Abstract
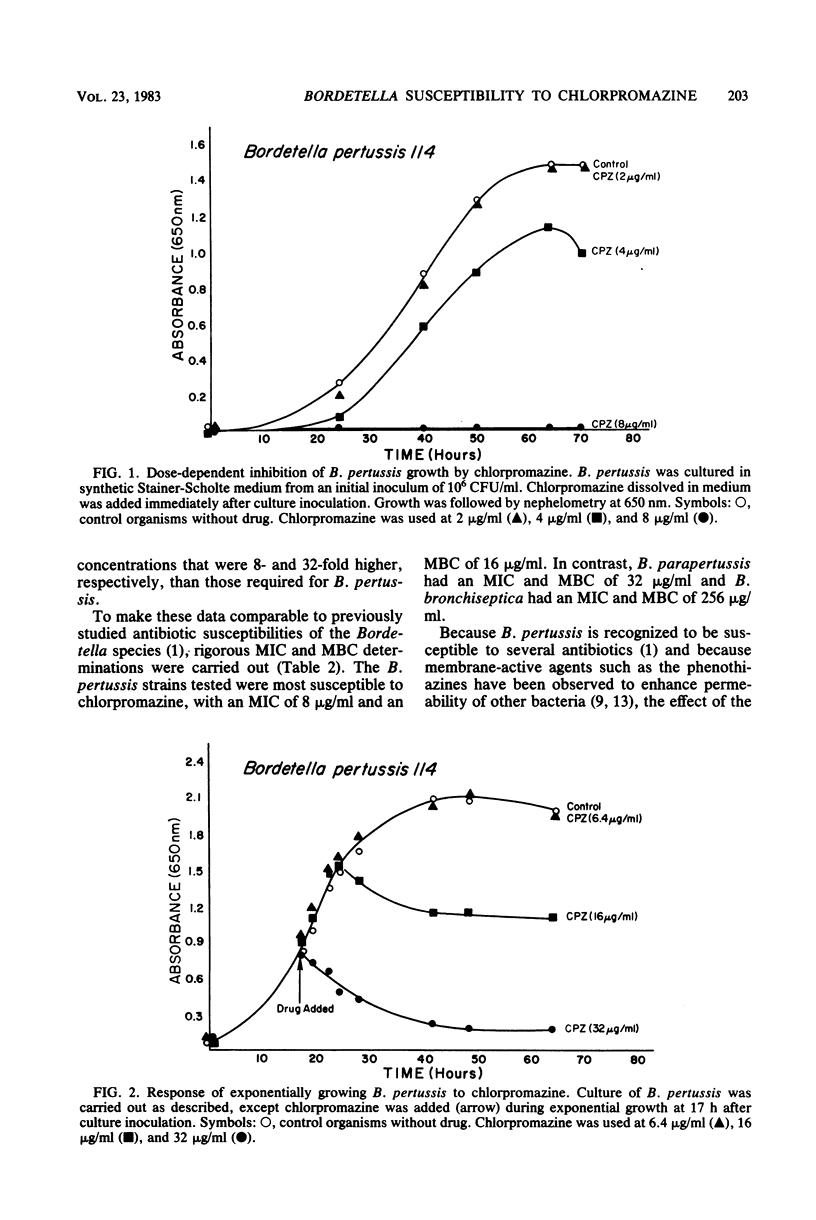
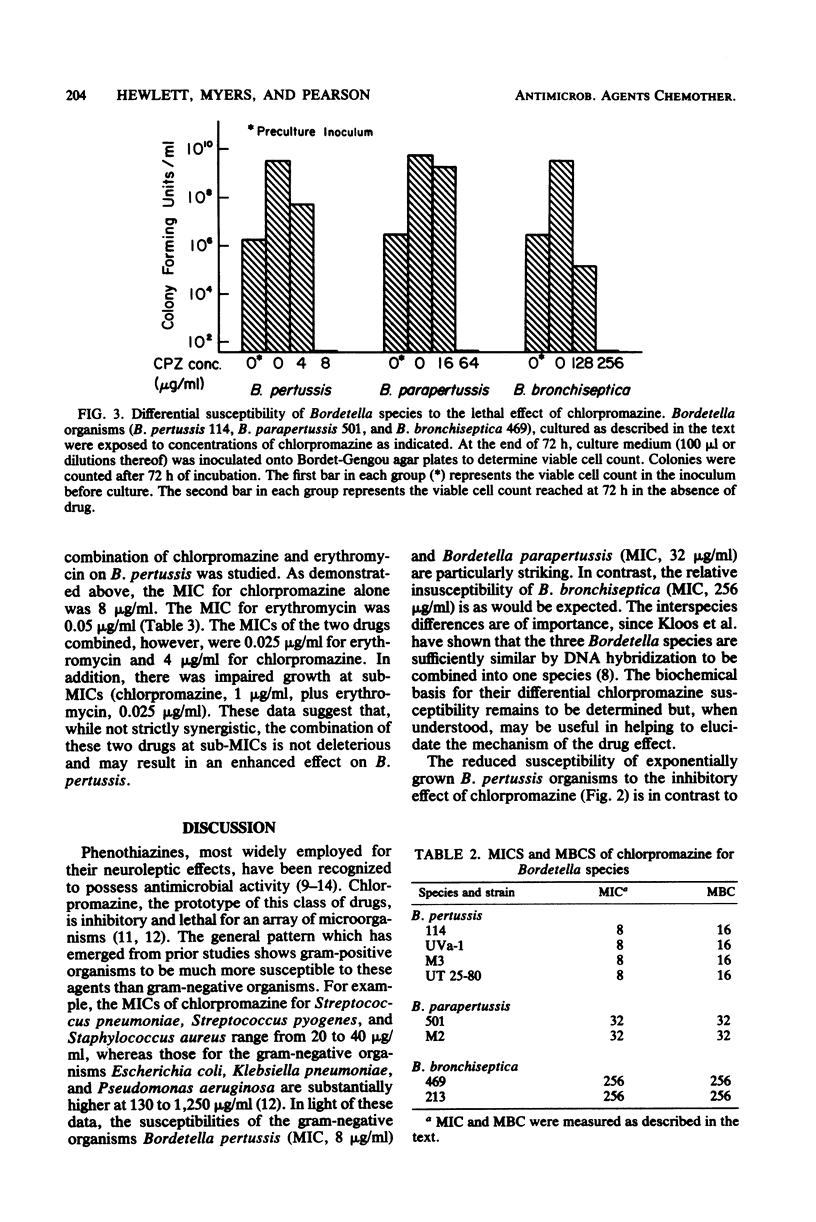
Chlorpromazine, the prototype phenothiazine tranquilizer, inhibited the growth and killed organisms of the genus Bordetella. There were striking differences, however, among the three Bordetella species. Bordetella pertussis was most susceptible, with some inhibition of growth at greater than or equal to 4 micrograms/ml and killing at 16 micrograms of chlorpromazine per ml. Bordetella parapertussis and Bordetella bronchiseptica were less susceptible, with killing at 32 and 256 micrograms/ml, respectively. Although the phenothiazines were inhibitory for Bordetella extracytoplasmic adenylate cyclase, the lethal effect occurred at a lower concentration and did not appear to involve modification of the enzyme activity. Exposure of B. pertussis to combinations of chlorpromazine and erythromycin resulted in impaired growth at concentrations lower than that of either drug alone, but there was no evidence that the two drugs interacted either synergistically or antagonistically.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz S. A., Goldhammer A. R., Hewlett E. L., Wolff J. Activation of prokaryotic adenylate cyclase by calmodulin. Ann N Y Acad Sci. 1980;356:360–360. doi: 10.1111/j.1749-6632.1980.tb29626.x. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manion R. E., Bradley S. G., Hall W. H. Retardation of the emergence of isoniazid-resistant mycobacteria by phenothiazines and quinacrine. Proc Soc Exp Biol Med. 1969 Jan;130(1):206–209. doi: 10.3181/00379727-130-33522. [DOI] [PubMed] [Google Scholar]
- Molnár J., Mándi Y., Király J. Antibacterial effect of some phenothiazine compounds and R-factor elimination by chlorpromazine. Acta Microbiol Acad Sci Hung. 1976;23(1):45–54. [PubMed] [Google Scholar]
- NATHAN H. A. Alteration of permeability of Lactobacillus plantarum caused by chlorpromazine. Nature. 1961 Nov 4;192:471–472. doi: 10.1038/192471b0. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Goldman M. Action of chlorpromazine on spore-forming Bacillus species. Can J Microbiol. 1974 Dec;20(12):1689–1693. doi: 10.1139/m74-261. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Manian A. A., Harcus J. L., Hall D., Hewlett E. L. Lethal effect of phenothiazine neuroleptics on the pathogenic protozoan Leishmania donovani. Science. 1982 Jul 23;217(4557):369–371. doi: 10.1126/science.6124040. [DOI] [PubMed] [Google Scholar]
- Suda T., Shimizu D., Maeda N., Shiga T. Decreased viscosity of human erythrocyte suspension induced by chlorpromazine and isoxsuprine. Biochem Pharmacol. 1981 Aug 1;30(15):2057–2064. doi: 10.1016/0006-2952(81)90223-9. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]



