Abstract
Background/aims
The conjunctival epithelial cell line, CCL20.2 (CCL), requires the presence of 10% fetal calf serum (FCS) in the medium to survive. To elucidate the molecular mechanism underlying such cell death, including the death signal for these cells, the activities of several caspases in the CCL were measured, and the effects of caspase inhibitors and serum components on cell death were examined.
Methods
CCL was grown in Medium 199 containing 10% FCS, and the medium was changed to Medium 199 with or without 10% FCS, or medium without 10% FCS but containing caspase inhibitors or serum components. After 24 hours' incubation, the enzyme activities of caspases 1, 3, 8, and 9 in the culture supernatants were measured, and the effects of caspase inhibitors and serum components—for example, growth factors, lactoferrin, retinoic acid, were investigated.
Results
DNA fragmentation was induced by serum deprivation, confirming that serum deprivation induces apoptosis in CCL. While the activities of caspases 3 and 8 were found to be increased, those of caspases 1 and 9 were not detected in the apoptotic cells. Z‐VAD completely suppressed the caspase 3 activation, and specific inhibitors of caspases 1, 8, and 9 partially suppressed the activation. Serum deprivation induced a decrease in the cellular viability, which, however, partially recovered in the presence of caspase inhibitors, epidermal growth factor and retinoic acid.
Conclusion
These results suggest that the apoptosis induced by serum deprivation involves caspases 1, 3, 8, and 9, and is suppressed by caspase inhibitors. EGF and retinoic acid have a key role in the maintenance of the ocular surface.
Keywords: apoptosis, caspase, cell culture, conjunctiva, dry eye
Severe dry eye results not only from desiccation of the ocular surface, but also from the lack of tear components, which are essential for maintaining the ocular surface.1,2,3,4,5 Artificial tears are useful for the treatment of dry eye, but the efficiency of the components included in artificial tears is not comparable to that of human tears. In a previous study,6 we used autologous serum as a substitute for tears for the treatment of severe dry eye, and observed a dramatic improvement in the ocular surface. A comparative analysis of the components of serum and tears revealed that the serum components were similar to the tear components.7 The efficient therapeutic effects of serum in cases of severe dry eye also suggest that the components of serum that are essential for maintaining viability of the ocular surface may be the same or similar to those of tears.
It is well known that deprivation of serum or growth factors from the medium causes apoptotic death of cultured cells. Myc and p53 appear to be involved in the mechanism underlying such apoptotic cell death, but the precise mechanism is still unknown.8,9,10,11 Some components of serum, such as growth factors, cause attenuation of apoptosis in cell cultures.12,13,14,15 Epidermal growth factor (EGF) has been shown to protect cultured cells against apoptosis induced by serum deprivation,14 and also fas induced apoptosis.13 The EGF receptor is also known to be distributed in human ocular surface epithelial cells.16,17 Glucocorticoids protect granulosa cells against apoptosis induced by serum deprivation via an increase of bcl‐2 expression levels.18 Retinoic acid is another major components found in tears. In previous clinical studies, retinoic acid was shown to be useful for the treatment of dry eye19,20 and Stevens‐Johnson syndrome.21
Although the precise biochemical pathway involved in mammalian cell death remains undefined by serum deprivation, it is clear that caspases, a family of cysteine proteases that cleave the carboxyl terminus of aspartic acid, have essential roles in apoptosis. Fourteen distinct caspases have been identified and have been grouped into three subfamilies based on their substrate specificities.22,23,24 Group I caspases prefer the tetrapeptide sequence WEHD, and are believed to play a part mainly in inflammation, whereas members of groups II and III, with the optimal peptide recognition motifs DexD and (I/L/V) ExD, respectively, are mainly involved in apoptosis.22,23,24 These caspases are produced as precursors and activated either by autocatalytic processing and/or cleavage by other caspases in response to a variety of death stimuli, including tumour necrosis factor (TNF), Fas ligand, staurosporine, or ultraviolet irradiation. This activation then triggers a cascade reaction. Caspases 8 and 9 are placed at the most upstream position in the caspase cascade,25,26,27,28 on the other hand, caspase‐3 is placed at the most downstream position in the cascade, as the “executioner.”29,30,31
To elucidate the mechanism of the maintenance of ocular surface epithelial cells by human serum, we used the conjunctival epithelial cell line, CCL‐20.2 (CCL), as a model system of the ocular surface. When serum is removed from the culture medium, CCL undergo apoptosis. In this study, we measured the activities of four caspase species using artificial fluorescent substrates in CCL, and determined the effects of caspase inhibitors, and serum and tear components, such as growth factors, retinoic acid, and lactoferrin, on caspase dependent apoptosis.
Methods
Reagents
EGF, FGF (fibroblast growth factor), IGF (insulin‐like growth factor), and PDGF (platelet derived growth factor) were purchased from Invitrogen Corporation (Carlsbad, CA). HGF (hepatocyte growth factor), NGF (nerve growth factor), and TGF‐β (transforming growth factor‐β) were purchased from PeproTech EC Ltd (London, UK). SCF (stem cell factor) was purchased from Strathmann Biotech GmbH (Hamburg, Germany). Retinoic acid and lactoferrin were obtained from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Other reagents were of the highest quality commercially available.
Cell stimulation
CCL was obtained from the American Type Culture Condition (Manassas, VA, USA) and grown to 70–80% confluence in 75 cm2 flasks in Medium 199 (Gibco BRL Life Technologies, Rockville, MD, USA) containing 10% FCS. It is known that the CCL does not survive when serum is removed from the medium. There are many common components between serum and tears, such as growth factors and vitamin A. We attempted to examine the influences of components of serum and tears on the apoptosis of CCL. The medium was changed to Medium 199 with 10% FCS (serum (+)), to Medium 199 without FCS (serum (−)), or to Medium 199 with tear factors (growth factors, lactoferrin, and retinoic acid) or caspase inhibitors (Enzyme System Products, Livermore, CA, USA) instead of FCS. After 24 hours of incubation, the cells were harvested and viability of the cells was measured by trypan blue staining. Harvested cells were solubilised and cell lysate were centrifuged to assay DNA fragmentation and the caspase activities.
Cell death detection assay
DNA fragmentation was quantified by measuring DNA fragmentation using the Cell Death Detection ELISA kit (Roche Diagnostics GmbH, Mannheim, Germany). This assay was conducted according to the manufacturer's protocol. Briefly, DNA fragments were bound to peroxidase labelled anti‐DNA antibody, and the production of antibody‐DNA fragment complex was measured photometrically using 2,2′‐azinobis(3‐ethylbenzothiazolin‐6‐sulfonic acid (ABTS) as the substrate.
Caspase activity assay
Since DNA fragmentation is catalysed by DNA endonucleases which are activated by the caspase cascade, we measured the activity of caspases 1, 3, 8, and 9. The caspase activities in the cell lysate were measured using artificial fluorescent substrates (Enzyme System Products) with a fluorescent microplate reader. Harvested cells were solubilised with 50 mM HEPES buffer (pH 7.4) containing 0.1% CHAPS, 0.1 mM PMSF, 1 mM DTT and 0.1 mM EDTA for 5 minutes in an ice bath. They were then centrifuged at 10000×g for 10 minutes at 4°C, and the caspase activities were measured in the supernatants.
Cell lysates were mixed with 50 mM HEPES buffer (pH 7.4) containing 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 0.1 mM EDTA, and 10% glycerol, and then 0.1 mM artificial substrate was added to begin the reaction catalysed by caspases. The artificial substrate used for measurement of caspase‐1 activity was acetyl‐Trp‐Glu‐His‐Asp‐AFC (WEHD‐AFC), for that of caspase‐3 was acetyl‐Asp‐Glu‐Val‐Asp‐AFC (DEVD‐AFC), for that of caspase‐8 was acetyl‐Leu‐Glu‐Thr‐Asp (LETD‐AFC), and for that of caspase‐9 was acetyl‐Leu‐Glu‐His‐Asp (LEHD‐AFC).32 Fluorescence was measured immediately and after incubation for 1 hour at 37°C, excitation wavelength 400 nm and emission wavelength 505 nm.
Inhibition assay
Caspase inhibitors were used to clarify the role of caspases in apoptotic cell death induced by serum deprivation. N‐benzyloxycarbonyl‐Trp‐Glu‐His‐Asp‐FMK (Z‐WEHD), a specific inhibitor of caspase‐1, Z‐Leu‐Glu‐Thr‐Asp‐FMK (Z‐LETD), a specific inhibitor of caspase‐8, Z‐Leu‐Glu‐His‐Asp‐FMK (Z‐LEHD), a specific inhibitor of caspase‐9, and Z‐Val‐Ala‐Asp (O‐methyl)‐FMK (Z‐VAD), a pan‐caspase inhibitor, were employed in the study. Caspase inhibitor, 10 μM, was added to Medium 199 without FCS, and the CCL was incubated for 24 hours at 37°C and harvested to measure the activity of caspase‐3.
Statistical analysis
Data are expressed as the mean (SD). Dunnett's test was used to determine the significance of the differences.
Results
DNA fragmentation assay
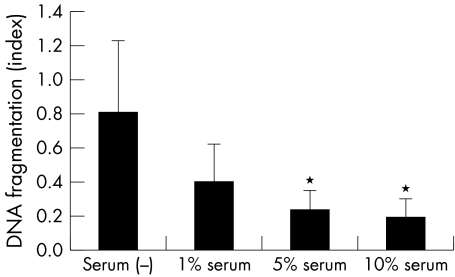
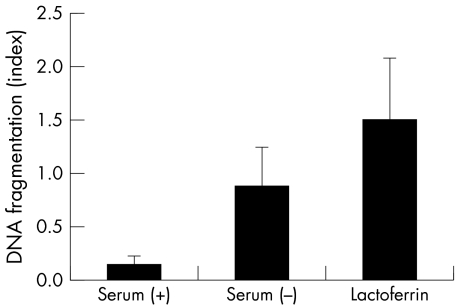
The viability of CCL incubated in serum (+) medium was 96.8% (1.3%), and that in serum (−) medium was 67.8% (SD 4.7%). To confirm that this cell death is apoptosis, DNA fragmentation, which is a hallmark of apoptosis, was measured with an ELISA kit (fig 1). DNA fragmentation was gradually induced with progressive serum deprivation, confirming that serum deprivation induces apoptosis in the CCL.
Figure 1 DNA fragmentation induced by serum deprivation. DNA fragmentation was estimated by measuring the change of absorbance at 405 nm. Results are the mean (SD) (n = 4). *Indicates a significant difference from the result in serum (−), p<0.05.
Caspase activity assay
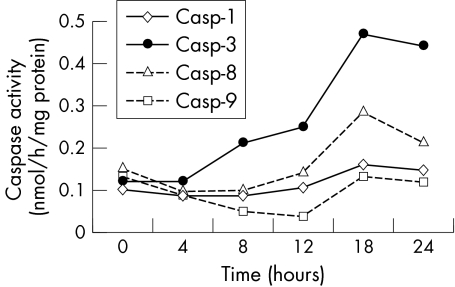
Figure 2 shows the temporal profile of the activity of caspases induced by serum deprivation. The activity of caspase‐3 increased markedly and that of caspase‐8 increased slightly. On the other hand, the activity of caspases 1 and 9 showed no change.
Figure 2 Temporal profiles of the caspase activities induced by serum deprivation. The activity of caspases was estimated by measuring AFC cleavage from AFC labelled artificial substrate.
Effects of caspase inhibitors
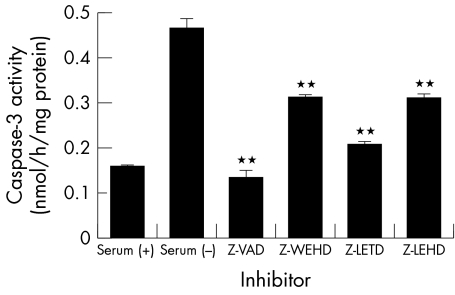
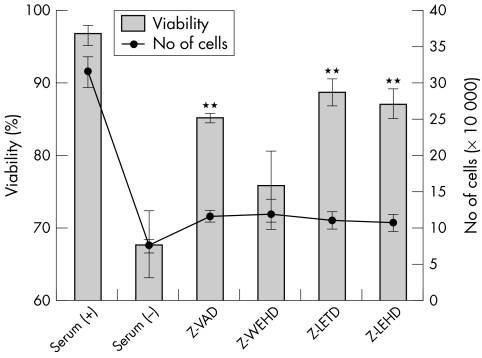
To confirm that caspases 1, 8, and 9 are involved in apoptosis induced by serum deprivation, the influence of caspase inhibitors on caspase‐3 activation was examined (fig 3). Z‐VAD, which is a pan‐caspase inhibitor, completely inhibited the activity of caspase‐3, and Z‐LEHD also inhibited the activity of caspase‐3 markedly. Z‐WEHD and Z‐LETD also inhibited the activity of caspase‐3. The activity of caspases 1, 8, and 9 are specifically inhibited by each inhibitor in this concentration. These results indicate that Z‐WEHD and Z‐LETD blocked the activation of caspase‐3 by inhibition of the reaction of caspase‐1 and caspase 9, respectively. Figure 4 shows the influences of caspase inhibitors on the cellular viability and number of cells. All the inhibitors restored cellular viability (fig 4, bars) by inhibiting cellular apoptosis. On the other hand, none of the inhibitors increased the number of cells (fig 4, solid line).
Figure 3 Suppression of caspase‐3 activation by caspase inhibitors. Activity of caspase‐3 was estimated by measuring the AFC production. Caspase inhibitors blocked activation of caspase‐3 by inhibition specific caspase inhibition (caspase‐1; Z‐WEHD, caspase‐8; Z‐LETD, and caspase‐9; Z‐LEHD) or all caspases pan‐caspase inhibition (Z‐VAD). Results are the mean (SD) (n = 3). **Indicates a significant difference from the result in serum (−), p<0.01.
Figure 4 Effect of caspase inhibitors on cellular viability and number of cells. The number of cells was counted after trypan blue staining. Bars indicate viability and the solid line indicates the number of cells. Results are the mean (SD) (n = 3). **Indicates a significant difference for viability from the result in serum (−), p<0.01.
Suppressive effects of serum and tear components on apoptosis
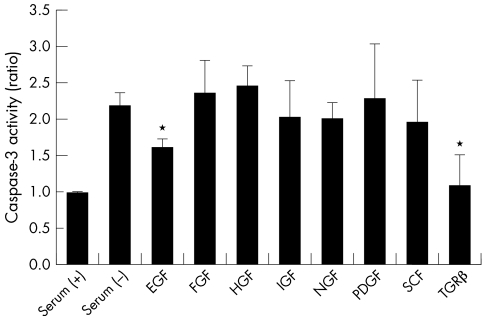
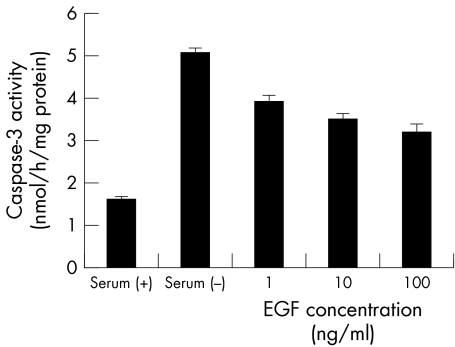
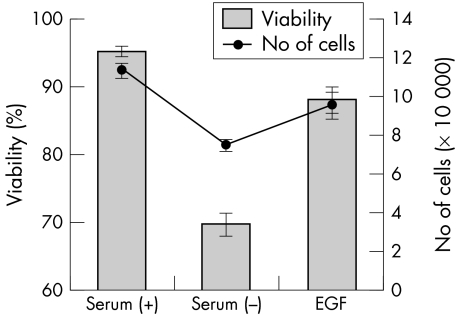
Figure 5 shows the effect of growth factors on DNA fragmentation of CCL induced by serum deprivation. EGF and TGF‐β significantly suppressed apoptosis. IGF, NGF, and SCF slightly suppressed apoptosis, whereas FGF, HGF, and PDGF did not suppress apoptosis. Since EGF is the major growth factor in tears,7 the effects of EGF on activity of caspase‐3 and the cellular viability were examined. Figure 6 shows dose dependency of EGF on suppression of caspase‐3 activity. EGF, 10 ng/ml and 100 ng/ml, significantly suppressed caspase‐3 activity. Figure 7 shows the influence of the cellular viability and the number of cells. These results were different from those obtained with caspase inhibitors; EGF restored cellular viability and the number of cells, and there was no significant difference between serum (+) and EGF (p>0.05).
Figure 5 Effect of growth factors on caspase‐3 activity induced by serum deprivation. Apoptosis caused by serum deprivation was suppressed by the addition of some growth factors to the medium. The concentration of EGF was 10 ng/ml, FGF 5 ng/ml, HGF 50 ng/ml, IGF 10 ng/ml, NGF 50 ng/ml, PDGF 10 ng/ml, SCF 50 ng/ml, and TGF‐β 1 ng/ml, respectively. Results are the mean (SD) (n = 5). *Indicates a significant difference from the result in serum (−), p<0.05.
Figure 6 Effect of EGF on caspase‐3 activity. Caspase‐3 activties were significantly suppressed by the addition of 10 ng/ml and 100 ng/ml EGF. Results are the mean (SD) (n = 3).
Figure 7 Effect of EGF on viability and number of cells. The number of cells was counted after trypan blue staining. Bar indicates viability and the solid line indicates the number of cells. Results are the mean (SD) (n = 3).
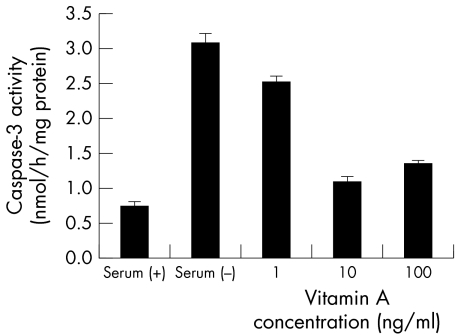
Figures 8 and 9 show the influence of retinoic acid and lactoferrin on apoptosis in the CCL, respectively. Retinoic acid significantly suppressed caspase‐3 activation, but lactoferrin did not suppress DNA fragmentation. It can be concluded that EGF and vitamin A are useful for cellular survival.
Figure 8 Effect of retinoic acid on caspase‐3 activity. Caspase‐3 activties were significantly suppressed by the addition of 10 ng/ml and 100 ng/ml vitamin A. Results are the mean (SD) (n = 3).
Figure 9 Effect of lactoferrin on apoptosis. Apoptosis was not suppressed by the addition of lactoferrin.
Discussion
Severe dry eye, which is caused by a lack of tear components, can be improved by supplying the components essential for maintaining the ocular surface. We used autologous serum to supply these components to the ocular surface.6 We tried to elucidate the effects of serum on the ocular surface by using a conjunctival epithelial cell line as a model system. Because components in the serum are similar to those in tears, elucidation of the effects of serum on CCL may be useful for understanding the mechanisms of maintenance of the ocular surface by tears.7
Serum deprivation from the tissue culture medium elicits many responses in cultured cells. In many cell lines, apoptosis induced by serum deprivation is observed.8,9,10,11 In this study, serum deprivation was found to induce apoptosis in the CCL, and this apoptosis appeared to involve the caspase cascade. Because serum consists of many components, CCL cultured in medium containing serum could be affected by various signals. Caspase‐8 and caspase‐9 are the initial enzymes of the caspase cascade, which are concerned with the death signal pathway and mitochondrial pathway, respectively. Caspase‐3 is an effector enzyme which activates DNA endonucleases.33 Partial inhibition of caspase‐3 activation by inhibitors of caspases 1, 8, and 9 suggests this apoptosis is at least a participated death signal pathway and mitochondrial pathway. The activities of caspase‐1 and caspase 9 were not detected (fig 2). We think that the activities of these caspases were too weak to be detected by our assay technique. Caspase inhibitors could affect cell death via inhibition of caspase‐3 activation, but they did not affect cellular proliferation.
Growth factors included in serum and tears are effective, at least partially, for suppressing apoptosis induced by serum deprivation (figs 5, 6, and 7). This indicates that these growth factors are also at least partially responsible for maintaining cellular homeostasis. Loo et al showed that EGF induced bcl‐2 expression in serum‐free mouse embryo cell line.34 We think one of the protection mechanisms of EGF against apoptosis is caused by induced bcl‐2 expression. The other protection mechanism of EGF may be proliferation effect on cells.35 A study of repression of the EGF receptor by anti‐EGF receptor antibody reveals that anti‐EGF receptor antibodies can induce apoptosis associated with activation of the caspase cascade.36 NGF has also been reported to rescue cultured cells from serum deprivation induced apoptosis.15 TGF‐β protected against cellular apoptosis via the mitogen activated protein kinase pathway; however, the anti‐apoptotic effect of EGF was not involved in this pathway.12
Kitamura et al showed the anti‐apoptotic mechanism of retinoic acid in mesanginal cells. Retinoic acid inhibits oxidative stress induced apoptosis via suppression of c‐fos/c‐jun expression and JNK activation.37 Retinoic acid was shown to regulate proliferation and differentiation of cultured corneal epithelium as a physiological modulator.38 Anti‐apoptotic effects of retinoic acid may be the result of this physiological role. The effects of retinoic acid on cells are mediated by nuclear retinoic acid receptors which were identified in rabbit corneal epithelial cells.39 It is possible that EGF and retinoic acid are key components of autologous serum eye drops that have a different but important role in the maintenance of the ocular surface in severe dry eye.
Abbreviations
ABTS - 2,2′‐azinobis(3‐ethylbenzothiazolin‐6‐sulfonic acid
AFC - 7‐amino‐4‐trifluoromethyl coumarin
CCL - conjunctival epithelial cell line
CHAPS - 3‐[(3‐cholamidopropyl)dimethylammonio]‐2‐hydroxy‐1‐propanesulfonic acid
EGF - epidermal growth factor
FCS - fetal calf serum
FGF - fibroblast growth factor
FMK - fluoromethyl ketone
HGF - hepatocyte growth factor
IGF - insulin‐like growth factor
NGF - nerve growth factor
PDGF - platelet derived growth factor
SCF - stem cell factor
TGF‐β - transforming growth factor‐β
Z‐LEHD - Z‐Leu‐Glu‐His‐Asp‐FMK
Z‐LETD - Z‐Leu‐Glu‐Thr‐Asp‐FMK
Z‐WEHD - N‐benzyloxycarbonyl‐Trp‐Glu‐His‐Asp‐FMK
Z‐VAD - Z‐Val‐Ala‐Asp (O‐methyl)‐FMK
Footnotes
Grant support: Research on Eye and Ear Sciences, Immunology, Allergy and Organ Transplantation.
References
- 1.Ubels J L, Foley K M, Rismond V. Retinal secretion by the lacrimal grand. Invest Ophthalmol Vis Sci 1986271261–1268. [PubMed] [Google Scholar]
- 2.Ohashi Y, Motokura M, Kinoshita Y.et al Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci 1989301879–1882. [PubMed] [Google Scholar]
- 3.Van Setten G B, Macauley S, Humphreys‐Beher M.et al Detection of transforming growth factor‐alpha mRNA and protein in rat lacrimal glands and characterization of transforming growth factor‐alpha in human tears. Invest Ophthalmol Vis Sci 199637166–173. [PubMed] [Google Scholar]
- 4.Yoshino K, Garg R, Monroy D.et al Production and secretion of transforming growth factor beta (TGF‐beta) by the human lacrimal gland. Curr Eye Res 199615615–624. [DOI] [PubMed] [Google Scholar]
- 5.Wilson S E, Liang Q, Kim W J. Lacrimal grand HGF, KGF, and EGF mRNA levels increase after corneal epitherial wounding. Invest Ophthalmol Vis Sci 1999402185–2190. [PubMed] [Google Scholar]
- 6.Tsubota K, Goto E, Fujita H.et al Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol 199983390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsubota K, Higuchi A. Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin 200040113–122. [DOI] [PubMed] [Google Scholar]
- 8.Evan G I, Brown L, Whyte M.et al Apoptosis and the cell cycle. Curr Opin Cell Biol 19957825–834. [DOI] [PubMed] [Google Scholar]
- 9.Packham G, Cleveland J L. c‐Myc and apoptosis. Biochim Biophys Acta 1995124211–28. [DOI] [PubMed] [Google Scholar]
- 10.Packham G, Porter C W, Cleveland J L. c‐Myc induces apoptosis and cell cycle progression by separable, yet overlapping, pathways. Oncogene 199613461–469. [PubMed] [Google Scholar]
- 11.Elliott K, Sakamuro D, Basu A.et al Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene 1999183564–3573. [DOI] [PubMed] [Google Scholar]
- 12.McClellan M, Kievit P, Auersperg N.et al Regulation of proliferation and apoptosis by epidermal growth factor and protein kinase C in human ovarian surface epithelial cells. Exp Cell Res 1999246471–479. [DOI] [PubMed] [Google Scholar]
- 13.Gibson S, Tu S, Oyer R.et al Epidermal growth factor protects epithelial cells against Fas‐induced apoptosis. J Biol Chem 199927417612–17618. [DOI] [PubMed] [Google Scholar]
- 14.Tong L, Werrbach‐Perez K, Perez‐Polo J R. Prolonged activation of transcription factor AP‐1 during NGF‐mediated rescue from apoptotic cell death in PC12 cells. Neurochem Res 1999241431–1441. [DOI] [PubMed] [Google Scholar]
- 15.Chin B Y, Petrache I, Choi A M.et al Transforming growth factor beta1 rescues serum deprivation‐induced apoptosis via the mitogen‐activated protein kinase (MAPK) pathway in macrophages. J Biol Chem 199927411362–11368. [DOI] [PubMed] [Google Scholar]
- 16.Offord E A, Sharif N A, Mace K.et al Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci 1999401091–1101. [PubMed] [Google Scholar]
- 17.Liu B, Fang M, Schmidt M.et al Induction of apoptosis and activation of the caspase cascade by anti‐EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c‐jun N‐terminal kinase activity. Br J Cancer 2000821991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasson R, Tajima K, Amsterdam A. Glucocorticoids protect against apoptosis induced by serum deprivation, cyclic adenosine 3′,5′‐monophosphate and p53 activation in immortalized human granulosa cells: involvement of Bcl‐2. Endocrinology 2001142802–811. [DOI] [PubMed] [Google Scholar]
- 19.Tseng S C, Maumenee A E, Stark W J.et al Topical retinoid treatment for various dry‐eye disorders. Ophthalmology 198592717–727. [DOI] [PubMed] [Google Scholar]
- 20.Selek H, Unlu N, Orhan M.et al Evaluation of retinoic acid ophthalmic emulsion in dry eye. Eur J Ophthalmol 200010121–127. [DOI] [PubMed] [Google Scholar]
- 21.Singer L, Brook U, Romem M.et al Vitamin A in Stevens‐Johnson syndrome. Ann Ophthalmol 198921209–210. [PubMed] [Google Scholar]
- 22.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci 199722299–306. [DOI] [PubMed] [Google Scholar]
- 23.Talanian R V, Quinlan C, Trautz S.et al Substrate specificities of caspase family proteases. J Biol Chem 19972729677–9682. [DOI] [PubMed] [Google Scholar]
- 24.Thornberry N A, Rano T A, Peterson E P.et al A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 199727217907–17911. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes‐Alnemri T, Armstrong R C, Krebs J.et al In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD‐like domains. Proc Natl Acad Sci USA 1996937464–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boldin M P, Goncharov T M, Goltsev Y V.et al Involvement of MACH, a novel MORT1/FADD‐interacting protease, in Fas/APO‐1‐ and TNF receptor‐induced cell death. Cell 199685803–815. [DOI] [PubMed] [Google Scholar]
- 27.Zou H, Henzel W J, Liu X.et al Apaf‐1, a human protein homologous to C. elegans CED‐4, participates in cytochrome c‐dependent activation of caspase‐3. Cell 199790405–413. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Nijhawan D, Budihardjo I.et al Cytochrome c and dATP‐dependent formation of Apaf‐1/caspase‐9 complex initiates an apoptotic protease cascade. Cell 199791479–489. [DOI] [PubMed] [Google Scholar]
- 29.Faleiro L, Kobayashi R, Fearnhead H.et al Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J 1997162271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins L M, Kottke T, Mesner P W.et al Activation of multiple interleukin‐1beta converting enzyme homologues in cytosol and nuclei of HL‐60 cells during etoposide‐induced apoptosis. J Biol Chem 19972727421–7430. [DOI] [PubMed] [Google Scholar]
- 31.MacFarlane M, Cain K, Sun X M.et al Processing/activation of at least four interleukin‐1beta converting enzyme‐like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells. J Cell Biol 1997137469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stennicke H R, Salvesen G S. Biochemical characteristics of caspases‐3, ‐6, ‐7, and ‐8. J Biol Chem 199727225719–25723. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zou H, Slaughter C.et al DFF, a heterodimeric protein that functions downstream of caspase‐3 to trigger DNA fragmentation during apoptosis. Cell 199789175–184. [DOI] [PubMed] [Google Scholar]
- 34.Loo D T, Bradford S, Helmrich A.et al Bcl‐2 inhibits cell death of serum‐free mouse embryo cells caused by epidermal growth factor deprivation. Cell Biol Toxicol 199814375–382. [DOI] [PubMed] [Google Scholar]
- 35.Mao J, Smith M F, Rucker E B.et al Effect of epidermal growth factor and insulin‐like growth factor I on porcine preantral follicular growth, antrum formation, and stimulation of granulosal cell proliferation and suppression of apoptosis in vitro. J Anim Sci 2004821967–1975. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Carvajal M, Carraway C A.et al Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea 20012081–85. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura M, Ishikawa Y, Moreno‐Manzano V.et al Intervention by retinoic acid in oxidative stress‐induced apoptosis. Nephrol Dial Transplant (17 Suppl) 2002984–87. [DOI] [PubMed] [Google Scholar]
- 38.Kruse F E, Tseng S C. Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest Ophthalmol Vis Sci 1994352405–2420. [PubMed] [Google Scholar]
- 39.Bossenbroek N M, Sulahian T H, Ubels J L. Expression of nuclear retinoic acid receptor and retinoid X receptor mRNA in the cornea and conjunctiva. Curr Eye Res 199817462–469. [DOI] [PubMed] [Google Scholar]