Abstract
Background/aims
The authors investigated the expression of S100A1, S100A6, S100B, MelanA, and CEA in conjunctival naevi, primary acquired melanosis (PAM), conjunctival melanoma, and uveal melanoma in order to assess their potential usefulness in the pathological differential diagnosis of these entities.
Methods
Paraffin embedded sections of 18 conjunctival naevi, 14 PAM, 16 conjunctival melanomas, and 20 uveal melanomas were immunostained for S100A1, S100A6, S100B, MelanA, and CEA, and expression was scored semiquantitatively.
Results
Expression of S100A1 differed significantly between conjunctival naevi and conjunctival melanoma, with percentages of positive cells of 30.6% and 71.4%, respectively. Conjunctival melanomas had high average scores for S100A1 and S100B (71.4%, 62.9%, respectively), while uveal melanomas also had high S100A1 but low S100B scores (88.5%, 18.5%, respectively). MelanA was highly variable; naevi and uveal melanoma had higher average scores than conjunctival melanoma. CEA was hardly detectable in all four groups.
Conclusion
S100A1 seems to be a possible candidate to differentiate conjunctival naevi from conjunctival melanoma. S100B seems to differentiate between uveal melanoma and conjunctival melanoma. However, the study size was small and therefore the data have to be confirmed by others.
Keywords: uveal melanoma, conjunctival melanoma, conjunctival naevi, primary acquired melanosis
The most common melanocytic lesions of the conjunctiva include conjunctival naevus, primary acquired melanosis (PAM), and conjunctival melanoma. Clinically, their distinction may be difficult,1 and in case of doubt, histological investigation is required. PAM is subdivided histologically into a typical and an atypical form, and especially the latter, as well as conjunctival melanoma, has a high tendency for local recurrence after treatment.2,3 The mortality rate of patients with a conjunctival melanoma is about 30% in 10 years.2,3,4,5,6,7,8,9,10
Immunophenotypic markers might be of help in distinguishing between benign and malignant melanocytic lesions of the conjunctiva. Previous studies addressed S100, MelanA, and HMB45 expression in conjunctival melanoma,11,12,13,14,15,16,17 and a few studies compared expression patterns on conjunctival naevus or PAM with conjunctival melanoma.14,15,16,17 Although most markers have proved to be of value in the establishment of the melanocytic origin of various lesions, they are of much less help in the distinction between a melanocytic naevus and a melanoma.14,15,16
Calcium binding proteins like S100 have been implicated in establishing the malignant and metastatic phenotype of various tumours.18,19,20 The S100 protein family consists of over 20 members. The expression of S100A1, S100A2, S100A3, S100A4, S100A6, and S100B has been studied previously in cutaneous melanoma.21,22,23,24,25 S100A6 seems of some use in the distinction between a Spitz‐naevus and a cutaneous melanoma.25 The S100B level in serum is of considerable interest as a prognostic marker in cutaneous melanoma, and has been used to monitor patients with metastatic cutaneous melanoma.23
In this study we stained histological samples of conjunctival naevus, PAM, conjunctival melanoma, and uveal melanoma for S100A1, S100A6, S100B, MelanA, and CEA.
Materials and methods
Patients and tissues
Eighteen conjunctival naevi, 14 PAM (one with no atypia, four with mild atypia, six with moderate atypia, and three with severe atypia), 16 conjunctival melanomas, and 20 uveal melanomas (six epithelioid, eight spindle, and six mixed cell type) from different patients were collected from the pathology archives of the Leiden University Medical Centre, Leiden, the Netherlands. Patient records were used to obtain information on local or distant recurrence, and to investigate whether patients with PAM or naevus subsequently developed conjunctival melanoma.
Immunohistochemistry
Specimens were fixed in 4% neutral buffered formaldehyde and embedded in paraffin. Immunohistochemical reactions were performed using the streptavidin‐biotin method. Sections were cut at 4 µm and mounted on glass slides, and deparaffinised in xylene (four times, 5 minutes each) and ethanol 99% (twice, 5 minutes each). The endogenous peroxidase activity was blocked by incubating the slides with methanol/H2O2 0.3% for 20 minutes. After the slides were washed, antigen retrieval was performed by boiling in citrate buffer (Dako, Glostrup, Denmark) for 10 minutes. Slides were washed again in phosphate buffered saline (PBS), and they were incubated overnight with the first antibody at 4°C. Rabbit anti‐S100A1 polyclonal (dilution1:100) (A5109, Dako), mouse anti‐S100A6 mAb clone CACY‐100 (dilution 1:4000) (S‐5049, Sigma‐Aldrich, Steinheim, Germany), mouse anti‐S100B mAb clone DAK‐S100B/2 (dilution 1:100) (M7221, Dako), mouse anti‐MelanA mAb clone A103 (dilution 1:100) (M7196, Dako), and mouse anti‐CEA mAb clone 11‐7 (dilution 1:50) (M7072, Dako) were used. As negative control, the primary antibody was replaced by PBS. Slides were then incubated with biotinylated anti‐mouse anti‐rabbit Ig (Dako) for 30 minutes. After washing, the slides were labelled with Streptavidin‐HRP (Dako) for 30 minutes; hereafter the labelling was made visible with a 30 minute incubation in AEC (3‐amino‐9‐ethylcarbazole) or DAB (3,3 diaminobenzidine). Slides were counterstained with Mayer's haematoxylin and finally embedded in Kaiser's glycerine. For the CEA antibody, sections of colon carcinoma were used as a positive control; for S100A1, S100A6, S100B, and MelanA, sections of cutaneous melanoma were used.
Scoring
The immunolabelled slides were interpreted by determining the percentage positively staining cells, scored on a scale of 0% to 100%, in steps of 10%. Slides were independently scored by at least two people; in all cases agreement was reached.
Statistics
For statistical analysis, the mean (SEM) was used. Data were analysed in SPSS 11.0 (SPSS Inc, Chicago, IL, USA). A one way ANOVA test was used to calculate significance between the groups. A two tailed non‐parametric Mann‐Whitney U test was used to compare two variables.
Results
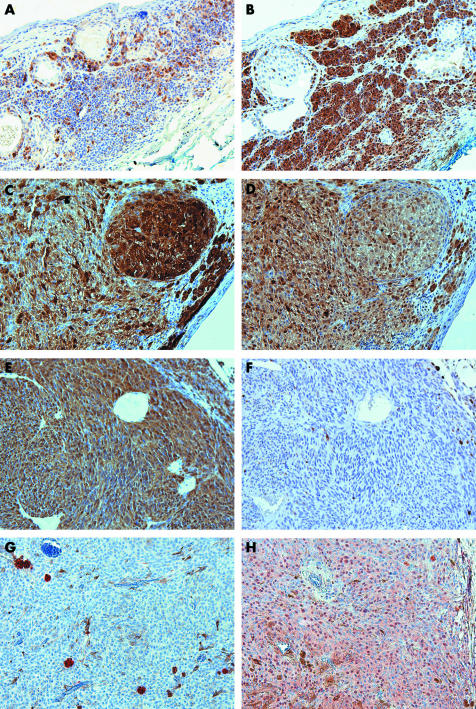
Of a total of about 400 slides, six were lost because of technical problems or lack of residual tissue in the blocks. As a consequence, only 13 of 14 PAM specimens could be stained with S100A6 and MelanA, 14 of 16 conjunctival melanoma were stained with S100A1 and S100B. Figure 2 shows the staining results for S100A1, S100A6, S100B, and CEA staining in representative sections of conjunctival naevi, PAM, conjunctival melanoma, and uveal melanoma.
Figure 2 (A) and (B) represent the S100A1 and S100B expression on conjunctival naevi, respectively. (C) and (D) show the expression of S100A1 and S100B on conjunctival melanoma, respectively. (E–H) represent the S100A1, S100B, CEA, and S100A6 expression on uveal melanoma, respectively. Mark the difference in S100A1 expression between conjunctival naevi and conjunctival melanoma, also the S100B expression between uveal and conjunctival melanoma is remarkably different. All figures are with a magnification of ×200.
S100A1
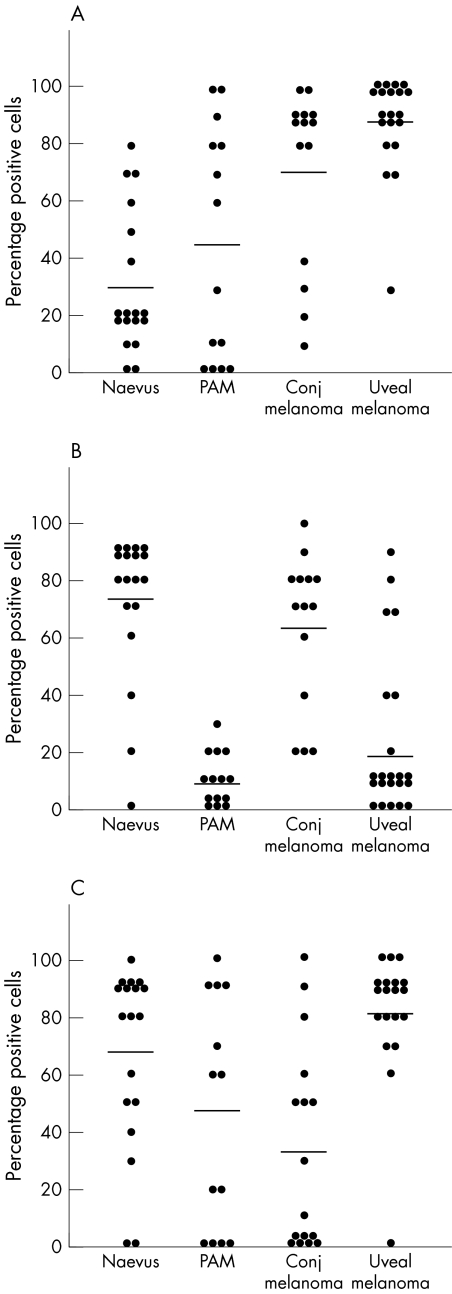
S100A1 positively stained more than 10% of the lesional cells of 16 of the 18 conjunctival naevi (89%), of 10 of 14 PAM (71%), of all conjunctival melanomas (n = 14, 100%), and all uveal melanoma (n = 20, 100%) (fig 2). Significantly more cells were positive in conjunctival melanoma compared to conjunctival naevi (71.4%, and 30.6%, respectively) (p = 0.001 Mann‐Whitney) (fig 1), while the PAM reacted intermediately (45.0% positive cells).
Figure 1 Percentage positive cells after staining for antibodies against S100A1 (A), S100B (B), and MelanA (C) in conjunctival naevus, PAM, conjunctival melanoma, and uveal melanoma specimens. Each dot represents a single case, the line represents the average score for each group of melanocytic lesions. (S100A1: naevus versus conjugated melanoma p = 0.001, Mann‐Whitney U test) (S100B: PAM versus naevus p<0.0001, PAM versus conjugated melanoma p<0.0001, conjugated melanoma versus uveal melanoma p<0.0001, Mann‐Whitney U test).
S100A6
In all uveal melanomas more than 10% of the cells stained positively; the average percentage of positive cells was very high (72.1%). All conjunctival tissues showed severe non‐specific staining, and could therefore not be scored: most epithelial cells stained positive, which has been described previously.21,22
S100B
More than 10% of the cells scored positively with S100B of 17 of the 18 conjunctival naevi (94%), of eight of 14 PAM (57%), of all conjunctival melanoma (n = 14, 100%), and of 15 of 20 uveal melanoma (75%). Comparing the average scores for S100B, naevus and conjunctival melanoma scored significantly higher than PAM (p<0.001) (fig 1). The average percentage positive cells in conjunctival melanoma was significantly higher than in the uveal melanoma (62.9%, 18.5%, respectively) (p<0.0001, Mann‐Whitney) MelanA
MelanA stained more than 10% of the cells in 16 of the 18 naevi (89%), nine of 13 PAM (69%), nine of 16 conjunctival melanoma (56%), and 19 of 20 uveal melanoma (95%). Most naevi and uveal melanoma had a good expression of MelanA, while in PAM and conjunctival melanoma expression was very variable (fig 1).
CEA
CEA was completely negative in most lesions, but in one naevus , two conjunctival melanomas, and 15 uveal melanomas, groups of non‐melanocytic cells were positive (fig 2). The total number of CEA positive cells was very low in all categories.
The atypia stage of the PAM lesions did not correlate with the expression levels of the tested antibodies. No significant difference in expression was seen between uveal or conjunctival melanomas in patients with or without metastasis, although for S100A1 and S100A6 there was a trend towards higher expression in uveal melanoma patients with metastatic disease.
Discussion
In this study, we set out to determine whether expression patterns of various markers may differentiate between benign and malignant conjunctival pigmented lesions, as previous studies did not yield a positive result.14,15,16,17 In our study, S100A1 staining differed between a naevus and a conjunctival melanoma. The naevi had low S100A1 expression (mean 30.6%), while in most conjunctival melanomas expression was strong (mean 71.4%) (figs 1 and 2). It must be noted that because of the rarity of histological sections of conjunctival naevi and conjunctival melanomas, the sample size is small. Since no other studies have been published about S100A1 staining of melanocytic conjunctival lesions, confirmation by others will be awaited. S100B staining did not differ between conjunctival naevi and conjunctival melanoma, however, a lower staining was seen in the PAM lesions (fig 1). Others also found no difference in S100 expression between conjunctival naevi and conjunctival melanoma.16,17,26 Steuhl et al did also find slightly lower expression of S100 in epithelial melanosis than in conjunctival naevi or conjunctival melanoma.16 However, Hitzer et al, and Sharara et al did not find any difference between PAM and conjunctival naevi , and conjunctival melanoma.17,26 The former studies used the “general” S100 antibody which probably represents the S100B used in our study.
To our surprise MelanA was not present in seven of the 16 conjunctival melanomas, while the expression in PAM and conjunctival naevi was higher. Others found higher expression of MelanA in conjunctival melanoma,12,13 although Heegaard et al found weak staining of the MelanA in most of the conjunctival melanomas.12 Our findings indicate that MelanA is not a good marker for conjunctival melanoma.
Whether conjunctival melanoma is biologically more closely related to the skin melanoma or the uveal melanoma is still a topic of discussion. We included uveal melanoma samples as a control and to compare expression of the conjunctival lesions with this tumour. The S100B was as abundantly expressed in conjunctival melanomas as the S100A1, while in uveal melanomas the expression of S100A1 was also high, but S100B was low. Others also found higher expression of S100B in conjunctival melanoma than in uveal melanoma.12,13 Iwamoto claims that conjunctival melanoma is most similar to the epithelioid phenotype of the cutaneous melanoma.13 Table 1 compares the S100A1, S100A6, and S100B data from this study with data on cutaneous melanoma in the literature. All lesions have similarities for one or two antibodies, but one cannot conclude that conjunctival melanoma resembles cutaneous melanoma more closely than uveal melanoma. Perhaps it is better to see them as three separate identities, although clinically the conjunctival melanoma has more similarities with cutaneous melanoma, since both have the tendency to metastasise to the regional lymph node first.
Table 1 Expression of S100A1, S100A6, and S100B of conjunctival melanoma, uveal melanoma, and cutaneous melanoma.
| S100A1 | S100A6 | S100B | |
|---|---|---|---|
| Conjunctival melanoma † | +++ | * | ++ |
| Uveal melanoma † | +++ | +++ | + |
| Cutaneous melanoma ‡ | 0 | ++ | ++ |
The tested antibodies may not only be used for differentiation between lesions but may also be potential serum metastases markers. Especially molecules with a high expression in the malignant lesion may be good candidates. Serum levels of S100B have proved to be reliable serum markers to follow metastasis in cutaneous melanoma.23 For both uveal and conjunctival melanoma good serum markers may also be of help in detecting and following primary and metastastic disease. For uveal melanoma, S100A1 and S100A6 could be candidate serum markers, since they are abundantly expressed on uveal melanoma, for conjunctival melanoma S100A1 and S100B could be good candidates to test in the serum. Van Ginkel already implied that S100A6 could be of influence on malignant transformation of the uveal melanoma.27 Serum S100B was not able to detect metastatic disease in patients with uveal melanoma,28 probably since S100B is not well expressed in uveal melanoma, but serum S100A1b was able to detect metastatic disease in uveal melanoma patients (GS Missotten et al, submitted). As far as we know, no serum markers have yet been analysed in conjunctival melanoma. In 1976 Michelson et al reported a slightly elevated blood CEA levels in 45% of the uveal melanoma patients, although the increase of CEA levels was only marginal.29 We also investigated the CEA expression of conjunctival and uveal lesions, but could not find any tumour labelling, although in some tumours we did find staining of a few non‐melanocytic cells (fig 2), which were microscopically identified as melanophages. As CEA is not expressed, using it as a serum marker seems inefficient.
In conclusion, we think that of those we studied S100A1 is the best marker to differentiate between a naevus and conjunctival melanoma, although it does not provide an absolute cut‐off value. Because of the small sample size, further studies will be needed to confirm these findings.
Acknowledgements
The authors thank Professor WJ Mooi and his laboratory for the S100A1 and S100B staining. We also appreciate the help of ER Barthen and CJM Krose with the S100A6, MelanA, and CEA stainings.
Abbreviations
DAB - 3,3 diaminobenzidine
PAM - primary acquired melanosis
PBS - phosphate buffered saline
Footnotes
Competing interests: none declared
References
- 1.Wolff‐Rouendaal de D. Management of conjunctival tumours. In: Oosterhuis JA, ed. Ophthalmic tumours. Dordrecht: Dr Junk Publishers, 1985159–171.
- 2.Wolff‐Rouendaal de D. Conjunctival melanoma in the Netherlands: a clinico‐pathological and follow‐up study. The Netherlands: Thesis, University of Leiden, 1990
- 3.Missotten G S, Keijser S, De Keizer R J.et al Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci 20054675–82. [DOI] [PubMed] [Google Scholar]
- 4.Paridaens A D, Minassian D C, McCartney A C.et al Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol 199478252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werschnik C, Lommatzsch P K. Long‐term follow‐up of patients with conjunctival melanoma. Am J Clin Oncol 200225248–255. [DOI] [PubMed] [Google Scholar]
- 6.Norregaard J C, Gerner N, Jensen O A.et al Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol 1996234569–572. [DOI] [PubMed] [Google Scholar]
- 7.Seregard S, Kock E. Conjunctival malignant melanoma in Sweden 1969–91. Acta Ophthalmol 199270289–296. [DOI] [PubMed] [Google Scholar]
- 8.Shields C L. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc 200098471–492. [PMC free article] [PubMed] [Google Scholar]
- 9.Shields C L, Shields J A, Gunduz K.et al Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol 20001181497–1507. [DOI] [PubMed] [Google Scholar]
- 10.Bobic‐Radovanovic A, Latkovic Z, Marinkovic J.et al Predictors of survival in malignant melanoma of the conjunctiva: a clinico‐pathological and follow‐up study. Eur J Ophthalmol 199884–7. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs U, Kivela T, Liesto K.et al Prognosis of conjunctival melanomas in relation to histopathological features. Br J Cancer 198959261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heegaard S, Jensen O A, Prause J U. Immunohistochemical diagnosis of malignant melanoma of the conjunctiva and uvea: comparison of the novel antibody against melan‐A with S100 protein and HMB‐45. Melanoma Res 200010350–354. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto S, Burrows R C, Grossniklaus H E.et al Immunophenotype of conjunctival melanomas. Arch Ophthalmol 20031201625–1629. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell J M, Sun Y Y, Wagner D. HMB‐45 immunohistochemical staining of conjunctival melanocytic lesions. Ophthalmology 199198453–458. [DOI] [PubMed] [Google Scholar]
- 15.Steuhl K P, Rohrbach J M, Knorr M. Distribution of melanoma‐associated antigens (HMB 45 and S 100) in benign and malignant melanocytic tumors of the conjunctiva. Klin Monatsbl Augenheilkd 1991199187–191. [DOI] [PubMed] [Google Scholar]
- 16.Steuhl K P, Rohrbach J M, Knorr M.et al Significance, specificity, and ultrastructural localization of HMB‐45 antigen in pigmented ocular tumors. Ophthalmology 1993100208–215. [DOI] [PubMed] [Google Scholar]
- 17.Sharara N A, Alexander R A, Luthert P J.et al Differential immunoreactivity of melanocytic lesions of the conjunctiva. Histopathology 200139426–431. [DOI] [PubMed] [Google Scholar]
- 18.Schafer B W, Heizmann C W. The S100 family of EF‐hand calcium‐binding proteins: functions and pathology. Trends Biochem Sci 199621134–140. [DOI] [PubMed] [Google Scholar]
- 19.Takenaga K, Nakamura Y, Sakiyama S. Expression of antisense RNA to S100A4 gene encoding an S100‐related calcium‐binding protein suppresses metastatic potential of high‐metastatic Lewis lung carcinoma cells. Oncogene 199714331–337. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Davies M P, Rudland P S.et al Transcriptional down‐regulation of the metastasis‐inducing S100A4 (p9Ka) in benign but not in malignant rat mammary epithelial cells by GC‐factor. J Biol Chem 199727220283–20290. [DOI] [PubMed] [Google Scholar]
- 21.Boni R, Burg G, Doguoglu A.et al Immunohistochemical localization of the Ca2+ binding S100 proteins in normal human skin and melanocytic lesions. Br J Dermatol 199713739–43. [PubMed] [Google Scholar]
- 22.Boni R, Heizmann C W, Doguoglu A.et al Ca(2+)‐binding proteins S100A6 and S100B in primary cutaneous melanoma. J Cutan Pathol 19972476–80. [DOI] [PubMed] [Google Scholar]
- 23.Hauschild A, Engel G, Brenner W.et al Predictive value of serum S100B for monitoring patients with metastatic melanoma during chemotherapy and/or immunotherapy. Br J Dermatol 19991401065–1071. [DOI] [PubMed] [Google Scholar]
- 24.Maelandsmo G M, Florenes V A, Mellingsaeter T.et al Differential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanoma. Int J Cancer 199774464–469. [DOI] [PubMed] [Google Scholar]
- 25.Ribe A, McNutt N S. S100A6 protein expression is different in Spitz nevi and melanomas. Mod Pathol 200316505–511. [DOI] [PubMed] [Google Scholar]
- 26.Hitzer S, Bialasiewicz A A, Richard G. Immunohistochemical markers for cytoplasmic antigens in acquired melanosis, malignant melanomas, and nevi of the conjunctiva. Klin Monatsbl Augenheilkd 1998213230–237. [DOI] [PubMed] [Google Scholar]
- 27.Van Ginkel P R, Gee R L, Walker T M.et al The identification and differential expression of calcium‐binding proteins associated with ocular melanoma. Biochim Biophys Acta 19981448290–297. [DOI] [PubMed] [Google Scholar]
- 28.Missotten G S, Tang N E, Korse C M.et al Prognostic value of S‐100‐beta serum concentration in patients with uveal melanoma. Arch Ophthalmol 20031211117–1119. [DOI] [PubMed] [Google Scholar]
- 29.Michelson J B, Felberg N T, Shields J A. Carcinoembryonic antigen. Its role in the evaluation of intraocular malignant tumors. Arch Ophthalmol 197694414–416. [DOI] [PubMed] [Google Scholar]