Abstract
Aims
To compare the theoretical retinal threshold time for endoilluminators and experimental phototoxic effect using A2e laden retinal pigment epithelial (RPE) cells.
Methods
The spectral irradiances of three types of 20 gauge and 25 gauge endoilluminators, currently commercially available from two manufacturers, were evaluated in conditions where the total beam spectral power was divided by the beam spot size at an estimated use distance of 5 mm from the retina. The retinal threshold time was calculated using the guidelines recommended by the International Commission on Non‐Ionizing Radiation Protection. In vitro, A2e laden cells were evenly exposed to light for 30 minutes with a standard endoilluminator positioned 1 cm above the cells and the cell viability was assessed by WST‐1 assay.
Results
The retinal threshold times were within 1 minute for all the endoilluminators tested. A significant decrease in the viability of A2e laden RPE cells was observed after they were exposed to light from two of the three 20 gauge endoilluminators. Cell viability was not affected by the exposure to 25 gauge endoilluminators under the same conditions. There was no correlation between the theoretical threshold times and experimental data.
Conclusions
Light exposure during vitrectomy can induce photochemical damage to the retina. Although the A2e laden RPE model may not correctly mimic a clinical situation, this model may be useful to estimate the possible photochemical damage to RPE cells that could not be deduced by a theoretical retinal hazard model.
Keywords: vitrectomy, endoilluminator, phototoxicity, A2e
It is generally assumed that radiation from ultraviolet and blue light is hazardous to the retina. Several studies have demonstrated that irradiation from surgical devices such as endoilluminators, exceed the existing safety standards.1,2 Actually, several clinical reports suggest that retinal phototoxic damage can occur in clinical situations.3,4 Previous investigations estimate the retinal threshold time of endoilluminator induced phototoxic damage by calculating the threshold phototoxic exposure time based on theoretical values offered by the International Commission on Non‐Ionizing Radiation Protection (ICNIRP)1 or the American Conference of Government Industrial Hygienists (ACGIH).2 To weight the phototoxic effects of blue light, van den Biesen et al,1 and Miller et al,2 employed the guidelines provided by the ICNIRP and the ACGIH, respectively. The integrated aphakic weighted irradiance and the threshold limit value (TLV) time were calculated, and it was concluded that the TLV time was between 0.2–3.5 minutes. This seems an unrealistically short exposure time for a vitrectomy. One reason may be that safety factors are incorporated and Van den Biesen et al1 do speculate that the safety factor is 33 for the ICNRP, and Miller et al,2 10 or more for the ACGIH. As an alternative approach to estimating the retinal threshold time for endoilluminators, Meyer et al5 and Miller et al both assumed the retinal threshold dose to be 200 J/cm2 based on experimental owl monkey models and that the calculated theoretical time required to produce an ophthalmoscopically visible lesion is as long as 75 minutes and 48 minutes, respectively. However, these assumptions neglect the fact that retinal phototoxicity is dependent on the spectral irradiance of the light. To the best of our knowledge, there has been no other clinically relevant model system to assess the retinal phototoxicity induced by light irradiation from endoilluminators. With the advent of new types of endoilluminators, the proper assessment of phototoxicity is becoming increasingly important.
Previous investigations1,2,5 have suggested that photochemical reactions, but not photothermal reactions, induced by the irradiation of endoilluminator play a major part in retinal damage. Among several endogeneous photosensitisers that mediate photochemical reactions in retina, one of the major intracellular chromophores responsible for the blue light sensitivity is the lipofuscin constituent, A2e.6 A2e forms by sequential condensation of all‐trans‐retinalaldehyde with phosphatidylethanolamine.7 In vivo, A2e accumulates in retinal pigment epithelium (RPE) with age, and is considered to be involved in age related macular degeneration.7 In vitro, the viability of A2e laden RPE cells is reduced when they are exposed to blue light, suggesting that A2e mediates blue light toxicity, at least in part.8
This study investigates the usefulness of an A2e laden RPE model to assess A2e mediated phototoxic effects of the irradiation from endoilluminators. Using several fibre optics and light sources that output light with different irradiances, the theoretical retinal hazard was examined and the results obtained from the A2e laden model compared.
Materials and methods
Light probes
In the present study, two illumination devices , produced by two different manufacturers (Alcon and Bausch & Lomb), were used. One illumination device was equipped with a halogen lamp and the other with a metal halide lamp. Note that the latter device had two types of output light through two different filters and these are referred to as “halogen‐like” and “xenon‐like”, as designated by the manufacturer. For both devices 20 gauge fibre light guides and 25 gauge fibre light guides, provided by the manufacturers, were tested. The 25 gauge fibre light guides were developed for a transconjunctival sutureless vitrectomy system.9 Given the attenuation of light by the endoilluminators of the 25 gauge system vitrectomy, it is likely that light toxicity would be less than that of the conventional 20 gauge system. Thus, in total, six types of endoilluminators were tested (20G and 25G halogen, 20G and 25G xenon‐like, and 20G and 25G halogen‐like; see table 1).
Table 1 Integrated aphakic weighted irradiance and calculated time to threshold limit value (TLV), total irradiance, and calculated time to damage.
| Endoilluminators | Manufacturer | Light source | Filter type | System | Aphakic weighted irradiance (300–700 nm) | Time to TLV (minutes) | Total irradiance (250–800 nm) | Time to damage (minutes) |
|---|---|---|---|---|---|---|---|---|
| 20G halogen | Brand A | Halogen | Halogen | 20G | 125 | 0.2 | 165 | 12.6 |
| 25G halogen | 25G | 41 | 0.6 | 88 | 37.8 | |||
| 20G xenon‐like | Brand B | Metal halide | Xenon‐like | 20G | 219 | 0.11 | 421 | 7.9 |
| 25G xenon‐like | 25G | 47 | 0.53 | 110 | 30.2 | |||
| 20G halogen‐like | Brand B | Metal halide | Halogen‐like | 20G | 108 | 0.23 | 211 | 15.8 |
| 25G halogen‐like | 25G | 32 | 0.79 | 68 | 48.7 |
In all experiments, the 25 gauge endoilluminator was held at the same distance as the 20 gauge endoilluminator and the highest light intensities were chosen. Note that this may not be the case during an actual vitrectomy, as the brighter light of the 20 gauge may result in it being held further away. It must also be considered that vitrectomy procedure times may differ between the 20 gauge and 25 gauge systems.
Spectral irradiance
Spectral irradiance was measured with a spectroradiometer. Measurements were taken from 300–800 nm at 5 nm intervals, similar to previous reports.1,2 Briefly, the delivery fibres were positioned in front of the input aperture at a distance of 2 mm, such that the entire beam was measured by the spectroradiometer. The total beam spectral power was divided by the beam spot size at an estimated use distance of 5 mm from the retina.
Theoretical retinal hazard analysis
Retinal exposure limits for blue light photochemical retinal hazard were calculated using the guidelines given by the International Commission on Non‐Ionizing Radiation Protection (ICNIRP), similar to a previous report.1 The aphakic hazard function from the ICNIRP was employed. To calculate the time to damage, the total irradiance over the 250–800 nm was determined for comparison with the experimental threshold data of 200 J/cm2 similar to previous reports.2,5
Cell culture
A human retinal pigment epithelial cell line, ARPE‐19, was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). Cell cultures were maintained in DMEM/F12 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL), penicillin (100 U/ml), and streptomycin (100 mg/ml) (Gibco BRL).
Preparation of A2e laden RPE cells
A2e was synthesised and purified as has been previously reported.7 A2e laden ARPE cells were prepared as had been previously reported with minor modifications.8 Briefly, after ARPE‐19 cells were grown confluent on 24 well plates, they were incubated with A2e to allow its intracellular accumulation. Preliminary experiments demonstrated that 24 hour incubation with 50 mM A2e was the most appropriate to assess the phototoxicity of the endoilluminators. Under these conditions, the intracellular concentration of A2e is similar to those observed in human RPE.8
Light exposure
After washing with phosphate buffered saline (PBS), the cells were exposed to light with a standard endoillumination probe connected to a light source. The probe was positioned at 1 cm above the cells and the cells were evenly exposed for 30 minutes at 37°C. One hour after the irradiation, the viability of the cells was determined by the WST‐1 assay (Roche). The assay was performed according to the manufacturer's instructions. Samples that were not exposed to light illumination served as the control. All groups consisted of six wells and the results were averaged.
Statistical analysis
Statistical analysis was done by an analysis of variance followed by Bunnett's post hoc test. A p value of <0.05 or less was considered significant.
Results
Theoretical retinal hazard
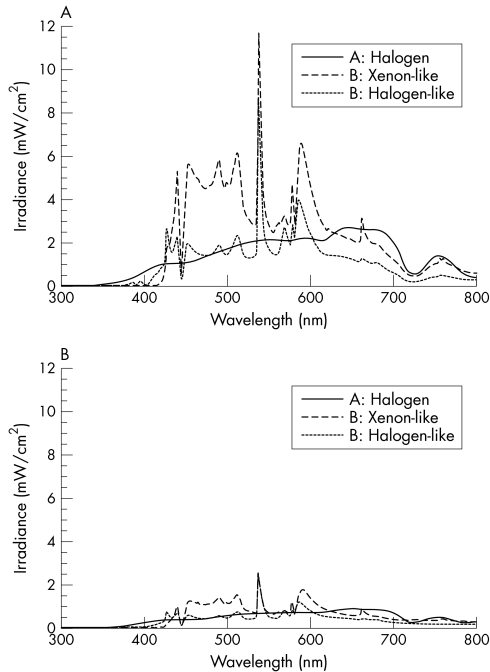
Theoretical retinal hazard was evaluated under the conditions in which the total beam spectral power was divided by the beam spot size at an estimated use distance of 5 mm from the retina. Figure 1A shows the typical spectral irradiances from the 20 gauge endoilluminators. Note that “halogen‐like light” and “xenon‐like light” are not actually from halogen light and xenon light sources, respectively, but are from a metal halide light source. The colour temperatures of the filtered lights resemble those of halogen and xenon, and are thus referred to as “halogen‐like” and “xenon‐like.” Table 1 shows the integrated power of the illuminators, the time to reach the ICNIRP guideline thresholds for blue light retinal hazard (TLV time), and the calculated time to damage using the experimental data on owl monkeys. With the 20 gauge system vitrectomy endoilluminators, the integrated power ranged from 528–1052 mW/cm2. All of the sources showed TLV times of less than 1 minute. The time to damage was between 7.9–15.8 minutes. As expected, the total irradiance and the integrated aphakic weighted irradiance from the 25 gauge light system was 26–32% and 21–33% less than that of the 20 gauge system, respectively (fig 1B and table 1). Accordingly, the calculated TLV times and time to damage increased to 0.53–0.79 minutes and 30.2–48.7 minutes, respectively (table 1).
Figure 1 Spectral irradiance of the endoilluminators. Absolute spectral irradiance of 20 gauge (A) and 25 gauge (B) endoilluminators evaluated. Note that the spectral irradiance was reduced to approximately 30% of the endoilluminators of the 20 gauge vitrectomy system
Experimental light toxicity to RPE cells
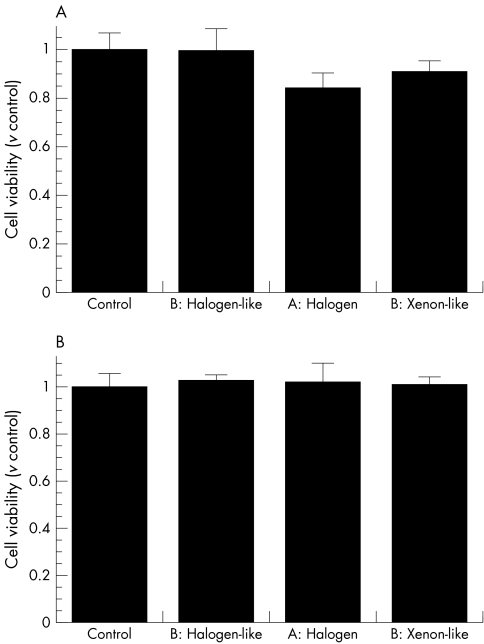
In the RPE cell viability analysis, the cells were exposed to light with standard endoillumination positioned at 1 cm above the cells and the cells were evenly exposed for 30 minutes at 37°C. The light exposure did not affect the viability of RPE cells that were not preincubeted with A2e (data not shown). When the A2e laden cells were irradiated with the 20 gauge xenon‐like light or halogen light for 30 minutes, the viability of the cells was less than that of the control (fig 2A). Interestingly, the viability of the cells treated with the 20 gauge halogen‐like light was no less than the control under the same conditions (fig 2A). Thus, two of the three 20 gauge endoilluminators tested, led to a significant decrease in the cell viability of A2e laden RPE cells. In contrast, no obvious changes were found in the viability of the cells treated with the 25 gauge endoilluminators under the same conditions (fig 2B). Note that the 25 gauge endoilluminator was held at the same distance as the 20 gauge endoilluminator in all experiments.
Figure 2 Phototoxic effects of endoilluminators on A2e laden RPE cells. After ARPE‐19 cells were incubated with A2e to allow intracellular accumulation, they were exposed to light from 20 gauge (A) and 25 gauge (B) endoilluminators for 30 minutes. Cell viability was examined by WST‐1 assay. Cells not exposed to light illumination served as the control. Bars represent mean; error bars indicate standard deviation (n = 6 wells in each experiment). *p<0.05 by ANOVA followed by Bunnett's post hoc test (v non‐irradiated group).
Discussion
The results from the theoretical retinal hazard models were similar to previous studies.1 Using the guidelines provided by ICNIRP, the threshold time was calculated to be less than 1 minute for all 20 gauge endoilluminators tested in this study. Even with the attenuated light of the 25 gauge endoilluminators, the threshold time was less than 1 minute. These results, together with previous reports1,2 demonstrating that the threshold time was between 0.2–2.1 minutes and 0.27–3.5 minutes using the ICNIRP and ACGIH guidelines, respectively, suggest that the retinal threshold time is relatively low. However, considering this extremely short threshold time, the fact that the guidelines of the ICNIRP and the AHGIH were not intended for the light induced damage of endoilluminators and the fact that safety factors were probably incorporated in these guidelines, it remains questionable whether it is appropriate to apply the value obtained using this model to estimate the theoretical retinal hazard of endoilluminators. Comparing the threshold values with the calculated time to damage, perhaps the incorporation of a safety factor of 33 into the ICNIRP guidelines should be considered, as has been suggested by van den Biessen et al.1
This is the first study investigating A2e mediated retinal phototoxicity using the A2e laden RPE model. Our in vitro experiments demonstrated that with an exposure time of 30 minutes, light from the 20 gauge endoilluminators induces phototoxic damage to A2e laden RPE cells to varying degrees. Although there is a trend for the endoilluminators with the more intense light to induce more damage, the reduction of A2e laden cell viability correlated with neither the total irradiance nor the aphakic weighted irradiance. In particular, the following two observations suggest that the parameters obtained by the theoretical retinal hazard model are not useful for predicting the phototoxic effects on A2e laden RPE cells. Firstly, although the total irradiance from the 20 gauge xenon‐like light is higher and thus its TLV time shorter than the 20 gauge halogen light, the phototoxic effects of the two endoilluminators are comparable. Secondly, although the TLV time and time to damage of 20 gauge halogen light and 20 gauge halogen‐like light were comparable, cell viability was affected by the 20 gauge halogen light but not by the 20 gauge halogen‐like light (see fig 1A). Moreover, it is worth mentioning that while it has been stated that the light instruments induce similar levels of A2e mediated damage the 20 gauge xenon‐like light appears brighter in a clinical setting and may thus actually induce more photochemical damage. This might be because the xenon‐like filter cuts out blue light with a wavelength shorter than 420 nm, whereas the halogen filter allows shorter wavelength light through (see fig 1A). Therefore, the use of short wavelength light filters may be important in reducing A2e mediated phototoxicity. Additionally, because both the halogen‐like and xenon‐like lights are from an identical light source and both filter out blue light with a wavelength below 420 nm, the total irradiance over 420 nm (see fig 1) is important in reducing A2e mediated RPE damage. This idea is supported by the fact that A2e laden cells do absorb light with a wavelength over 420 nm, despite the fact that their peak absorbance is below 380 nm.8
The light from the endoilluminators in the 25 gauge transconjunctival vitrectomy system was attenuated and the total irradiance was 32–34% of the 20 gauge system endoilluminators. In the current study, it was assumed that the length of the intraocular portion of the procedure was the same for a 20 gauge as for a 25 gauge system, which may limit this study. However, considering that the average surgical time of the 25 gauge system vitrectomy is 17 minutes9 and that 30 minutes' exposure did not affect the viability of A2e laden cells in the in vitro experiments, the A2e mediated light damage is likely to be negligible. This may be one of the factors in explaining why earlier postoperative visual recovery is obtained by the 25 gauge transconjunctival vitrectomy system.10
A disadvantage of this study is that a steady fixation of the light on the retina was assumed and the highest light intensities chosen, but in practice these settings are unlikely. Additionally, this study neglects the interaction between the photoreceptors and RPE, which may exacerbate or alleviate the effects. This study does demonstrate however, that light exposure during vitrectomy can induce photochemical damage to the retina. The A2e laden RPE model is useful in estimating the possible photochemical damage to RPE cells that could not be deduced using the theoretical retinal hazard model. In the current study, endoilluminators that are currently commercially available were used. In near future, with proper optical design, 25 gauge light sources should be able to output light of a similar intensity to that of the 20 gauge system. Additionally, this experiment system is suitable for recently developed non‐movable light sources such as the chandelier and torpedo type endoilluminators. The phototoxic effects of such endoilluminators are issues for future research.
Abbreviations
FBS - fetal bovine serum
PBS - phosphate buffered saline
RPE - retinal pigment epithelium
TLV - threshold limit value
Footnotes
Competing interest: none declared
References
- 1.Van den Biesen P R, Berenschot T, Verdaasdonk R M.et al Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol 2000841372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller S A, Landry R J, Byrnes G A. Endoilluminators: evaluation of potential retinal hazards. Appl Opt 2004431648–1653. [DOI] [PubMed] [Google Scholar]
- 3.Michels M, Lewis H, Abrams G W.et al Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am J Ophthalmol 1992114287–296. [DOI] [PubMed] [Google Scholar]
- 4.Postel E A, Pulido J S, Byrnes G A.et al, eds. Long‐term follow‐up of iatrogenic phototoxicity. Arch Ophthalmol 199811675–77. [DOI] [PubMed] [Google Scholar]
- 5.Meyers S M, Bonner R F. Retinal irradiance from vitrectomy endoilluminators. Am J Ophthalmol 19829426–29. [DOI] [PubMed] [Google Scholar]
- 6.Sparrow J R, Fishkin N, Zhou J.et al A2E, a byproduct of the visual cycle. Vis Res 2003432983–2990. [DOI] [PubMed] [Google Scholar]
- 7.Parish C A, Hashimoto M, Nakanishi K.et al Isolation and one‐step preparation of A2E and iso‐A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA 19989514609–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow J R, Parish C A, Hashimoto M.et al A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci 1999402988–2995. [PubMed] [Google Scholar]
- 9.Fujii G Y, De Juan E, Jr, Humayun M S.et al A new 25‐gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109: 1807–12; discussion, 1813 [DOI] [PubMed]
- 10.Fujii G Y, De Juan E, Jr, Humayun M S.et al Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology 20021091814–1820. [DOI] [PubMed] [Google Scholar]