Abstract
Aim
To determine whether eyes implanted with the Lenstec KH‐3500 “accommodative” intraocular lenses (IOLs) have improved subjective and objective focusing performance compared to a standard monofocal IOLs.
Methods
28 participants were implanted monocularly with a KH‐3500 “accommodative” IOL and 20 controls with a Softec1 IOL. Outcome measures of refraction, visual acuity, subjective amplitude of accommodation, objective accommodative stimulus response curve, aberrometry, and Scheimpflug imaging were taken at ∼3 weeks and repeated after 6 months.
Results
Best corrected acuity with the KH‐3500 was 0.06 (SD 0.13) logMAR at distance and 0.58 (0.20) logMAR at near. Accommodation was 0.39 (0.53) D measured objectively and 3.1 (1.6) D subjectively. Higher order aberrations were 0.87 (0.85) μm and lower order were 0.24 (0.39) μm. Posterior subcapsular light scatter was 0.95% (1.37%) greater than IOL clarity. In comparison, all control group measures were similar except objective (0.17 (0.13) D; p = 0.032) and subjective (2.0 (0.9) D; p = 0.009) amplitude of accommodation. Six months following surgery, posterior subcapsular scatter had increased (p<0.01) in the KH‐3500 implanted subjects and near word acuity had decreased (p<0.05).
Conclusions
The objective accommodating effects of the KH‐3500 IOL appear to be limited, although the subjective and objective accommodative range is significantly increased compared to control subjects implanted with conventional IOLs. However, this “accommodative” ability of the lens appears to have decreased by 6 months post‐surgery.
Keywords: intraocular lens, presbyopia, ocular accommodation, eye focus
The proposed principal action for “accommodating” intraocular lenses (IOLs), presently marketed for the correction of presbyopia, is an anterior shift of the lens on contraction of the ciliary muscle. Subjective amplitude of accommodation has been found to be on average 1.33–2.36 D1,2,3,4,5,6 with “accommodating” compared to approximately 0.42–1.08 D for conventional non‐accommodative IOLs.3,6 Various attempts have been made to objectively quantify the range of accommodation with these IOLs.4,5,6,7 Biometry before and after pharmaceutically induced ciliary muscle contraction has suggested movement of, on average, between 100 μm and 1040 μm as measured with ultrasound,5,6,8,9,10 partial coherence interferometry,3,5,6,10,11 or image analysis.10 Dynamic aberrometry suggests changes in defocus of up to 1 D.7
Streak retinoscopy and photorefraction have shown apparent accommodation of on average ∼1.0–1.2 D for the 1CU IOL compared to 0.2–0.4 D for patients implanted with a conventional non‐accommodative IOL.3,5,6 However, the target at 0.35 metre (compared to a 5 metre baseline) will present a blurred target to the visual system at near for the majority of subjects, which is known to affect the accommodative accuracy and does not quantify the accommodation exerted to view intermediate targets. In addition, none of the previously published objective accommodation measures have been made on eyes implanted with the KH‐3500 IOL (Lenstec, St Petersburg, FL, USA).
Method
Informed consent was obtained from the subjects before inclusion in the study after explanation of the nature and possible consequences of the study. The inclusion criteria were patients undergoing routine cataract surgery to remove a lenticular opacity affecting the visual demand of the patient. Patients were excluded from the study if they had associated ocular co‐morbidity. The research followed the tenets of the Declaration of Helsinki and was approved by the Solihull local research ethics committee.
Twenty eight subjects aged 42–88 years (average 72.9 (12.2)) from a single centre were randomised to have phacoemulsification cataract surgery and implantation of a KH‐3500 “accommodative” IOL in one eye. A further 20 subjects aged 57–92 years (average 71.1 (9.7)) had phacoemulsification cataract surgery and implantation of a conventional non‐accommodative IOL (Softec1, Lenstec) in one eye (control group). Each subject underwent a full subjective binocular refraction at 6 metres.
The KH‐3500 and Softec1 are single piece, spherical, acrylic IOLs with refractive indices of 1.46. The central optic portion is 5.75 mm and the overall size 12.0 mm in diameter. However, the KH‐3500 IOL has a flexible haptic that is designed to allow the whole lens to move anteriorly in the capsular bag secondary to ciliary muscle contraction, unlike the hinged haptics in the 1CU “accommodating” IOL design.
Three weeks and 6 months (plus or minus 1.5 weeks) post‐implantation, following retinoscopy and subjective refraction, optimally distance corrected threshold letter acuity at distance and near threshold word acuity at near (40 cm) was measured with logMAR progression charts. Contrast sensitivity was measured with a Pelli‐Robson chart at 1 metre. Amplitude of accommodation was measured three times with an RAF binocular gauge and averaged (ClementClarke/ Haag‐Streit, UK).
Monocular objective accommodative responses were measured using the SRW‐5000 (Shin‐Nippon Commerce Inc, Tokyo, Japan) through undilated pupils. Subjects viewed a static 90% contrast Maltese cross (100 lux) located at 0.00 D, 0.50 D, 1.00 D, 1.50 D, 2.00 D, 2.50 D, 3.00 D, 3.50 D, and 4.00 D accommodative demand through a Badal optical system in a random order.
Low and high (up to sixth) order Zernike aberrations across the dilated pupil were quantified using an optical path difference (OPD) skiascopy wavefront sensing device (Nidek, Gamagori, Japan) over a 6 mm pupil. Pupil size was measured with the OPD before dilatation.
Lenticular posterior capsular light scatter was assessed through dilated pupils using a Pentacam (Oculus, Wetzlar, Germany) rotating Scheimpflug technique. Twenty five anterior chamber sections of the eye (separated by ∼7°) were captured and the light scatter from the centre of the IOL subtracted from that of the posterior capsule.
Results
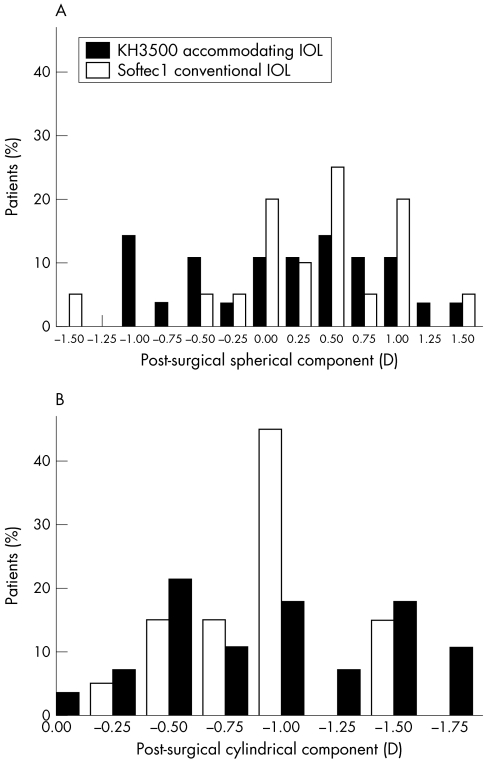
The mean spherical equivalent refractive error of the “accommodative” IOL implanted subjects was −0.23 (0.69) D (average (SD)) compared to −0.06 (0.69) D with the Softec1 (p = 0.568; fig 1).
Figure 1 (A) Frequency of residual spherical refractive error and (B) cylindrical error following IOL implantation surgery (n = 28 KH‐3500 “accommodative” IOL, n = 20 Softec1 conventional IOL).
The best corrected distance acuity was on average +0.06 (0.13) logMAR with the KH‐3500 compared to +0.08 (0.15) logMAR with the Softec1 (p = 0.519). The near word acuity at 40 cm with their optimal distance prescription was on average 0.58 (0.20) logMAR with the KH‐3500 compared to 0.62 (0.25) logMAR with the Softec1 (p = 0.684). Contrast sensitivity was similar in KH‐3500 (+1.57 (0.27) log units) and Softec1 (+1.58 (0.15) log units, p = 0.913) implanted subjects.
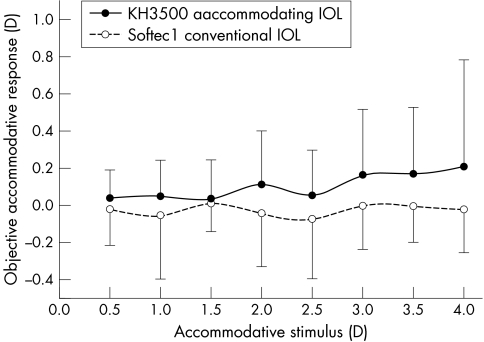
The objective amplitude of accommodation was significantly greater (p = 0.032) for the “accommodative” IOL (average 0.39 (0.53) D; range 0.00–2.69 D) compared to the Softec1 (average 0.17 ((0.13) D; range 0.00–0.44 D; fig 2) implanted subjects. Examining individual stimulus response curves of eyes implanted with the KH‐3500 “accommodative” IOL identified several different profiles. There was a linear increase in accommodative response with increasing stimulus demand in four eyes, an increase followed by a flattening/decrease in seven eyes, an increase only at higher levels of stimulus demand in six eyes, and no apparent increase in accommodative response in 11 eyes.
Figure 2 Accommodative stimulus response curve as measured with the SRW‐5000 (n = 28 KH‐3500 “accommodative” IOL, n = 20 Softec1 conventional IOL). Error bars = plus or minus 1 SD
The subjective amplitude of accommodation was significantly greater (p = 0.009) for the KH‐3500 (average 3.1 (1.6) D; range 1.0–6.4 D) compared to the conventional IOL (2.0 (0.9) D; 0.5–3.2 D). The correlations between subjective and objective amplitude of accommodation and other measures for the KH‐3500 and Softec1 IOLs are shown in table 1. The average undilated pupil size was similar (p = 0.586) for subjects implanted with the KH‐3500 (4.0 (0.9) mm) and with the Softec1 IOL (3.8 (1.3) mm).
Table 1 Pearson product moment correlation of objective and subjective accommodation with the KH‐3500 “accommodative” IOL and Softec1 conventional IOL, 2–3 weeks after lens implantation.
| Subjects | IOL power | MSE refraction | Distance VA | Near VA | CS | Pupil size | Higher order aberrations | Lower order aberrations | Subcap scatter | Patient age | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Objective accommodation | |||||||||||
| KH‐3500 | 28 | 0.18 | −0.24 | −0.06 | −0.31 | −0.17 | −0.27 | −0.25 | −0.14 | −0.18 | 0.09 |
| p = 0.379 | p = 0.259 | p = 0.770 | p = 0.126 | p = 0.429 | 0 = 0.288 | p = 0.230 | p = 0.492 | p = 0.383 | p = 0.656 | ||
| Softec1 | 20 | 0.54 | −0.28 | 0.00 | 0.29 | −0.13 | 0.30 | −0.68 | −0.50 | 0.35 | 0.51 |
| p = 0.021* | p = 0.265 | p = 0.999 | p = 0.242 | p = 0.598 | p = 0.286 | p = 0.006* | p = 0.061 | p = 0.170 | p = 0.031* | ||
| Subjective accommodation | |||||||||||
| KH‐3500 | 28 | 0.37 | −0.19 | −0.18 | −0.44 | 0.08 | −0.15 | 0.08 | 0.03 | 0.23 | 0.05 |
| p = 0.054 | p = 0.356 | p = 0.357 | p = 0.019* | p = 0.704 | p = 0.567 | p = 0.691 | p = 0.871 | p = 0.269 | p = 0.820 | ||
| Softec1 | 20 | 0.60 | −0.13 | −0.07 | −0.06 | 0.15 | 0.03 | −0.00 | 0.00 | 0.37 | 0.20 |
| p = 0.009* | p = 0.600 | p = 0.774 | p = 0.815 | p = 0.556 | p = 0.917 | p = 0.988 | p = 0.991 | p = 0.143 | p = 0.426 | ||
IOL power (D); MSE, mean spherical equivalent refraction (D); VA, visual acuity (logMAR); CS, contrast sensitivity (log units); Pupil size (mm); Aberrations (μm); Subcap scatter, lenticular posterior subcapsular light scatter (% compared to IOL clarity); Patient age (years). *Indicates significance at p<0.05.
Aberrometry showed no statistical difference in higher (0.87 (0.85) v 0.98 (0.59); p = 0.939) or lower (0.24 (0.39) v 0.28 (0.30) μm; p = 0.935) order aberrations between the KH‐3500 and the Softec1 IOL. Lens densitometry showed no significant difference in posterior capsular light scatter for the KH‐3500 (0.95% (1.37%) of the IOL clarity) compared to the Softec1 IOL (1.03% (1.61%); p = 0.418). Light scatter was not strongly correlated with either distance (r = 0.11, p = 0.542) or near (r = 0.22, p = 0.145) visual acuity.
Follow up after 6 months showed no significant change from zero in data except for a decrease in the smallest print seen at near (p = 0.031) accompanied by a decrease in the subjective amplitude of accommodation, which approached significance, and a significant increase in posterior capsular light scatter in subjects implanted with the KH‐3500 “accommodating” IOL (table 2).
Table 2 Change (average (SD)) in ocular measures from 2–3 weeks to 6 months after lens implantation.
| MSE refraction | Distance VA | Near VA | CS | Subjective accom | Objective accom | Higher order aberrations | Lower order aberrations | Subcap scatter | |
|---|---|---|---|---|---|---|---|---|---|
| KH‐3500 | 0.08 (0.76) | 0.00 (0.17) | −0.10 (0.14) | −0.09 (0.17) | 1.7 (2.2) | 0.23 (0.99) | −0.84 (2.28) | −0.31 (1.22) | 1.82 (1.18) |
| p = 0.918 | p = 0.783 | p = 0.031* | p = 0.160 | p = 0.062 | p = 0.345 | p = 0.402 | p = 0.527 | p = 0.008* | |
| Softec1 | 0.08 (0.69) | 0.04 (0.11) | −0.16 (0.31) | −0.06 (0.22) | 0.3 (0.8) | −0.14 (0.35) | −0.11 (0.65) | −0.02 (0.27) | 1.18 (1.70) |
| p = 0.764 | p = 0.400 | p = 0.227 | p = 0.526 | p = 0.314 | p = 0.317 | p = 0.682 | p = 0.846 | p = 0.206 |
MSE, mean spherical equivalent refraction (D); VA, visual acuity (logMAR); CS, contrast sensitivity (log units); Accom, amplitude of accommodation (D); Aberrations (μm); Subcap scatter, lenticular posterior subcapsular light scatter (% compared to IOL clarity). *Indicates significance compared to no change with time at p<0.05.
Discussion
Patients implanted with the KH‐3500 “accommodating” IOL and with the conventional non‐accommodating Softec1 both had a relatively moderate residual distance prescription (ranging from −2.00 D to +1.50 D), a relatively good best corrected (−0.2–0.5 logMAR) distance visual acuity (Snellen equivalent ∼20/13–20/64) and a good contrast sensitivity (0.9–2.0 log units). Multifocal IOLs tend to induce glare and reduced contrast.12,13 The near word acuity at a fixed working distance, optimally corrected for distance vision, was similar with both IOLs, although it varied greatly between individuals (0.1–1.1 logMAR, or N4–N40 size print at 40 cm). The unexpectedly good near vision achieved by some subjects implanted with the conventional non‐accommodative IOL is supported by the subjective amplitude of accommodation, which was similar to that found in a previous large cohort study of pseudophakic eyes.14 Despite the spherical design of the Softec1 and KH‐3500 IOLs, both show greater (p<0.05) aberrations compared to a population of 30 subjects (average age 21.0 (3.0) years) with healthy eyes and clear ocular media (also measured with the OPD). Therefore, these aberrations are likely to contribute to the subjective depth of focus of eyes implanted with these IOLs, and hence the increased subjective amplitude of accommodation and near visual acuity.
The subjective amplitude of accommodation shown in this study suggests a closer near point, of between 16 cm and 100 cm, is achieved with the KH‐3500 “accommodating” IOL compared to 31–200 cm with the conventional IOL. Anterior shift of the IOL is one of a number of factors contributing to the subjective amplitude of accommodation. Intriguingly, objective and subjective accommodation measured with the conventional Softec1 IOL was significantly correlated with the implanted IOL power whereas that measured with the “accommodating” KH‐3500 IOL was not. However, KH‐3500 subjective accommodation was better correlated with improved near acuity than the Softec1 IOL.
The objective amplitude of accommodation was calculated from stimulus response curves measured by a well validated autorefractor.15 This technique avoids the use of pharmacological agents as it seems that the mechanism and magnitude of pharmacologically induced ciliary muscle contraction is not similar to that manifest physiologically.16 The objective amplitude of accommodation achieved was relatively small, on average ∼0.4 D, but over double that achieved with the non‐accommodative IOL. Therefore, some measurable restoration of voluntary accommodation seems to occur with the KH‐3500 IOL design. The stimulus response curve profile varies between subjects, indicating that previous methods of measuring the difference in refractive power of the eye at only two viewing distances is likely to lead to misleading results.3,5,6
Six months following IOL implantation, there was little change in refraction, distance visual acuity, contrast sensitivity, and aberrations in KH‐3500 implanted eyes. However, there was a significant increase in lenticular posterior capsular light scatter accompanied by a reduction in the smallest print size that can be read at near and a suggested decrease in the subjective amplitude of accommodation. No other postoperative complications were observed. Previous studies that have followed up patients implanted with “accommodating” IOLs for more than one time point after surgery, have shown a stable refraction and subjective accommodation over a 1 year period with the 1CU IOL17 and stable subjective accommodation over a 6 month period with the Crystalens AT‐45 IOL.18 This study with the KH‐3500 suggests lens capsule fibrosis occurs with time, reducing the limited objective and subjective accommodative benefits over conventional non‐accommodating lenses.
Abbreviations
IOL - intraocular lens
OPD - optical path difference
Footnotes
No financial support was received for this study and none of the authors have any commercial connection with any of the products or companies mentioned in this paper.
References
- 1.Nakazawa M, Ohtsuki K. Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses. Am J Ophthalmol 198396435–438. [DOI] [PubMed] [Google Scholar]
- 2.Nakazawa M, Ohtsuki K. Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses: optical analysis. Invest Ophthalmol Vis Sci 1984251458–1460. [PubMed] [Google Scholar]
- 3.Kuchle M, Nguyen N X, Langenbucher A.et al Implantation of a new accommodative posterior chamber intraocular lens. J Refract Surg 200218208–216. [DOI] [PubMed] [Google Scholar]
- 4.Mastropasqua L, Toto L, Nubile M.et al Clinical study of the 1CU accommodating intraocular lens. J Cataract Refract Surg 2003291307–1312. [DOI] [PubMed] [Google Scholar]
- 5.Langenbucher A, Huber S, Nguyen N X.et al Cardinal points and image‐object magnification with an accommodative lens implant (1CU). Ophthalmic Physiol Opt 20032361–70. [DOI] [PubMed] [Google Scholar]
- 6.Langenbucher A, Huber S, Nguyen N X.et al Measurement of accommodation after implantation of an accommodating posterior chamber intraocular lens. J Cataract Refract Surg 200329677–685. [DOI] [PubMed] [Google Scholar]
- 7.Dick H B, Kaiser S. Dynamic aberrometry during accommodation of phakic eyes and eyes with potentially accommodative intraocular lenses. Ophthalmologe 200299825–834. [DOI] [PubMed] [Google Scholar]
- 8.Lea S J H, Rubenstein P M, Snead M P.et al Pseudophakic accommodation? A study of the stability of the capsular bag supported, one piece rigid tripod or soft flexible implants. Br J Ophthalmol 19907422–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravalico G, Baccara F. Apparent accommodation in pseudophakic eyes. Acta Ophthalmol (Copenh) 199068604–606. [DOI] [PubMed] [Google Scholar]
- 10.Auffarth G U, Schmidbauer J, Becker K A.et al Miyake‐Apple video analysis of movement patterns of an accommodative intraocular lens implant. Ophthalmologe 200299811–814. [DOI] [PubMed] [Google Scholar]
- 11.Findl O, Kiss B, Petternel V.et al Intraocular lens movement caused by ciliary muscle contraction. J Cataract Refract Surg 200329669–670. [DOI] [PubMed] [Google Scholar]
- 12.Montés‐Micó R, Alió J L. Distance and near contrast sensitivity function after multifocal intraocular lens implantation. J Cataract Refract Surg 200329703–711. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz S, Dick H B, Krummenauer F.et al Contrast sensitivity and glare disability by halogen light after monofocal and multifocal lens implantation. Br J Ophthalmol 2000841109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuyama M, Oshika T, Amano S.et al Relationship between apparent accommodation and corneal multifocality in pseudophakic eyes. Ophthalmology 19991061178–1181. [DOI] [PubMed] [Google Scholar]
- 15.Mallen E A H, Wolffsohn J S, Gilmartin B.et al Clinical evaluation of the Shin‐Nippon SRW‐5000 autorefractor in adults. Ophthalmic Physiol Opt 200121101–107. [PubMed] [Google Scholar]
- 16.Kriechbaum K, Findl O, Koeppl C.et al Stimulus‐driven versus pilocarpine‐induced biometric changes in pseudophakic eyes. Ophthalmology 2005112453–459. [DOI] [PubMed] [Google Scholar]
- 17.Kuchle M, Seitz B, Langenbucher A.et al Stability of refraction, accommodation, and lens position after implantation of the 1CU accommodating posterior chamber intraocular lens. J Cataract Refract Surg 2003292324–2329. [DOI] [PubMed] [Google Scholar]
- 18.Marchini G, Pedrotti E, Sartori P.et al Ultrasound biomicroscopic changes during accommodation in eyes with accommodating intraocular lenses. J Cataract Refract Surg 2004302476–2482. [DOI] [PubMed] [Google Scholar]