular symptoms (burning, foreign body sensation, dryness, itching, mucous secretion, photophobia, hyperaemia, tearing) were administered.
Ocular tests
The tests were performed, during routine ambulatory sections, by an observer who was masked about the time of observation of the study and who was different from the person who collected the symptom questionnaire.
The following ocular tests were performed: tear film break up time (BUT) (seconds); corneal fluorescein vital staining (score 0–15),23 Schirmer I test (SNO strips, Chauvin Pharmaceuticals Ltd, Kingston Upon Thames, UK) normal values above 10 mm/5 minutes; basal secretion test (SNO strips, Chauvin Pharmaceuticals Ltd) normal values above10 mm/5 minutes24; conjunctival imprinting.
For conjunctival imprinting, Supor 200 filters (Gelmann, France) were cut in half. Samples were obtained from each patient from the conjunctiva at the 12 o'clock position, 2 mm far from the limbus. The cells obtained were immediately transferred on a polylysinate slide and fixed with 4% paraformaldehyde. The specimen was then stained by haematoxylin and eosin stain (Merck, Darmstadt, Germany) and observed and photographed with a Zeiss Axiovert 10, light microscope. The observer was masked about the time of collection of the specimens under study. A cytological score was obtained as previously described considering the following cellular parameters: specimen cellularity, cell to cell junction, nucleus/cytoplasm ratio, nuclear chromatin, goblet cells distribution, inflammatory cells presence. For each parameter a score was attributed: 0 for normal pattern, 1 for borderline pattern, 2 and 3 (only for inflammatory cells presence) for abnormal patterns. A total score was obtained adding the results of each parameter.25,26,27 For goblet cells count three microscopic fields were randomly chosen from each slide at a final magnification of ×200.
Statistical analysis
Primary efficacy variables were the conjunctival cytology with particular regard to the goblet cells distribution. The statistical analysis of the results was carried out in a masked method, using the software SAS (version 8.1). Only the results of the right eyes were considered for statistical analysis. Student's t test and Mann‐Whitney U test were used as appropriate. A value of p⩽0.05 was considered statistically significant.
Results
The effect of oral pilocarpine on systemic symptoms was reported in table 1. The only statistically significant improvement was observed for xerostomia starting at T1 and lasting to T2. At T3 the symptom xerostomia was raised again towards basal values.
Table 1 VARS for systemic symptoms.
| Mouth dryness | Skin dryness | Vagina dryness | |
|---|---|---|---|
| T0 | 75.7 (13.7) | 32.6 (38.6) | 31.6 (31.4) |
| T1 | 50.8 (12.5)* | 26.8 (35.6) | 27.0 (32.1) |
| T2 | 47.6 (10.8)* | 13.6 (28.3) | 19.6 (20.6) |
| T3 | 63.5 (16.4)† | 28.4 (22.5) | 21.5 (18.6) |
*p<0.001 v T0; †p = 0.03 v T2.
As to ocular discomfort symptoms, a statistically significant reduction of burning, foreign body sensation, and ocular dryness was observed, starting from T1; also ocular dryness was statistically significantly reduced at T2. At the follow up visit at T3, the mean VARS score for foreign body sensation was still statistically significantly lower than the baseline value (table 2).
Table 2 VARS for ocular symptoms.
| Burning | Foreign body | Dryness | Itching | Mucus secretion | Photophobia | Hyperaemia | Tearing | |
|---|---|---|---|---|---|---|---|---|
| T0 | 51.1 (27.9) | 29.6 (23.1) | 52.1 (36.2) | 32.0 (33.6) | 8.6 (14.8) | 14.7 (14.5) | 9.4 (20.8) | 0 (0) |
| T1 | 24.1 (12.2)* | 7.4 (9.4)* | 20.4 (26.6) | 11.4 (12.6) | 4.1 (8.9) | 6.8 (9) | 1.3 (3.4) | 7.4 (19.6) |
| T2 | 21.7 (9.8)* | 3.1 (5.8)* | 14.6 (21.5)* | 9.6 (11.6) | 0.7 (1.9) | 3.6 (6.3) | 1.1 (3) | 6.4 (17) |
| T3 | 36.5 (19) | 12.5 (14.5) | 35.8 (36.3) | 20.6 (21.7) | 5.7 (11.3) | 12.3 (13) | 5.0 (13.2) | 0 (0) |
*p<0.02 v T0.
For ocular tests only the BUT showed a statistically significant increase at time T2. No other statistically significant changes were observed for the remaining tests (table 3).
Table 3 Ocular tests results.
| BUT | Corneal fluorescein stain | Schirmer's I test | Basal secretion test | |
|---|---|---|---|---|
| T0 | 1.8 (0.7) | 1.4 (1) | 13.3 (8.4) | 7.5 (10.6) |
| T1 | 2.4 (1) | 1.3 (0.9) | 13.5 (7) | 10.0 (11.3) |
| T2 | 2.6 (0.8)* | 0.7 (0.7) | 15.3 (9.8) | 11.0 (12.7) |
| T3 | 2.0 (1.3) | 1.1 (0.5) | 14.0 (8.3) | 7.8 (9.8) |
*p<0.05 v T0.
As to the conjunctival cytology the scoring system showed at time T2, a statistically significant amelioration of all parameters with the exception of the presence of inflammatory cells.
At time T3 all parameters showed a statistically significant increased score (table 4).
Table 4 Conjunctival cytology parameters results.
| Cellularity | Cell‐cell junctions | Nucleus/cytoplasm ratio | Chromatin | Goblet cells | Inflammatory cells | Total score | |
|---|---|---|---|---|---|---|---|
| T0 | 1.9 (0.3) | 1.8 (0.4) | 1.8 (0.4) | 1.8 (0.4) | 1.7 (0.5) | 0.9 (1.2) | 12.3 (2.5) |
| T1 | 1.6 (0.5) | 1.6 (0.5) | 1.5 (0.5) | 1.5 (0.5) | 1.5 (0.5) | 0.7 (1) | 10.1 (2.4) |
| T2 | 0.7 (0.6)* | 0.7 (0.5)* | 0.6 (0.5)* | 0.5 (0.5)* | 0.5 (0.5)* | 0.3 (0.7) | 3.8 (2.2)* |
| T3 | 1.4 (0.5)† | 1.5 (0.5)† | 1.3 (0.4)† | 1.3 (0.5)† | 1.5 (0.5)† | 0.4 (0.8) | 8.6 (2.2)† |
*p<0.001 v T0 and T1; †p<0.01 v T2.
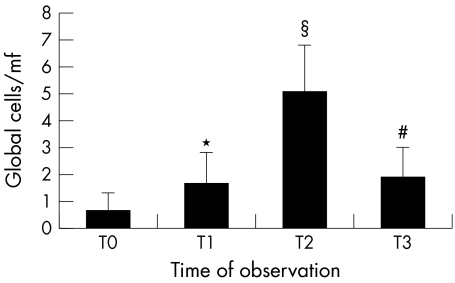
The goblet cell number was 0.6 (SD 0.7) at T0. At T1 a statistically significant increase was observed (1.6 (1.2); p = 0.025 v T0). At T2 a further increase in goblet cells number was observed (5.1 (1.7); p<0.001 v T0 and T1). The observation 1 month after treatment suspension (T3) revealed a statistically significant decrease of goblet cells number (1.9 (1.1); p<0.001 versus T2), although it remained statistically significantly higher than the T1 visit (p = 0.030) (figs 1 and 2).
Figure 1 Number of goblet cells/microscopic field (mf) (magnification of observation ×200). *p = 0.025 v T0; § = p<0.001 v T0, T1, T3; # = p<0.001 v T0.
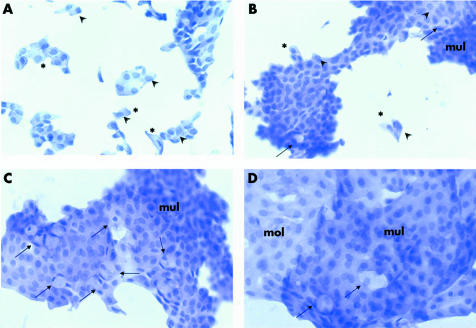
Figure 2 (A) Conjunctival imprinting obtained before pilocarpine treatment. Epithelial cells appear organised in a monolayer with small clumps or isolated cells, it is often possible to distinguish cell borders. Some cells show normal nucleus/cytoplasm ratio others an abnormally reduced nucleus/cytoplasm ratio (*). Many cells show uneven nuclear chromatin with evident nucleoli, sign of cell activation (arrowhead). No goblet cells are present. (haematoxylin and eosin ×200). (B) After 1 month of treatment: epithelial cells are arranged in wider area of multilayered cells (mul), sign of good cell to cell connection, less distinguishable borders and normal nucleus/cytoplasm ratio. Some isolated cells still persist with an altered nucleus/cytoplasm ratio (*) and unevenly dispersed nuclear chromatin (arrowhead). Few goblet cells are present (arrow) (haematoxylin and eosin ×200). (C) After 2 months of treatment. Large sheets of multilayered epithelium (mul) with cells with tight cell to cell connection and undistinguishable borders, normal nucleus/cytoplasm ratio, evenly dispersed chromatin. Goblet cells are well represented (arrow) (haematoxylin and eosin ×200). (D) Follow up visit 15 days after treatment suspension. Large sheets of epithelial cells are present, both multilayered (mul) and monolayered (mol). Several cells have a slightly reduced nucleus/cytoplasm ratio but evenly dispersed chromatin. Goblet cells are still present (arrow) although their number appears reduced (haematoxylin and eosin ×320).
During the course of the treatment with oral pilocarpine the most common adverse event was sweating (in six patients, 40%); other adverse effects were chill (in three patients, 20%), nausea (in two patients, 13.3%), oversalivation (in two patients, 13.3%), gastritis (in one patient, 6.6%). None of these adverse events caused the affected patients to drop out of the study.
Discussion
Pilocarpine was proposed to increase tear and saliva production in patients with dry eye and dry mouth consequent to SS or gland irradiation for the treatment of cranial tumours. It was shown, by binding the muscarinic receptors M3 and M1, to stimulate the watery secretions of lacrimal and salivary glands and to prevent the acinar apoptosis.28 Furthermore, it was shown that pilocarpine interferes with the deleterious effects of pro‐inflammatory cytokines.29 The use of oral pilocarpine as an agonist of the muscarinic receptor for the treatment of the oral and ocular dryness was recently approved by the US Food and Drug Administration.1
Controlled studies showed that oral pilocarpine significantly improves sicca symptoms in the eyes, mouth, and other sites. It was shown that pilocarpine, at 20 mg/day, significantly improved the patients' ability to expectorate mucus; release of Ach from parasympathetic nerves activates postjunctional muscarinic receptors present on airway smooth muscle, submucosal glands and blood vessels, and causes respectively bronchoconstriction, mucus secretion, and vasodilatation.30
The results of this study showed a statistically significant amelioration of conjunctival cytological features after 2 months of pilocarpine treatment. This amelioration was not accompanied by an increased tear production as demonstrated by the unchanged results of Schirmer's I test and tear basal secretion test. This seems to indicate that the effect of pilocarpine on symptoms of discomfort and ocular surface is not mediated only by an increased tear secretion as stated in other studies.31,32 Therefore, it seems possible that it is the direct effect of pilocarpine on the conjunctival epithelium and, in particular, on goblet cells. In fact, it was demonstrated that goblet cells are surrounded by both parasympathetic and sympathetic nerves, which can have a role in the regulation of goblet cell secretion. Furthermore, muscarinic receptor subtypes were detected on goblet cells of human conjunctiva.13,14 Goblet cell activity plays a key part in maintaining a healthy ocular surface through mucus production. In particular, goblet cells produce some glycoproteins, such as mucin 5 and specifically express the MUC5AC gene encoding for a gel forming glycoprotein essential for tear film stability. The main result of our study was the amelioration of conjunctival epithelium, as shown by impression cytology, with an increased number of goblet cells and the better condition of the epithelial cells. The stimulation of muscarinic receptors, apart from the stimulation of the secretory function, may help prevent the apoptosis,33 so allowing a better function of the residual goblet cells. The improved condition of the conjunctival epithelium might account for the reduced ocular discomfort encountered in the course of pilocarpine treatment, even in absence of increased tear secretion.
Abbreviations
Ach - acetylcholine
BUT - break up time
IL - interleukin
RM - muscarinic receptors subtypes
SS - Sjögren's syndrome
TGF - transforming growth factor
TNF - tumour necrosis factor
VARS - visual analogue rating scales
Footnotes
Competing interests: none declared
References
- 1.Fox R I, Stern M, Michelson P. Update in Sjögren's syndrome. Curr Opin Rheumatol 200012391–396. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K. Pathogenesis of Sjögren's syndrome. Autoimmun Rev 2003213–18. [DOI] [PubMed] [Google Scholar]
- 3.Brignole F, Pisella P J, Goldschild M.et al Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalml Vis Sci 2000411356–1363. [PubMed] [Google Scholar]
- 4.Jones D T, Monroy D, Ji Z, Atherton S S.et al Sjögren's syndrome: cytokine and Epstein‐Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci 1994353493–3504. [PubMed] [Google Scholar]
- 5.Jonsson R, Haga H J, Gordon T P. Current concepts on diagnosis, autoantibodies and therapy in Sjögren's syndrome. Scand J Rheumatol 200029341–348. [DOI] [PubMed] [Google Scholar]
- 6.Fox P C, Tornwall J, Maruyama T.et al Evolving concept of diagnosis, pathogenesis and therapy of Sjögren's Syndrome. Curr Opin Rheumatol 199810446–456. [DOI] [PubMed] [Google Scholar]
- 7.Fox R I, Kang H I, Andor D.et al Cytokine mRNA expression in salivary gland biopses of Sjögren's Syndrome. J Immunol 19941525532–5539. [PubMed] [Google Scholar]
- 8.Baudouin C, Haouat N, Brignole F.et al Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br J Ophthalmol 199276545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baudouin C, Brignole F, Becquet F.et al Flow cytometry in impression cytology specimens: a new method for evaluation of conjunctival inflammation. Invest Ophthalmol Vis Sci 1997381458–1464. [PubMed] [Google Scholar]
- 10.Aragona P, Scullica L. Attività della Interleuchina‐1 nelle lacrime di pazienti affetti da sindrome di Sjögren secondaria. In: Proceedings of the 73rd Congress of the Società Oftalmologica Italiana. Roma,1993261–266.
- 11.Lu G, Beuerman R W, Zhao S.et al Tumor necrosis factor‐alpha and interleukin‐1 induce activation of MAP kinase and SAP kinase in human neuroma fibroblasts. Neurochem Int 199730401–410. [DOI] [PubMed] [Google Scholar]
- 12.Bacman S, Sterin‐Borda L, Camuso J J.et al Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren's syndrome. Clin Exp Immunol 1996104454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rios J D, Forde K, Diebold Y.et al Development of conjunctival goblet cells and their neuroreceptor subtype expression. Invest Ophthalmol Vis Sci 2000412127–2137. [PubMed] [Google Scholar]
- 14.Diebold Y, Rios J D, Hodges R R.et al Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci 2001422270–2282. [PubMed] [Google Scholar]
- 15.Manduit P, Jannes H, Rassiguel B. M(3) muscarinic acetilcholine‐receptor compling to PLC in rat exorbital lacrimal acinar‐cells. Am J Physiol 1993264550–560. [DOI] [PubMed] [Google Scholar]
- 16.Bacman S, Berra A, Sterin‐Borda L.et al Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjögren syndrome. Invest Ophthalmol Vis Sci 200142321–327. [PubMed] [Google Scholar]
- 17.Walcott B, Cameron R H, Brink P R. The anatomy and innervation of lacrimal glands. Adv Exp Med Biol 199435011–18. [DOI] [PubMed] [Google Scholar]
- 18.Wiseman L R, Faulds D. Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerstomia. Drugs 199549143–155. [DOI] [PubMed] [Google Scholar]
- 19.Tsifetaki N, Kitsos G, Paschides C A.et al Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren's syndrome: a randomised 12 week controlled study. Ann Rheum Dis 2003621204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argueso P, Gipson I K. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res 200173281–289. [DOI] [PubMed] [Google Scholar]
- 21.Inatomi T, Spurr‐Michaud S, Tisdale A S.et al Expression of the secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci 1996371684–1692. [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R.et al Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemp M A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J 199521221–232. [PubMed] [Google Scholar]
- 24.Aragona P, Cannavò S P, Borgia F.et al Usefulness of the ocular surface study in patients with acne vulgaris treated with oral isotretinoin. Br J Dermatol 2005152576–578. [DOI] [PubMed] [Google Scholar]
- 25.Aragona P, Romeo G F, Puzzolo D.et al Impression cytology of the conjunctival epithelium in patients with vernal conjunctivitis. Eye 19961082–85. [DOI] [PubMed] [Google Scholar]
- 26.Aragona P, Ferreri G, Micali A.et al Morphological changes of the conjunctival epithelium in contact lens wearers evaluated by impression cytology. Eye 199812461–466. [DOI] [PubMed] [Google Scholar]
- 27.Aragona P, Di Stefano G, Ferreri F.et al Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in sjögren's syndrome patients. Br J Ophthalmol 200286879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox R I, Konttinen Y, Fisher A. Use of muscarinic agonists in the treatment of Sjögren's syndrome. Clinical Immunol 2001101249–263. [DOI] [PubMed] [Google Scholar]
- 29.Fisher A. Therapeutic strategies in Alzheimer's disease: M1 muscarinic agonists. Jpn J Pharmacol 200084101–112. [DOI] [PubMed] [Google Scholar]
- 30.Coulson F R, Fryer A D. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther 20039859–69. [DOI] [PubMed] [Google Scholar]
- 31.Vivino F B. The treatment of Sjögren's syndrome patients with pilocarpine‐tablets. Scand J Rheumatol 2001115(Suppl)1–9. [DOI] [PubMed] [Google Scholar]
- 32.Vivino F B, Al‐Hashimi I, Khan Z.et al Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren's syndrome: a randomized, placebo‐controlled, fixed‐dose, multicenter trial. P92‐01 Study Group. Arch Intern Med 1999159174–181. [DOI] [PubMed] [Google Scholar]
- 33.Budd D C, Spragg E J, Ridd K.et al Signalling of the M3‐muscarinic receptor to the anti‐apoptotic pathway. Biochem J 2004381(Pt 1)43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]