Abstract
Aim
To isolate autoantigens possibly involved in the pathogenesis of Vogt‐Koyanagi‐Harada (VKH) disease.
Methods
Autoantigens recognised by immunoglobulin G antibodies (IgG Ab) in sera from VKH patients were isolated by screening the lambda phage cDNA libraries made from melanocytes and a highly pigmented melanoma cell line with the patients' sera. Presence of IgG specific for the autoantigens in sera from patients with various panuveitis and healthy individuals was evaluated. Relation between the specific IgG and various clinicopathological features was examined.
Results
KU‐MEL‐1 was found to be one of the 81 isolated positive clones representing 35 distinct genes, which is a previously isolated melanoma antigen preferentially expressed in melanocytes. The IgG Ab specific for KU‐MEL‐1 was detected in sera from patients with VKH in significantly higher amounts than in sera from patients with Behçet's disease, sarcoidosis, and from healthy individuals. Positive serum KU‐MEL‐1 Ab was significantly associated with HLA‐DRB1*0405 and male VKH patients.
Conclusion
KU‐MEL‐1 was identified as a new autoantigen for VKH. The highly frequent induction of IgG Ab for KU‐MEL‐1 in HLA‐DRB1*0405 positive VKH patients may suggest the possible involvement of KU‐MEL‐1 specific CD4+ T cells in the pathogenesis of VKH, suggesting the possible use in the development of diagnostic and therapeutic treatments for VKH patients.
Keywords: Vogt‐Koyanagi‐Harada disease, KU‐MEL‐1, DNA cloning, autoimmunity, uveitis
Vogt‐Koyanagi‐Harada disease (VKH) is an idiopathic systemic disorder involving melanocyte containing organs, including uvea, skin, inner ears, and choroid plexus. The clinical features of VKH are characterised by non‐traumatic bilateral diffuse granulomatous uveitis, tinnitus, vitiligo, alopecia, poliosis, pleocytosis, and meningism, caused by destruction of local melanocytes.1,2 Histopathologically, the eyes from VKH patients showed scattering infiltration of lymphocytes in the thickened choroid with a remarkable depletion of choroidal melanocytes.3,4 Lymphocytes of VKH patients proliferate when stimulated with crude extracts from melanocytes.5 A strong association between an HLA‐DRB1*0405‐DQB1*0401 haplotype and susceptibility to VKH has been reported in Japan6 and other countries.7,8 Thus, VKH is considered to be a T cell mediated autoimmue disorder directed against antigens expressed in melanocytes.
Identification of autoantigens involved in VKH is important for the understanding of autoimmune pathogenesis and the development of diagnostic and therapeutic methods. Tyrosinase has been reported to be an autoantigen in VKH patients because peptides derived from these proteins stimulated proliferation of lymphocytes from VKH patients, and immunisation of rats with these peptides induced autoimmunity resembling VKH.9,10,11 A T cell line isolated from peripheral blood mononuclear cells (PBMC) of a VKH patient recognised a tyrosinase peptide presented by HLA‐DRB1*0405,12 showing possible involvement of autoreactive CD4+ T cells in the development of VKH. However, systematic identification of autoantigens has not easily been performed.
We have previously identified many human melanoma antigens using cDNA expression cloning with melanoma reactive T cells or patients' serum immunoglobulin G antibodies (IgG Ab).13,14,15,16 The latter method with patients' sera is established as a useful technique for the identification of tumour antigens or autoantigens recognised by T cells. The presence of IgG Ab indicates that CD4+ helper T cells specific for the same antigens are activated in the patients. In addition, CD8+ cytotoxic T cells (CTL) are sometimes activated by the same antigens. Thus, the isolated antigens with the patients' sera are possible antigens for both CD4+ and CD8+ T cells activated in patients with tumour or autoimmune diseases. In this study, we attempted to isolate autoantigens that induced immune response in VKH patients by screening lambda phage cDNA libraries of melanocytes and pigmented melanoma cell lines with sera from VKH patients.
Materials and methods
Profile of patients
Thirty five VKH patients investigated in this study underwent ophthalmological examination to determine the clinical diagnosis. Table 1 showed the profiles of the patients whose sera were used for screening cDNA libraries. Twenty five patients with Behçet's disease, 20 patients with sarcoidosis, and 52 healthy individuals served as controls. Informed consent was obtained from all subjects before blood sampling. This study was performed according to the tenets of the Declaration of Helsinki.
Table 1 Profile of patients whose sera were used for screening cDNA libraries made from cultured melanocytes and melanoma.
| Patient | Screening libraries | HLA‐ DRB1*0405 | Disease status | SRD* | Pleocytosis | |
|---|---|---|---|---|---|---|
| Melanocyte | Melanoma | |||||
| VKH‐1 | + | + | + | Initial onset | + | + |
| VKH‐2 | − | + | − | Recurrence | − | Not done |
| VKH‐3 | + | + | + | Initial onset | + | − |
| VKH‐4 | − | + | + | Recurrence | − | Not done |
| VKH‐5 | − | + | + | Initial onset | + | + |
| VKH‐6 | − | + | − | Recurrence | − | − |
| VKH‐7 | + | + | − | Recurrence | + | − |
| VKH‐8 | + | + | − | Initial onset | + | + |
*Serous retinal detachment.
Cell lines
Human melanoma cell line SKmel23 was kindly provided by Dr Rosenberg, NCI, Bethesda, MD, USA, and cultured in 10% fetal bovine serum RPMI1640, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Human melanocytes kindly provided by Dr Honjou, Morinaga, Yokohama, Japan, were cultured in serum free MM‐4 medium.
Construction of cDNA libraries
The cDNA libraries were constructed as previously described.15 The cDNA library from SKmel23 was constructed with 5 μg of poly(A)+ RNA. First strand synthesis was performed using an oligodeoxythymidylate primer with an internal XhoI site and 5‐methylCTP. The cDNA was ligated to EcoRI adapters and digested with XhoI. The cDNA fragments were directionally inserted into the bacteriophage expression vector lambda zap II (Stratagene, La Jolla, CA, USA), packaged into phage particles, and used to transform Escherichia coli, resulting in 3×106 primary recombinants. The cDNA library from cultured melanocytes was constructed with 5 μg of poly(A)+ RNA. First strand synthesis was performed using HindIII random primer (Novagen, Madison, WI, USA) and 5‐methylCTP. The cDNA was treated with T4 DNA polymerase to blunt the ends and ligated with directional EcoRI/HindIII. The cDNA fragments were directionally inserted into the bacteriophage expression vector lambda screen (Novagen) and resulted in 1.3×106 primary recombinants.
Immunoscreening of a cDNA library with serum
The immunoscreening method was performed as previously described.15 Briefly, recombinant phages were seeded at a concentration of 1.0×104/150 mm agar plates and incubated for 5 hours at 42°C. The plaques were transferred to nitrocellulose membranes (Hybond‐C; Amersham Pharmacia, Bucks, UK) pretreated with isopropyl β‐d‐thiogalactoside (Wako, Osaka, Japan), then incubated for 3–4 hours at room temperature in the serum diluted 1:100 in 5% skim milk/TBS‐T. After washing with TBS‐T, the membranes were then incubated with 1:4000 diluted goat antihuman IgG (Fc) antibody conjugated with alkaline phosphatase (Cappel, Aurola, OH, USA). Nitro blue tetrazolium (Boehringer Mannheim, GmbH, Germany) and 5‐bromo‐4‐chloro‐3‐indolyl phosphate (Sigma Chemical Co, St Louis, MO, USA) were used for the enzymatic detection. Positive plaques were picked from the plates and purified through secondary and tertiary rounds of additional screening. The purified cDNAs were amplified by polynerase chain reaction (PCR) using the Ex Taq kit (Takara Shuzo) and sequenced using the Big Dye Terminator Cycle Sequencing Ready Reaction Kit and an ABI Prism automated sequencer (Perkin‐Elmer, Branchburg, NJ, USA). The sequenced DNAs were analysed by a BLAST search of genetic databases at the National Center for Biotechnology Information.
Detection of antigen specific IgG Ab
For detecting specific IgG Ab for the identified antigens in sera from patients and healthy individuals, the phages that inserted the identified antigen cDNAs 2000 plaque forming units (pfu)/150 mm plate were mixed with the no insert phages as the internal negative controls 3000 pfu/150 mm plate, and the plaques were transferred to nitrocellulose membranes. The sera from patients and healthy individuals were diluted 1:100 in 5% (w/v) skim milk in TBS‐T and the membranes were incubated in the diluted sera. Positive plaques were detected as described above. The sera which clearly stained the positive plaques over the negative plaques were defined as IgG positive sera.
Results
Isolation of autoantigens recognised by IgG antibodies in the sera of VKH patients using cDNA expression cloning
The patients with VKH are likely to develop autoimmune responses to melanocyte specific molecules. Thus, we attempted to isolate antigens recognised by IgG Ab in sera from VKH patients, from melanocytes and melanoma. A total of 4.0×106 cDNA clones of two lambda phage cDNA libraries made from primary cultured melanocytes and a highly pigmented melanoma cell line, SKmel23, that expresses melanocyte specific melanosomal proteins, including tyrosinase, TRP1, TRP2, gp100, and MART‐1/Melan‐A, were screened with sera from eight VKH patients as described in table 1. Thirty two positive clones and 49 positive clones, representing 35 distinct genes, were isolated from the melanocyte and melanoma cDNA libraries, respectively (table 2). Among them, popular melanosomal proteins, including tyrosinase, TRP1, TRP2, gp100, and MART‐1, were not included. However, a melanocyte/melanoma specific molecule, KU‐MEL‐1, which has previously been isolated by our group from a patient who had favourable prognosis along with development of extensive vitiligo after chemo‐immunotherapy using IFN‐β possibly through anti‐melanocyte/melanoma immune responses,15 was isolated from the melanoctyte cDNA library. Lens epithelium derived growth factor (LEDGF), which was previously isolated using cDNA expression cloning with sera from VKH patients,17 was also isolated from the melanoma cDNA library.
Table 2 cDNAs isolated by screening with sera from VKH patients.
| Gene | UniGene | Number of clone | |
|---|---|---|---|
| Melanocyte library | |||
| Meningioma expressed antigen 5 (hyaluronidase) (MGEA5) | Hs.5734 | 22 | |
| EST; weakly similar to hypothetical protein FLJ20378 | Hs.399590 | 2 | |
| KU‐MEL‐1 | Hs.9788 | 1 | |
| Nuclear factor of activated T cell 5, tonicity responsive | Hs.86998 | 1 | |
| FLJ23538 | Hs.240443 | 1 | |
| Inhibitor of kappa light polypeptide gene enhancer in B cells | Hs.31323 | 1 | |
| KIAA1323 protein | Hs.34892 | 1 | |
| Lectin, galactoside binding, solbule, 3 (galectine 3) | Hs.621 | 1 | |
| Chromodomain helicase DNA binding protein 2 (CDH2) | Hs.36787 | 1 | |
| Coated vesicle membrane protein (RNP24) | Hs.75914 | 1 | |
| SKmel23 melanoma library | |||
| S164 protein mRNA | Hs.236051 | 11 | |
| Galectin‐3 (lectin,galctoside binding,soluble, 3) | Hs.621 | 9 | |
| Lens epithelium derived growth factor (LEDGF) | Hs.82110 | 3 | |
| Myeloid/lymphoid or mixed lineage leukaemia 2 (MLL2) | Hs.153638 | 2 | |
| Heat shock 70 kD protein 4 (Hsp70) | Hs.90093 | 2 | |
| COP9 subunit 6 (MOV34 homolog, 34 kD) | Hs.15591 | 2 | |
| Mitogen activated protein kinase 9 | Hs.246857 | 2 | |
| Cold inducible RNA binding protein | Hs.119475 | 1 | |
| KIAA0556 | Hs.30512 | 1 | |
| KIAA0690 | Hs.60103 | 1 | |
| DKFZP564O123 protein | Hs.11449 | 1 | |
| H‐2K binding factor‐2 | Hs.347340 | 1 | |
| Machado‐Joseph disease | Hs.66521 | 1 | |
| Ornithine decarboxylase antizyme 1 | Hs.380062 | 1 | |
| HIG1 Likely orthologue of mouse hypoxia induced gene 1 | Hs.7917 | 1 | |
| Tumour rejection antigen (gp96) 1 | Hs.82689 | 1 | |
| FLJ32844 | Hs.351403 | 1 | |
| Hu antigen R | Hs.12379 | 1 | |
| Chromosome 20 open reading frame 21 | Hs.11747 | 1 | |
| Splicing factor, arginine/serine rich 10 | Hs.30035 | 1 | |
| Translation initiation factor IF2 | Hs.158688 | 1 | |
| Eukaryotic translation elongation factor 1 epsilon 1 | Hs.298581 | 1 | |
| Casein kinase II β subunit | Hs.165843 | 1 | |
| PHD finger protein 3 | Hs.78893 | 1 | |
| Translocase of outer mitochondrial membrane 40 homologue | Hs.30928 | 1 |
Frequent induction of IgG Ab in VKH patients
IgG responses to KU‐MEL‐1, which we identified in this study, tyrosinase for which specific T cell response was reported in VKH patients,10,12 and NY‐ESO‐1, a cancer testis antigen expressed in melanoma, but not in melanocytes,18 were evaluated by screening sera from VKH patients and healthy individuals using the plaque assay (fig 1). As shown in table 3, IgG specific for KU‐MEL‐1 was detected in sera from 22 of 35 (63%) VKH patients, but two of 52 (4%) healthy individuals (p<0.01). In contrast, IgG for tyrosinase and NY‐ESO‐1 were not detected in any VKH patients or any healthy individuals, although serum from a melanoma patient used as a positive control reacted with these antigens. IgG for LEDGF was frequently detected in both the patients (4/12) and healthy donors (3/6). Thus, anti‐KU‐MEL‐1 IgG response is frequently induced in VKH patients. The presence of the KU‐MEL‐1 specific IgG Ab in sera from patients with other panuveitis, including Behçet's disease and sarcoidosis, was then examined. The phage containing the full length KU‐MEL‐1 was used for screening sera from the patients (table 4). The KU‐MEL‐1 specific IgG Abs were detected in sera from six of 25 (24%) patients with Behçet's disease and from nine of 30 (30%) patients with sarcoidosis. However, the frequencies were significantly lower than that of VKH patients in which the KU‐MEL‐1 specific IgG Abs were detected in 22 of 35 (63%) (p<0.01). These results suggest that anti‐KU‐MEL‐1 responses may play a part in the development of VKH, although the immune responses to KU‐MEL‐1 could be induced by extensive damage of the melanocytes.
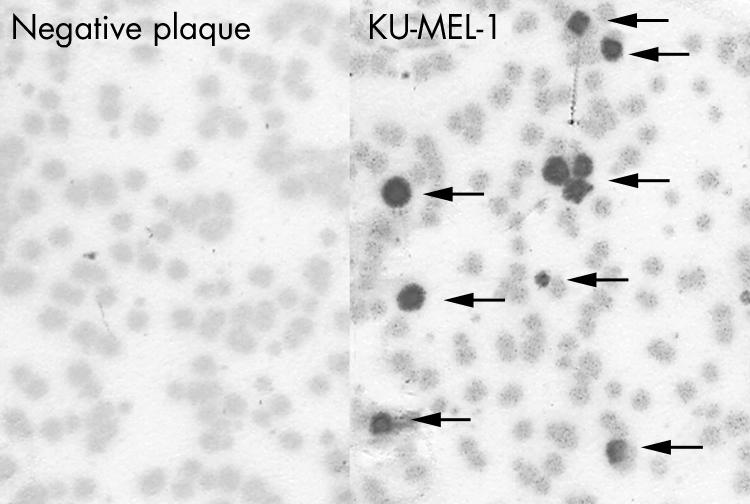
Figure 1 Detection of IgG antibody specific for KU‐MEL‐1 in sera from VKH patients. IgG specific for KU‐MEL‐1 was detected by the plaque assay as described in the materials and methods. Briefly, a mixture of KU‐MEL‐1 expressing plaques and negative plaques were reacted with sera from VKH patients. Left: the result with negative serum; and right: the result with anti‐KU‐MEL‐1 IgG Ab positive serum. Arrows indicate positive plaques.
Table 3 Presence of serum IgG antibodies specific for KU‐MEL‐1 and other related antigens in VKH patients and healthy individuals†.
| Antigen | VKH | Healthy individuals |
|---|---|---|
| Positive/total (%) | Positive/total (%) | |
| KU‐MEL‐1 | 22/35 (63%)* | 2/52 (4%) |
| Tyrosinase | 0/20 (0%) | 0/22 (0%) |
| NY‐ESO‐1 | 0/26 (0%) | 0/22 (0%) |
| LEDGF | 4/12 (33%) | 3/6 (50%) |
†Presence of IgG specific for each antigen was evaluated by the method similar to that the cDNA library screening with sera at 1:100 dilution.
*p<0.01 (v healthy donor by Fisher's exact probability test).
Table 4 Presence of IgG antibodies specific for KU‐MEL‐1 in sera from patients with panuveitis and healthy individuals.
| Disease | Positive/total (%) |
|---|---|
| VKH | 22/35 (63%) |
| Behçet's disease | 6/25 (24%)* |
| Sarcoidosis | 9/30 (30%)* |
| Healthy individuals | 2/52 (4%)* |
*p<0.01 (v VKH by Fisher's exact probability test).
Frequent induction of IgG response to KU‐MEL‐1 in HLA‐DRB1*0405 positive male patients with VKH
Associations of positive serum KU‐MEL‐1 Ab with clinicopathological features were examined in the VKH patients. As shown in table 5, positive KU‐MEL‐1 Ab was not significantly associated with age of onset (44.7 v 40.2), clinical patterns (initial onset or recurrence), SRD, and pleocytosis, although a tendency that initial onset patients or the patients with SRD raised the KU‐MEL‐1 Ab was observed. KU‐MEL‐1 Ab was found to be significantly positive in male patients (p<0.05) and HLA‐DRB1*0405 patients (p<0.05). Twelve of 13 male patients had KU‐MEL‐1 Ab, while 10 of 22 female patients had Ab. A strong association between HLA‐DRB1*0405 and susceptibility to VKH has been reported. The HLA‐DRB1*0405 haplotype was observed in 25 of 35 (71%) VKH patients in this study, while the frequency of HLA‐DRB1*0405 in the Japanese population is 15.5%.19 Nineteen of 25 (76%) HLA‐DRB1*0405 positive patients had KU‐MEL‐1 Ab, while three of 10 (30%) HLA‐DRB1*0405 negative patients had Ab (p<0.05), indicating that HLA‐DRB1*0405 restricted helper CD4+ T cells may be frequently involved in the production of the KU‐MEL‐1 specific IgG Ab and possibly involved in pathogenesis of VKH.
Table 5 Association between KU‐MEL‐1 specific serum IgG Ab and various clinicopathological features in VKH patients.
| Clinical features and genotype | KU‐MEL‐1 (+) | KU‐MEL‐1 (−) | p Value | |
|---|---|---|---|---|
| Age (mean (SD)) | 44.7 (15.1) | 40.2 (10.0) | ||
| Sex | Male | 12/13 (92%) | 1/13 (8%) | <0.05† |
| Female | 10/22 (45%) | 12/22 (55%) | ||
| Critical pattern | Initial onset | 20/30 (67%) | 10/30 (33%) | 0.52† |
| Recurrence | 2/5 (40%) | 3/5 (60%) | ||
| SRD* | Positive | 20/29 (69%) | 9/29 (31%) | 0.24† |
| Negative | 2/6 (33%) | 4/6 (67%) | ||
| Pleocytosis | Positive | 17/26 (65%) | 9/26 (35%) | 1.00† |
| Negative | 5/7 (71%) | 2/7 (29%) | ||
| HLA‐DRB1*0405 | Positive | 19/25 (76%) | 6/25 (24%) | <0.05† |
| Negative | 3/10 (30%) | 7/10 (70%) | ||
*Serous retinal detachment.
†Fisher's exact probability test.
Discussion
VKH is considered to be an autoimmune disorder against melanocytes, since various organs containing melanocytes, are systemically damaged when VKH progresses. T cell responses against a melanocyte specific melanosomal enzyme, tyrosinase, have previously been reported in VKH patients. However, the exact role of tyrosinase in the pathogenesis of VKH has not yet been investigated. In this study, we attempted to identify additional autoantigens involved in the anti‐melanocyte immune responses involved in VKH. By screening cDNA libraries made from melanocytes and a highly pigmented melanoma cell line SKmel23 with sera from VKH patients, conventional melanosomal antigens, including tyrosinase, TRP1, TRP2, gp100, and MART‐1, were not isolated, but KU‐MEL‐1, which was previously isolated using a similar cDNA cloning technique with sera from a melanoma patient who developed anti‐melanocyte/melanoma immune responses,15 was isolated. The further analysis of the immune response to KU‐MEL‐1 revealed that the KU‐MEL‐1 induced IgG response was associated with HLA‐DRB1*0405 in VKH patients. Although IgG for KU‐MEL‐1 was also detected in patients with other panuveitis, including Behcet's disease and sarcoidosis, this immune response may be the secondary phenomenon to non‐specific inflammatory destruction of uveal melanocytes, which led to the exposure of KU‐MEL‐1 and antigen presentation to the immune system. However, significantly higher rates of the KU‐MEL‐1 IgG response and a strong association of HLA‐DRB1*0405 in VKH patients may suggest the possible involvement of KU‐MEL‐1 in pathogenesis of VKH. Although the precise mechanism for the induction of immune response to KU‐MEL‐1 in VKH patients is not clear, high and preferential expression of KU‐MEL‐1 in melanocytes may result in the autoimmune response against KU‐MEL‐1.
KU‐MEL‐1 is preferentially expressed in melanocytes and melanoma cells, and was isolated in serum from a patient with melanoma who developed extensive vitiligo. It is a cytoplasmic protein containing armadillo repeats for interaction with other proteins and its function is not yet known. IgG Ab specific for KU‐MEL‐1 may not function as an effector against melanocytes, since KU‐MEL‐1 is a cytoplasmic protein. However, the presence of IgG Ab indicated activation of KU‐MEL‐1 specific helper CD4+ T cells, possibly restricted by HLA‐DRB1*0405 in VKH patients. These CD4+ helper T cells may influence the development of uveitis through the secretion of various cytokines. In addition, the KU‐MEL‐1 specific IgG Ab may also be involved in the augmentation of KU‐MEL‐1 immune responses with efficient antigen presentation by the specific B cells through efficient Ab mediated uptake of KU‐MEL‐1.
In the analysis of relation between KU‐MEL‐1 Ab and other clinical features of the VKH patients, the KU‐MEL‐1 Ab was detected significantly in male patients (p<0.05). Occurrence of VKH was not associated with sex. It is not clear why the induction of KU‐MEL‐1 Ab correlated with males in this study. KU‐MEL‐1 Ab appeared to be induced more frequently in patients with initial onset (67%) or patients with SRD (69%) compared with patients with recurrence (40%) and patients without SRD (33%). This may suggest that severe inflammation might induce anti‐KU‐MEL‐1 Ab or the immune response to KU‐MEL‐1 might accelerate inflammation leading to SRD.
Other melanocyte specific antigens were not isolated, and tyrosinase, which was reported to be recognised by T cells from VKH, was not recognised by sera from VKH patients enrolled in this study. The role of conventional melanosomal antigens in VKH remains to be investigated. Using a similar cDNA cloning method with a cDNA library made from bovine uvea and sera from VKH patients, two autoantigens, LEDGF,17 and uveal autoantigen with coiled coil domains and ankyrin repeats (UACA) have previously been isolated.20 Different from KU‐MEL‐1, those autoantigens are expressed in various normal tissues. Although LEDGF was also isolated in this study, specific IgG Abs were detected in three of six (50%) healthy donors. Thus, those antigens may have little role in the pathogenesis of VKH.
In summary, we have demonstrated that the IgG response to KU‐MEL‐1 preferentially expressed in melanocytes was frequently induced in VKH patients, particularly male patients with HLA‐DRB1*0405, suggesting its possible involvement in more severe uveitis in VKH. Thus, KU‐MEL‐1 may be useful for the development of diagnostic and therapeutic methods for patients with VKH and other autoimmune diseases against melanocytes.
Acknowledgements
This work was supported in part by the grants in aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from the Ministry of Health Labour and Welfare, and by the Keio Gijuku Academic Development Funds.
Abbreviations
Ab - antibody
CTL - cytotoxic T cells
IgG - immunoglobulin G
LEDGF - lens epithelium derived growth factor
PBMC - peripheral blood mononuclear cells
PCR - polynerase chain reaction
pfu - plaque forming units
SRD - serous retinal detachment
UACA - uveal autoantigen with coiled coil domains and ankyrin repeats
VKH - Vogt‐Koyanagi‐Harada
References
- 1.Ohno S, Minakawa R, Matsuda H. Clinical studies of Vogt‐Koyanagi‐Harada's disease. Jpn J Ophthalmol 198832334–343. [PubMed] [Google Scholar]
- 2.Moorthy R S, Inomata H, Rao N A. Vogt‐Koyanagi‐Harada syndrome. Surv Ophthalmol 199539265–292. [DOI] [PubMed] [Google Scholar]
- 3.Perry H D, Font R L. Clinical and histopathologic observations in severe Vogt‐Koyanagi‐Harada syndrome. Am J Ophthalmol 197783242–254. [DOI] [PubMed] [Google Scholar]
- 4.Inomata H, Sakamoto T. Immunohistochemical studies of Vogt‐Koyanagi‐Harada disease with sunset sky fundus. Curr Eye Res 19909(Suppl)35–40. [DOI] [PubMed] [Google Scholar]
- 5.Hammer H. Cellular hypersensitivity to uveal pigment confirmed by leucocyte migration tests in sympathetic ophthalmitis and the Vogt‐Koyanagi‐Harada syndrome. Br J Ophthalmol 197458773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindo Y, Ohno S, Yamamoto T.et al Complete association of the HLA‐DRB1*04 and ‐DQB1*04 alleles with Vogt‐Koyanagi‐Harada's disease. Hum Immunol 199439169–176. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg A C, Yamamoto J H, Chiarella J M.et al HLA‐DRB1*0405 is the predominant allele in Brazilian patients with Vogt‐Koyanagi‐Harada disease. Hum Immunol 199859183–188. [DOI] [PubMed] [Google Scholar]
- 8.Kim M H, Seong M C, Kwak N H.et al Association of HLA with Vogt‐Koyanagi‐Harada syndrome in Koreans. Am J Ophthalmol 2000129173–177. [DOI] [PubMed] [Google Scholar]
- 9.Gocho K, Kondo I, Yamaki K. Identification of autoreactive T cells in Vogt‐Koyanagi‐Harada disease. Invest Ophthalmol Vis Sci 2001422004–2009. [PubMed] [Google Scholar]
- 10.Yamaki K, Gocho K, Hayakawa K.et al Tyrosinase family proteins are antigens specific to Vogt‐Koyanagi‐Harada disease. J Immunol 20001657323–7329. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Ishikawa M, Yamaki K. Ultrastructural changes in rat eyes with experimental Vogt‐Koyanagi‐Harada disease. Jpn J Ophthalmol 200448222–227. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Kokubo T, Takahashi M.et al Tyrosinase epitope recognized by an HLA‐DR‐restricted T‐cell line from a Vogt‐Koyanagi‐Harada disease patient. Immunogenetics 199847398–403. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami Y, Eliyahu S, Delgado C H.et al Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 1994913515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami Y, Eliyahu S, Delgado C H.et al Identification of a human melanoma antigen recognized by tumor‐infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA 1994916458–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiniwa Y, Fujita T, Akada M.et al Tumor antigens isolated from a patient with vitiligo and T‐cell‐infiltrated melanoma. Cancer Res 2001617900–7907. [PubMed] [Google Scholar]
- 16.Kawakami Y, Fujita T, Matsuzaki Y.et al Identification of human tumor antigens and its implications for diagnosis and treatment of cancer. Cancer Sci 200495784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada K, Senju S, Shinohara T.et al Humoral immune response directed against LEDGF in patients with VKH. Immunol Lett 200178161–168. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y‐T, Matthew J, Scanlanet al A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA 1997941914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto M, Kinoshita T, Yamasaki M.et al Gene frequencies and haplotypic associations within the HLA region in 916 unrelated Japanese individuals. Tissue Antigens 199444166–173. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Senju S, Nakatsura T.et al Identification of a novel autoantigen UACA in patients with panuveitis. Biochem Biophys Res Commun 20012801169–1176. [DOI] [PubMed] [Google Scholar]


