Abstract
Aim
To analyse how intraocular lens (IOL) chromophores affect retinal photoprotection and the sensitivity of scotopic vision, melanopsin photoreception, and melatonin suppression.
Methods
Transmittance spectra of IOLs, high pass spectral filters, human crystalline lenses, and sunglasses are used with spectral data for acute ultraviolet (UV)‐blue photic retinopathy (“blue light hazard” phototoxicity), aphakic scotopic luminous efficiency, melanopsin sensitivity, and melatonin suppression to compute the effect of spectral filters on retinal photoprotection, scotopic sensitivity, and circadian photoentrainment.
Results
Retinal photoprotection increases and photoreception decreases as high pass filters progressively attenuate additional short wavelength light. Violet blocking IOLs reduce retinal exposure to UV (200–400 nm) radiation and violet (400–440 nm) light. Blue blocking IOLs attenuate blue (440–500 nm) and shorter wavelength optical radiation. Blue blocking IOLs theoretically provide better photoprotection but worse photoreception than conventional UV only blocking IOLs. Violet blocking IOLs offer similar UV‐blue photoprotection but better scotopic and melanopsin photoreception than blue blocking IOLs. Sunglasses provide roughly 50% more UV‐blue photoprotection than either violet or blue blocking IOLs.
Conclusions
Action spectra for most retinal photosensitisers increase or peak in the violet part of the spectrum. Melanopsin, melatonin suppression, and rhodopsin sensitivities are all maximal in the blue part of the spectrum. Scotopic sensitivity and circadian photoentrainment decline with ageing. UV blocking IOLs provide older adults with the best possible rhodopsin and melanopsin sensitivity. Blue and violet blocking IOLs provide less photoprotection than middle aged crystalline lenses, which do not prevent age related macular degeneration (AMD). Thus, pseudophakes should wear sunglasses in bright environments if the unproved phototoxicity‐AMD hypothesis is valid.
Keywords: intraocular lens, phototoxicity, scotopic, macular degeneration, melanopsin
Optical radiation includes ultraviolet (UV) radiation (200–400 nm) and visible light (400–700 nm).1 Violet (400–440 nm) and blue (440–500 nm) light comprise the shorter wavelength part of the visible spectrum.2,3 The cornea prevents UV radiation shorter than 300 nm from reaching the retina.4 The crystalline lens blocks most UV between 300 nm and 400 nm.4,5,6 Light transmission by the crystalline lens decreases with ageing, particularly at shorter wavelengths.4,5,6,7,8 The first poly(methylmethacrylate) intraocular lenses (IOLs) transmitted UV in addition to visible light.9 UV does not provide useful vision but it can harm the retina in acute intense exposures.9,10,11,12,13 Most IOLs incorporated UV blocking chromophores by 1986.14
Interest in blocking visible light as well as UV is motivated by the unproved hypothesis that phototoxicity from environmental light exposure can cause or accelerate age related macular degeneration (AMD).11,13,15,16,17,18,19,20,21,22,23,24,25 This phototoxicity‐AMD hypothesis is popular in part because lipofuscin accumulates with ageing in the retinal pigment epithelium (RPE), perhaps increasing the retinal phototoxicity risks of older adults.26,27,28 None the less, six of the eight major epidemiological studies found no correlation between AMD and lifelong light exposure,29,30,31,32,33,34,35,36,37 caused by (1) its absence, (2) difficulty in accurately estimating a subject's cumulative light exposure retrospectively, (3) variability in genetic susceptibility, or (4) other potentially obfuscating factors such as differences in the age at which subjects experience bright environmental light exposure.
Light can damage the retina by photomechanical, photothermal, or photochemical mechanisms.1,11,20,38 The two classic types of acute retinal photochemical injuries (“photic retinopathies” or “retinal phototoxicities”) can be distinguished by their action spectra, as shown in figure 1.1,3,20,39,40,41,42 An action spectrum characterises the variation in potential phototoxicity with wavelength.
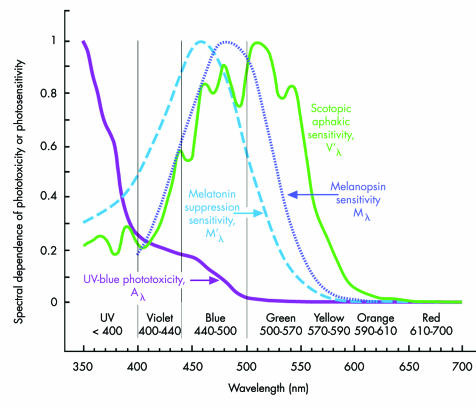
Figure 1 Acute aphakic UV‐blue phototoxicity (Aλ),10,12,73 aphakic scotopic luminous efficiency (V'λ),46 melanopsin spectral sensitivity (Mλ, peak sensitivity, 479–483 nm),68,69,70,71,188 and melatonin suppression sensitivity (M'λ, peak sensitivity, 459–464 nm).66,67,72 Original rather than smoothed aphakic scotopic luminous efficiency (V'λ) data are shown.46 The potential hazardousness of acute UV‐blue type phototoxicity increases with decreasing wavelength. Acute blue‐green retinal phototoxicity has an action spectrum similar to aphakic scotopic sensitivity because rhodopsin mediates both processes.43,44 Melatonin suppression and melanopsin sensitivity are more heavily dependent on blue light than rod (rhodopsin) mediated visual functions.
The first type of phototoxicity is blue‐green (“Noell‐type,” “class 1,” or “white light”) photic retinopathy. Its action spectrum is similar to aphakic scotopic sensitivity because rhodopsin mediates both processes. Thus, blue‐green phototoxicity hazardousness actually decreases in the blue and violet part of the spectrum below rhodopsin's peak sensitivity around 500 nm (cf, fig 1).43,44,45,46 Furthermore, any spectral filter that reduces blue‐green phototoxicity causes an equivalent percentage decrease in scotopic sensitivity.
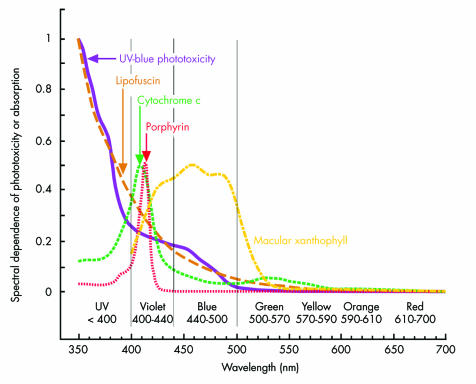
The second type of phototoxicity is UV‐blue (“Ham‐type,” “class 2,” or “blue light hazard”) photic retinopathy.10,12,20,25,40,47 As shown in figure 1, its severity increases with decreasing wavelength, similar to that of lipofuscin which is one of its primary mediators.23,25,48 Figure 2 illustrates that macular xanthophyll protection declines rapidly in the violet part of the spectrum,49,50,51,52 where porphyrin and cytochrome oxidase phototoxicity peak.23,43,53,54,55,56,57,58 The weakly phototoxic25 pyridinium bisretinoid A2E component of lipofuscin also has peak phototoxicity around 430 nm in the violet part of the spectrum.59,60 Separating acute photic retinopathy into the two preceding categories is useful heuristically, but it may oversimplify phototoxic interactions currently used to study retinal degeneration and cell biology.22,57,61,62,63
Figure 2 Acute UV‐blue10,12,73 and lipofuscin23,25 phototoxicity rise rapidly in the violet part of the spectrum, where porphyrin23,56 and cytochrome oxidase23,58 phototoxicities peak and macular xanthophyll protection declines.75 Conversely, as shown in figure 1, violet light is much less important than blue light for circadian photoentrainment and vision in dim environments.
There are three types of retinal photopigments: (1) cone photoreceptor photopigments that provide photopic (bright light) and mesopic (intermediate light) vision,64,65 (2) rhodopsin in rod photoreceptors responsible for mesopic and scotopic (dim light) vision,45,46 and (3) melanopsin in blue light sensitive retinal ganglion cells that modulate circadian photoentrainment, pupillary function, and possibly conscious vision.66,67,68,69 IOLs that block UV and visible light potentially reduce the risk of acute UV‐blue phototoxicity.3,11,14,42 They also decrease the light reaching S‐cones, light sensitive retinal ganglions, and rod photoreceptors,3,42 which have peak spectral sensitivities around 426 nm (violet),64,65 480 nm (blue),69,70,71 and 500 nm (blue‐green),45,46 respectively. Melanopsin containing light sensitive retinal ganglion cells control circadian photoentrainment through melatonin suppression. Melatonin suppression has a peak sensitivity in human subjects of roughly 460 nm (blue), approximately 20 nm shorter than the peak sensitivity measured experimentally for the photopigment melanopsin.66,67,68,69,70,71,72
The action spectra for acute experimental blue‐green and UV‐blue phototoxicities are well characterised, but if there is chronic light damage in humans, its action spectrum is unknown. If the phototoxicity‐AMD hypothesis is valid and chronic retinal damage arising from lifelong repetitive acute phototoxic injury does have a significant role in AMD, then action spectra for the two classic types of photic retinopathy can be used to estimate the relative protection afforded by different IOL spectral filters. UV‐blue phototoxicity is characterised by the international standard aphakic retinal hazard function (Aλ, fig 1),73 based on Ham's studies of light damage in young primates.10,12 Blue‐green phototoxicity may be specified by the aphakic scotopic sensitivity function governed by rhodopsin light absorption (fig 1).46
Retinal photoprotection, scotopic sensitivity, and circadian photoentrainment relative to a conventional UV only blocking IOL were computed for: (1) hypothetical UV + violet blocking high pass filters that have different cut‐off wavelengths, (2) UV + violet and UV + violet + blue blocking IOLs, and (3) crystalline lenses of different ages,4 using (1) spectral data for acute UV‐blue retinal phototoxicity,73 aphakic scotopic luminous efficiency,46 melanopsin sensitivity,69 and melatonin suppression,67 (2) transmittance spectra measured for each IOL, and (3) published data on the spectral transmittance of crystalline lenses4 and sunglasses.74 The terms “violet blocking” and “blue blocking” will be used for IOLs that attenuate UV + violet and UV + violet + blue light, respectively.
Materials and methods
A Beckman‐Coulter DU 800 UV/visible microcomputer controlled spectrophotometer (Beckman‐Coulter, Fullerton, CA, USA) was used to measure the spectral transmittance of UV transmitting (eyeonics Crystalens AT‐45), UV only blocking (AMO Clariflex), violet blocking (AMO OptiBlue), and blue blocking (Alcon AcrySof SN60AT and Hoya AF‐1) IOLs. Each IOL was aligned in a saline filled cuvette. Transmittance spectra were recorded from 350 nm to 700 nm. Three independent spectral transmittance measurements were performed for three IOLs of each type. Differences in the spectral transmittances of IOLs of the same type were less than 0.2% (spectral bandwidth ⩽1.8 nm; wavelength repeatability +/− 0.1 nm).
Acute aphakic UV‐blue phototoxicity (Aλ),73 aphakic scotopic luminous efficiency (V'λ),46 melanopsin spectral sensitivity (Mλ),69 and melatonin suppression sensitivity (M'λ) 67,72 were used to estimate the effect of each IOL, crystalline lens, or hypothetical violet blocking filter on phototoxicity or photoreception. Hypothetical high pass filters were used to study how photoprotection and photoreception are affected by blocking optical radiation below 400, 410, 420, 430, and 440 nm. Each hypothetical violet filter blocks all optical radiation below but transmits 99% of the optical radiation above the specified cut‐off wavelength.
Areas under the Aλ, V'λ, Mλ, and M'λ curves in figure 1 represent total UV‐blue phototoxicity, aphakic scotopic sensitivity, melanopsin sensitivity, and melatonin suppression sensitivity, respectively. Aλ, V'λ, Mλ, and M'λ were multiplied wavelength by wavelength with the transmittance of each spectral filter to determine how much the filter decreased phototoxicity, scotopic sensitivity, melanopsin photoreception, or melatonin suppression, respectively. Calculations were performed from 350–700 nm for Aλ and V'λ using an isoquantal spectrum42 and from 400–600 nm for Mλ and M'λ using daylight illumination.72 Results are expressed in terms of the percentage difference between the performance of a particular filter and a conventional UV only blocking filter (AMO ClariFlex).
Results
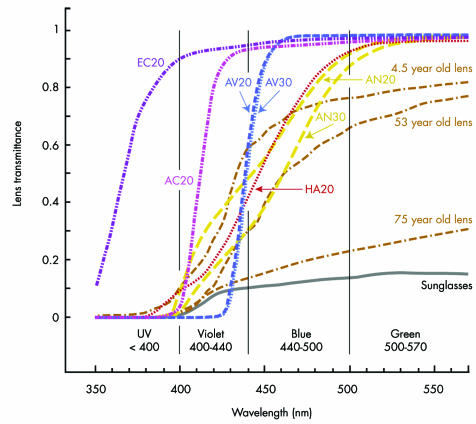
Spectral transmittances measured for IOLs are shown in figure 3, along with classic human crystalline lens transmittance data.4 Table 1 presents the computed photoprotection and photoreception of IOLs, crystalline lenses, and hypothetical high pass violet blocking filters, relative to a conventional UV only blocking IOL. Results generally agree with previous calculations,42 which used (1) the international standard CIE phakic scotopic sensitivity curve75 rather than the aphakic scotopic luminous efficiency data of Griswold and Stark,46 and (2) blue blocking IOL spectral transmittance data for measurements made in air rather than saline. Table 1 also presents the percentage of UV, violet, blue, or green light that each filter blocks.
Figure 3 The spectral transmittance of UV transmitting (Eyeonics Crystalens AT‐45: EC20), UV only blocking (AMO Clariflex: AC20), violet blocking (AMO OptiBlue: AV20 and AV30), and blue blocking (Alcon AcrySof SN60AT: AN20 and AN30; Hoya AF‐1: HA20) IOLs. The number 20 or 30 in each IOL label is the power in dioptres of the IOL tested. Also shown are the spectral transmittance of (1) neutral grey sunglasses (Sunglasses)74 and (2) 4.5, 53, and 75 year old crystalline lenses.4
Table 1 Photoprotection and photoreception relative to a UV only blocking IOL (AMO ClariFlex 20D): positive and negative percentages indicate better and worse performance, respectively. Percentage of optical radiation blocked in a particular wavelength band.
| UV‐blue photoprotection | Aphakic scotopic sensitivity* | Melanopsin sensitivity | Melatonin suppression | UV blocked† | Violet blocked† | Blue blocked† | Green blocked† | ||
|---|---|---|---|---|---|---|---|---|---|
| Eyeonics Crystalens AT‐45 | −150% | +10% | +4% | +14% | 39% | 7% | 3% | 2% | |
| AMO Clariflex 20D | – | – | – | – | 100% | 34% | 5% | 3% | |
| Violet 400 nm‡ | −33% | +6% | +6% | +11 | 95% | 1% | 1% | 1% | |
| Violet 410 nm‡ | −12% | +4% | +5% | +7 | 100% | 19% | 1% | 1% | |
| Violet 420 nm‡ | +6% | +2% | +3% | +1 | 100% | 44% | 1% | 1% | |
| Violet 430 nm‡ | +23% | −1% | 0% | −5% | 100% | 69% | 1% | 1% | |
| Violet 440 nm‡ | +39% | −5% | −5% | −13% | 100% | 94% | 1% | 1% | |
| AMO OptiBlue 20D | +40% | −6% | −7% | −15% | 100% | 90% | 6% | 1% | |
| AMO OptiBlue 30D | +42% | −6% | −7% | −16% | 100% | 92% | 6% | 1% | |
| Hoya AF‐1 | +43% | −15% | −18% | −27% | 98% | 78% | 27% | 4% | |
| Alcon AcrySof Natural 20D | +40% | −14% | −18% | −27% | 99% | 67% | 27% | 3% | |
| Alcon AcrySofNatural 30D | +57% | −21% | −27% | −38% | 100% | 83% | 40% | 5% | |
| 4.5 year old¶ | +35% | −24% | −26% | −30% | 97% | 69% | 30% | 21% | |
| 53 year old¶ | +61% | −37% | −41% | −48% | 100% | 86% | 48% | 28% | |
| 75 year old¶ | +82% | −76% | −78% | −80% | 99% | 91% | 81% | 73% | |
| Sunglasses§ | +89% | −86% | −86% | −87% | 100% | 93% | 88% | 85% |
*The percentage loss in aphakic scotopic sensitivity is the same as the percentage gain in blue‐green phototoxicity protection (rhodopsin mediates both processes).
†The percentage of optical radiation in a particular wavelength range that is blocked: UV (350–400 nm), violet (400–440 nm), blue (440–500 nm), and green (500–570 nm).
‡Hypothetical violet blocking high pass filters that block all optical radiation below but transmit 99% of radiation above the specified cut‐off wavelength.
¶Human crystalline lens spectral transmittance data from Boettner and Wolter.4
§Sunglasses spectral transmittance data from Marmor.74
High pass filter calculations in table 1 show that blocking an increasing amount of violet light from 400 nm to 440 nm increases UV‐blue and blue‐green photoprotection but decreases scotopic, melanopsin, and melatonin suppression sensitivity.
Table 1 also shows that the UV transmitting Crystalens provides 150% less UV‐blue photoprotection than a conventional UV only blocking IOL. Violet and blue blocking 20D IOLs offer approximately 40% more UV‐blue photoprotection than a UV only blocking IOL. They also provide roughly 50% less UV‐blue photoprotection than sunglasses and 20% less protection than a 53 year old crystalline lens.
Blue blocking IOLs offer about 20% better scotopic sensitivity and thus 20% less blue‐green phototoxicity protection than a 53 year old crystalline lens. The UV transmitting Crystalens provides 10% more scotopic sensitivity than conventional UV only blocking IOLs. Blue blocking 20D IOLs offer 15% less scotopic sensitivity than a standard IOL. Violet blocking IOLs reduce scotopic sensitivity loss to 7% but provide roughly the same acute UV‐blue phototoxicity protection as blue blocking IOLs.
Blue blocking IOLs provide 23% more melanopsin photoreception than a 53 year old lens but 18% less sensitivity than a conventional UV only blocking IOL. Violet blocking IOLs decrease melanopsin photoreception loss to 7% relative to a UV only blocking IOL. Blue blocking 20D and 30D IOLs provide 27% and 38% less melatonin suppression than a UV only blocking IOL, respectively, whereas violet blocking IOLs decrease melatonin suppression by only 15%–16%.
Discussion
Acute phototoxicity, IOLs, and AMD
The retina balances the production and removal of harmful reactive oxygen species in a hazardous oxidising environment that has high light levels and oxygen concentrations.20,21,76,77 Age related increasing accumulation of the photosensitiser lipofuscin may impair free radical control mechanisms and mechanically compromise cellular functions.21,23,60,78 AMD, blue‐green phototoxicity, and UV‐blue phototoxicity all probably involve direct or indirect oxidative damage,23,25,76,77 but this shared pathogenesis mechanism does not mean that phototoxicity causes AMD any more than it means that AMD causes phototoxicity. Additionally, both classic phototoxicities involve intense acute rather than lifelong normal light exposures.10,12,43,44 These acute exposures can injure the retina but they cannot simulate a lifetime of normal light exposure, just as scalding water can scar skin but it cannot simulate a lifetime of normal bathing.
AMD is a complex multifactorial process involving nutrition, smoking, genetics, and numerous influences other than light exposure.18,33,79,80 The relation between chronic light exposure and AMD is difficult to prove because of shared pathogenesis mechanisms, the size and duration of required epidemiological studies, and the difficulty of accurately estimating an individual's cumulative light exposure retrospectively. Two large population based studies did find a weak association.29,36 Four other large studies did not,31,33,34,37 including a later study by Taylor33 who first identified a potential association between light exposure and AMD in the Waterman study.29 Additionally, two large case‐control studies failed to show a correlation between AMD and environmental light exposure,30,32 one of which actually found that sunlight exposure was higher in the control group than in subjects with AMD.32
The risk of severe AMD has been reported to increase after cataract surgery,81,82,83 but this correlation is confounded by the possibility that cataract surgery may have been performed for decreased vision caused by AMD.82,84 Indeed, the AREDS study found no correlation between cataract surgery and AMD after specifically monitoring subjects for their AMD status before cataract surgery.84,85 If a correlation between AMD and cataract surgery does exist, it may be the result of the trauma and inflammation of operating microscope procedures and illumination on aged susceptible maculas.81,82,83
Despite 25 years of use, the evidence documenting the clinical advantage of UV only blocking versus UV transmitting IOLs remains limited. UV only blocking IOLs have been reported to reduce pseudophakic erythropsia, the transient reddish discoloration of vision that can occur after exposure to a bright outdoor environment.86,87 Short wave cone sensitivity was found to be lower in the UV transmitting IOL eye of seven bilateral pseudophakes who had a UV only blocking IOL in their other eye (no retinal abnormalities were observed).88 Vitreous fluorophotometry demonstrated less blood‐retinal barrier disruption in eyes with UV only or blue blocking IOLs than in those with UV transmitting IOLs.89 Early studies suggested that UV only blocking IOLs were associated with a lower risk of postoperative cystoid macular oedema than UV transmitting IOLs.90 Later studies failed to confirm that association.91 No significant difference in the incidence of exudative AMD was found in pseudophakic eyes with or without UV protection.92 IOL chromophores have been shown to decrease acute retinal phototoxic damage from intense violet light in cell culture and experimental animal studies.93,94
Table 1 summarises theoretical pseudophakic photoprotection. It shows that violet and blue blocking 20D IOLs provide similar acute UV‐blue photoprotection. Photoprotection varies with dioptric power for blue blocking AcrySof Natural IOLs but not violet blocking AMO OptiBlue IOLs. Blue‐green photoprotection and scotopic sensitivity are inversely proportional, so violet blocking IOLs offer 8–9% less blue‐green photoprotection than 20D blue blocking IOLs, although no IOL provides significant blue‐green phototoxicity protection.
Table 1 also shows that sunglasses provide roughly 50% more photoprotection than 20D violet or blue blocking IOLs. Sunglasses have the additional advantage of removability for optimal vision in dim environments. Visible light blocking IOLs provide roughly 20% less UV‐blue or blue‐green phototoxicity protection than a 53 year old crystalline lens.4 Most AMD occurs in people over 60 years of age,95 so 53 year old crystalline lenses do not prevent it. Thus, if acute UV‐blue phototoxicity (the “blue light hazard”) is a significant risk factor for AMD, then the Boettner and Wolter data4 used to design blue blocking IOLs96 show that they do not reduce an older adult's risks, and pseudophakes regardless of IOL type should wear sunglasses in bright environments.
Scotopic sensitivity and IOLs
The human retina contains approximately five million cone and 90 million rod photoreceptors.97,98 Rod and cone photoreceptors are responsible primarily for scotopic and photopic vision, respectively. They both provide mesopic vision.75 Rod photoreceptors influence cone mediated visual functions even at photopic luminances.99,100,101,102,103,104 Rod photoreceptor populations and sensitivity decrease with ageing, diminishing scotopic sensitivity and other rod mediated visual functions.105,106,107,108 Pupil diameter also decreases with ageing,72,109,110,111 further reducing available light.
Rod photoreceptor mediated vision is important in modern society. Cone photoreceptors provide information on headlight illuminated roads during night driving, but rod photoreceptors process the remaining visual field.112,113 When you arise at night and lighting is too dim to appreciate colour, you are using rod mediated vision. Aarnisalo demonstrated that filtering blue, in addition to violet, light can reduce scotopic sensitivity.114 Blue light provides 7% of photopic sensitivity and 35% of aphakic scotopic sensitivity. In comparison, violet light provides only 1% of photopic and 10% of aphakic scotopic sensitivity.
Table 1 shows that a UV transmitting Crystalens provides 10% better theoretical scotopic sensitivity than a UV only blocking IOL. Blue blocking 20D and 30D IOLs provide 14% and 21% less scotopic sensitivity than a UV only blocking IOL, respectively, in contrast with the 6% difference with violet blocking IOLs.
Is a 14–21% loss of scotopic sensitivity significant? This loss is difficult to measure clinically and is small in comparison with the broad range of visual sensitivity.115,116 None the less, (1) it is a loss, (2) standard static perimetric tests are poor surrogates for night vision tasks such as ambulation and driving, (3) scotopic vision loss is worse in people with AMD and diabetic retinopathy,117,118,119,120,121 (4) decreased night vision is well known to be a significant problem for older adults, prompting many to curtail night‐time driving and other activities,122,123,124,125,126,127 and (5) impaired dark adaptation increases the risk of falling in older adults.128 Forty per cent of people over 65 years of age fall each year,129 increasing their risk of debilitating injury, long term hospitalisation, and death.130 Additionally, a study by Jackson showed that AcrySof Natural IOL pseudophakes have decreased scotopic vision at violet and blue wavelengths,131 a type of vision loss correlated with night driving difficulties.132
Circadian rhythmicity and IOLs
Spectral filters also affect circadian rhythmicity. The importance of the predominantly blue light sensitive retinal photopigment melanopsin was not widely recognised until 2002, well after the design of current blue blocking IOLs.66,67,133,134,135,136 Melanopsin is contained in photosensitive retinal ganglion cells.66,67,133,134,135,136 These ganglion cells control pineal secretion and suppression of melatonin using signals sent through the retinohypothalamic tract to the master biological clock in the suprachiasmatic nucleus.66,67,68,69,134,135,137 Blue light is critical in controlling circadian photoentrainment, pupillary response, and the broad range of beneficial systemic effects of endogenous melatonin.69,71,134,136,138,139,140,141,142,143
Melatonin is a small, lipophilic indoleamine neurohormone that has a pivotal role in circadian rhythmicity. In response to twilight or darkness, the pineal gland secrets melatonin, core body temperatures fall, and sleep ensues.134,135,144 In response to bright light, melatonin secretion is suppressed, core body temperatures rise, and there is improved cognition and alertness.145,146,147,148
Endogenous melatonin is an important factor in systemic homeostasis. It is a potent free radical scavenger149 that modulates other antioxidants such as superoxide dismutase, catalase, and glutathione peroxidase.150,151 It may help protect the RPE against the oxidative stress implicated in AMD.152,153,154 It has numerous anti‐cancer effects155 and limits tumour cell proliferation by inhibiting telomerase.156,157 It has anti‐inflammatory actions.158,159 Stimulation of electron transport and ATP production in the inner mitochondrial membranes by melatonin may affect ageing.160
Disorganisation of circadian rhythmicity is more common in older adults and people with insomnia,161 depression,162,163 coronary artery disease,164 acute myocardial infarction,165 bronchial asthma,166 many cancer types,167,168,169 Alzheimer's disease,170,171,172 and dementia.173 Numerous clinical studies have shown the risks of disturbed circadian photoentrainment174,175 and the benefits of optimal rhythmicity.176,177
Age related pupillary miosis and crystalline lens yellowing limit the blue light reaching melanopsin for circadian rhythmicity, reducing older adults' effective retinal light exposure to one tenth that of younger people.72 This reduction is probably responsible for the decreased blue light melatonin suppression observed in older adults.178 Additionally, elderly lifestyles may average half the total daily luminance of young adults.179 All these factors conspire to weaken the photoentrainment of older adults' circadian clock. None the less, bright environmental light exposure can restore melatonin levels in older insomniacs to 21 year old control levels, with resolution of their insomnia.179 Furthermore, insomnia and depression have been shown to decrease after cataract surgery.180,181
Blue light is responsible for 35% of scotopic sensitivity and 53% of melanopsin photoreception. Blue blocking 20D IOLs provide 18% less melanopsin photoreception than a conventional UV only blocking IOL. Mishima found that older adults with insomnia averaged 19% less total daily environmental illuminance than age matched control subjects.179 Blue blocking IOLs provide 27–38% less melatonin suppression than a UV only blocking IOL, whereas the reduction is 15–16% for a violet blocking IOL.
Colour vision
Scotopic vision depends on a single photoreceptor, but three types of cone photoreceptors mediate normal photopic sensitivity.2,182 Normal cone photoreceptor reception and subsequent neural processing provide remarkable constancy of perceived colour, despite illumination changes.2,182,183,184 For example, a red apple appears red both in incandescent and outdoor illumination and the sky appears blue when viewed through different sunglass tints.
The spectral sensitivity of photopic vision is similar in aphakic and phakic patients, despite crystalline lens blockage of shorter wavelength light.185 Additionally, colour appearance returns largely to normal within a few months of implantation of a UV only blocking IOL, despite the IOL's increased transmittance of shorter wavelength light.186 Colour disparity problems that required explantation of a blue blocking AcrySof Natural IOL have been reported, however, in an individual with a UV only blocking IOL in their other eye.187 In general, most individuals readily adjust to vision with visible light blocking IOLs.
Conclusion
There is no conclusive clinical or experimental proof that (1) normal light exposure causes AMD, (2) pseudophakes are at increased risk for AMD, or (3) repetitive acute phototoxicity causes AMD. None the less, if IOLs can increase retinal protection without significantly compromising photoreception, people and society should benefit. The phototoxicity‐AMD hypothesis remains attractive because RPE lipofuscin concentration increases with ageing, perhaps compromising cellular function and increasing an older adults' risk of photic retinopathy.
UV, violet, and blue light are responsible for 67%, 18%, and 14% of acute UV‐blue phototoxicity, respectively, in the spectral region from 350–700 nm where optical radiation can potentially reach the retina of a pseudophakic eye. Lipofuscin phototoxicity increases rapidly and porphyrin, cytochrome oxidase and A2E phototoxicity all peak in the violet part of the spectrum. UV is potentially hazardous and provides no useful vision so it is logical to block it with IOL chromophores. Violet light causes an additional 18% of UV‐blue phototoxicity but provides only 10% of aphakic scotopic sensitivity. Thus, if the phototoxicity‐AMD hypothesis is valid and UV blue photoxicity (the “blue‐light hazard”) does have a significant role in macular ageing, violet blocking IOLs protect the retina from most potentially phototoxic violet light while transmitting light in the blue part of the spectrum where rhodopsin, melanopsin, and melatonin suppression sensitivities all are maximal. Blue blocking IOLs have spectral transmittances similar to adult crystalline lenses.
Cataract surgery is an older adult's once in a lifetime opportunity to have improved circadian rhythmicity and vision in dim environments. UV only blocking IOLs have provided patients with their best possible scotopic vision and melanopsin photoreception for over a quarter of a century. Visible light blocking IOLs should endeavour to continue this tradition, particularly since the phototoxicity‐AMD hypothesis remains unproved and blue light mediated melatonin may actually help protect the RPE from the oxidative stresses probably involved in AMD.
Acknowledgements
This research was supported in part by the Kansas Lions Sight Foundation, Inc, Manhattan, KS, USA. The author acknowledges the valuable assistance of Patricia L Turner, MD, Michael D Lowery, PhD, and Alan J Lang, PhD.
Abbreviations
AMD - age related macular degeneration
IOL - intraocular lens
RPE - retinal pigment epithelium
UV - ultraviolet
Footnotes
Competing interests: MAM serves as a consultant for Advanced Medical Optics, Inc, which manufactures intraocular lenses.
Presented in part at the 2005 Annual Meeting of the American Society of Cataract of Refractive Surgery in Washington DC and the 2005 Annual Meeting of the European Society of Cataract and Refractive Surgery in Lisbon, Portugal.
References
- 1.Mainster M A, Turner P L. Retinal injuries from light: mechanisms, hazards and prevention. In: Ryan SJ, Hinton DR, Schachat AP, et al, eds. Retina. Vol 2. London: Elsevier, 20061857–1870.
- 2.Pokorny J, Smith V C, Verriest G.et alCongenital and acquired color vision defects. New York: Grune & Stratton, 1979
- 3.Mainster M A. Intraocular lenses should block UV radiation and violet but not blue light. Arch Ophthalmol 2005123550–555. [DOI] [PubMed] [Google Scholar]
- 4.Boettner E A, Wolter J R. Transmission of the ocular media. Invest Ophthalmol 19621776–783. [Google Scholar]
- 5.van Norren D, Vos J J. Spectral transmission of the human ocular media. Vis Res 1974141237–1244. [DOI] [PubMed] [Google Scholar]
- 6.Mellerio J. Yellowing of the human lens: nuclear and cortical contributions. Vis Res 1987271581–1587. [DOI] [PubMed] [Google Scholar]
- 7.Weale R A. Age and the transmittance of the human crystalline lens. J Physiol 1988395577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Pokorny J, Smith V C. Optical density of the human lens. J Opt Soc Am A 199714953–960. [DOI] [PubMed] [Google Scholar]
- 9.Mainster M A. Spectral transmittance of intraocular lenses and retinal damage from intense light sources. Am J Ophthalmol 197885167–170. [DOI] [PubMed] [Google Scholar]
- 10.Ham W T, Jr, Mueller H A, Sliney D H. Retinal sensitivity to damage from short wavelength light. Nature 1976260153–155. [DOI] [PubMed] [Google Scholar]
- 11.Mainster M A. Solar retinitis, photic maculopathy and the pseudophakic eye. J Am Intraocul Implant Soc 1978484–86. [DOI] [PubMed] [Google Scholar]
- 12.Ham W T, Jr, Ruffolo J J, Jr, Mueller H A.et al he nature of retinal radiation damage: dependence on wavelength, power level and exposure time. Vis Res 1980201105–1111. [DOI] [PubMed] [Google Scholar]
- 13.Mainster M A, Ham W T, Jr, Delori F C. Potential retinal hazards. Instrument and environmental light sources. Ophthalmology 198390927–932. [DOI] [PubMed] [Google Scholar]
- 14.Mainster M A. The spectra, classification, and rationale of ultraviolet‐protective intraocular lenses. Am J Ophthalmol 1986102727–732. [DOI] [PubMed] [Google Scholar]
- 15.Van der Hoeve J. Eye lesions produced by light rich in ultraviolet rays: senile cataract, senile degeneration of the macula. Am J Ophthalmol 19203178–194. [Google Scholar]
- 16.Ts'o M O, La Piana F G, Appleton B. The human fovea after sungazing. Trans Am Acad Ophthalmol Otolaryngol 197478OP677. [PubMed] [Google Scholar]
- 17.Young R W. A theory of central retinal disease. In: Sears ML, ed. New directions in ophthalmic research. New Haven, CT: Yale University Press, 1981237–270.
- 18.Tso M O. Pathogenetic factors of aging macular degeneration. Ophthalmology 198592628–635. [DOI] [PubMed] [Google Scholar]
- 19.Marshall J. Radiation and the ageing eye. Ophthalmic Physiol Opt 19855241–263. [PubMed] [Google Scholar]
- 20.Mainster M A. Light and macular degeneration: a biophysical and clinical perspective. Eye 19871304–310. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J. The ageing retina: physiology or pathology. Eye 19871(Pt 2)282–295. [DOI] [PubMed] [Google Scholar]
- 22.Reme C, Reinboth J, Clausen M.et al Light damage revisited: converging evidence, diverging views? Graefes Arch Clin Exp Ophthalmol 19962342–11. [DOI] [PubMed] [Google Scholar]
- 23.Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B 200164144–161. [DOI] [PubMed] [Google Scholar]
- 24.Burkle A. Mechanisms of ageing. Eye 200115371–375. [DOI] [PubMed] [Google Scholar]
- 25.Margrain T H, Boulton M, Marshall J.et al Do blue light filters confer protection against age‐related macular degeneration? Prog Retin Eye Res 200423523–531. [DOI] [PubMed] [Google Scholar]
- 26.Feeney‐Burns L, Berman E R, Rothman H. Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol 198090783–791. [DOI] [PubMed] [Google Scholar]
- 27.Weiter J J, Delori F C, Wing G L.et al Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 198627145–152. [PubMed] [Google Scholar]
- 28.Feeney‐Burns L, Hilderbrand E S, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 198425195–200. [PubMed] [Google Scholar]
- 29.Taylor H R, West S, Munoz B.et al The long‐term effects of visible light on the eye. Arch Ophthalmol 199211099–104. [DOI] [PubMed] [Google Scholar]
- 30.The Eye Disease Case‐Control Study Group Risk factors for neovascular age‐related macular degeneration. Arch Ophthalmol 19921101701–1708. [DOI] [PubMed] [Google Scholar]
- 31.Hirvela H, Luukinen H, Laara E.et al Risk factors of age‐related maculopathy in a population 70 years of age or older. Ophthalmology 1996103871–877. [DOI] [PubMed] [Google Scholar]
- 32.Darzins P, Mitchell P, Heller R F. Sun exposure and age‐related macular degeneration. An Australian case‐control study. Ophthalmology 1997104770–776. [DOI] [PubMed] [Google Scholar]
- 33.McCarty C A, Mukesh B N, Fu C L.et al Risk factors for age‐related maculopathy: the Visual Impairment Project. Arch Ophthalmol 20011191455–1462. [DOI] [PubMed] [Google Scholar]
- 34.Delcourt C, Carriere I, Ponton‐Sanchez A.et al Light exposure and the risk of age‐related macular degeneration: the Pathologies Oculaires Liées à l'Age (POLA) study. Arch Ophthalmol 20011191463–1468. [DOI] [PubMed] [Google Scholar]
- 35.Cruickshanks K J, Klein R, Klein B E.et al Sunlight and the 5‐year incidence of early age‐related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2001119246–250. [PubMed] [Google Scholar]
- 36.Tomany S C, Cruickshanks K J, Klein R.et al Sunlight and the 10‐year incidence of age‐related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2004122750–757. [DOI] [PubMed] [Google Scholar]
- 37.Clemons T E, Milton R C, Klein R.et al Risk factors for the incidence of Advanced Age‐Related Macular Degeneration in the Age‐Related Eye Disease Study (AREDS) AREDS report no 19. Ophthalmology 2005112533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mainster M A. Retinal laser accidents: mechanisms, management and rehabilitation. J Laser Appl 2000123–9. [Google Scholar]
- 39.Kremers J J, van Norren D. Two classes of photochemical damage of the retina. Lasers Light Ophthalmol 1988241–52. [Google Scholar]
- 40.Mellerio J. Light effects on the retina. In: Albert DM, Jakobiec FA, eds. Principles and practice of ophthalmology. Vol 1. Philadelphia: WB Saunders, 19941326–1345.
- 41.Gorgels T G, Van Norren D. Two spectral types of retinal light damage occur in albino as well as in pigmented rat: no essential role for melanin. Exp Eye Res 199866155–162. [DOI] [PubMed] [Google Scholar]
- 42.Mainster M A, Sparrow J R. How much blue light should an IOL transmit? Br J Ophthalmol 2003871523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noell W K, Walker V S, Kang B S.et al Retinal damage by light in rats. Invest Ophthalmol 19665450–473. [PubMed] [Google Scholar]
- 44.Noell W K. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vis Res 1980201163–1171. [DOI] [PubMed] [Google Scholar]
- 45.Wald G. Human vision and the spectrum. Science 1945101653–658. [DOI] [PubMed] [Google Scholar]
- 46.Griswold M S, Stark W S. Scotopic spectral sensitivity of phakic and aphakic observers extending into the near ultraviolet. Vis Res 1992321739–1743. [DOI] [PubMed] [Google Scholar]
- 47.Reme C E, Grimm C, Hafezi F.et al Why study rod cell death in retinal degenerations and how? Doc Ophthalmol 200310625–29. [DOI] [PubMed] [Google Scholar]
- 48.Rozanowska M, Jarvis‐Evans J, Korytowski W.et al Blue light‐induced reactivity of retinal age pigment. In vitro generation of oxygen‐reactive species. J Biol Chem 199527018825–18830. [DOI] [PubMed] [Google Scholar]
- 49.Pease P L, Adams A J, Nuccio E. Optical density of human macular pigment. Vis Res 198727705–710. [DOI] [PubMed] [Google Scholar]
- 50.Werner J S, Donnelly S K, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vis Res 198727257–268. [DOI] [PubMed] [Google Scholar]
- 51.Mainster M A. Henle fibers may direct light toward the center of the fovea. Lasers Light Ophthalmol 1988279–86. [Google Scholar]
- 52.Handelman G J, Snodderly D M, Krinsky N I.et al Biological control of primate macular pigment. Biochemical and densitometric studies. Invest Ophthalmol Vis Sci 199132257–267. [PubMed] [Google Scholar]
- 53.Lawwill T. Three major pathologic processes caused by light in the primate retina: a search for mechanisms. Trans Am Ophthalmol Soc 198280517–579. [PMC free article] [PubMed] [Google Scholar]
- 54.Pautler E L, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem Photobiol 199051599–605. [DOI] [PubMed] [Google Scholar]
- 55.Gorgels T G, van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest Ophthalmol Vis Sci 199536851–863. [PubMed] [Google Scholar]
- 56.Anderson H L. Building molecular wires from the colours of life: conjugated porphyrin oligomers. Chem Commun 199992323–2330. [Google Scholar]
- 57.Grimm C, Wenzel A, Williams T.et al Rhodopsin‐mediated blue‐light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci 200142497–505. [PubMed] [Google Scholar]
- 58.Kaderbhai M A, Hopper D J, Akhtar K M.et al A cytochrome c from a lupanine‐transforming Pseudomonas putida strain is expressed in Escherichia coli during aerobic cultivation and efficiently exported and assembled in the periplasm. Appl Environ Microbiol 2003694727–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sparrow J R, Parish C A, Hashimoto M.et al A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci 1999402988–2995. [PubMed] [Google Scholar]
- 60.Sparrow J R, Nakanishi K, Parish C A. The lipofuscin fluorophore A2E mediates blue light‐induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000411981–1989. [PubMed] [Google Scholar]
- 61.Wenzel A, Grimm C, Marti A.et al c‐fos controls the “private pathway” of light‐induced apoptosis of retinal photoreceptors. J Neurosci 20002081–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm C, Wenzel A, Hafezi F.et al Gene expression in the mouse retina: the effect of damaging light. Mol Vis 20006252–260. [PubMed] [Google Scholar]
- 63.Reme C E. The dark side of light: rhodopsin and the silent death of vision the proctor lecture. Invest Ophthalmol Vis Sci 2005462671–2682. [DOI] [PubMed] [Google Scholar]
- 64.Merbs S L, Nathans J. Absorption spectra of human cone pigments. Nature 1992356433–435. [DOI] [PubMed] [Google Scholar]
- 65.Stockman A, Sharpe L T, Merbs S.et al Spectral sensitivities of human cone visual pigments determined in vivo and in vitro. Methods Enzymol 2000316626–650. [DOI] [PubMed] [Google Scholar]
- 66.Brainard G C, Hanifin J P, Greeson J M.et al Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 2001216405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thapan K, Arendt J, Skene D J. An action spectrum for melatonin suppression: evidence for a novel non‐rod, non‐cone photoreceptor system in humans. J Physiol 2001535261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hankins M W, Lucas R J. The primary visual pathway in humans is regulated according to long‐term light exposure through the action of a nonclassical photopigment. Curr Biol 200212191–198. [DOI] [PubMed] [Google Scholar]
- 69.Dacey D M, Liao H W, Peterson B B.et al Melanopsin‐expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005433749–754. [DOI] [PubMed] [Google Scholar]
- 70.Hattar S, Lucas R J, Mrosovsky N.et al Melanopsin and rod‐cone photoreceptive systems account for all major accessory visual functions in mice. Nature 200342476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu X, Kumbalasiri T, Carlson S M.et al Induction of photosensitivity by heterologous expression of melanopsin. Nature 2005433745–749. [DOI] [PubMed] [Google Scholar]
- 72.Charman W N. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol Opt 200323181–187. [DOI] [PubMed] [Google Scholar]
- 73.ACGIH Threshold limit values and biological eposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2000
- 74.Marmor M F. Double fault! Ocular hazards of a tennis sunglass. Arch Ophthalmol 20011191064–1066. [DOI] [PubMed] [Google Scholar]
- 75.Wyszecki G, Stiles W S.Color science. 2nd ed. New York: John Wiley & Sons, 1982
- 76.Winkler B S, Boulton M E, Gottsch J D.et al Oxidative damage and age‐related macular degeneration. Mol Vis 1999532. [PMC free article] [PubMed] [Google Scholar]
- 77.Beatty S, Koh H, Phil M.et al The role of oxidative stress in the pathogenesis of age‐related macular degeneration. Surv Ophthalmol 200045115–134. [DOI] [PubMed] [Google Scholar]
- 78.Shamsi F A, Boulton M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest Ophthalmol Vis Sci 2001423041–3046. [PubMed] [Google Scholar]
- 79.Seddon J M, Ajani U A, Sperduto R D.et al Dietary carotenoids, vitamins A, C, and E, and advanced age‐related macular degeneration. Eye Disease Case‐Control Study Group. JAMA 19942721413–1420. [PubMed] [Google Scholar]
- 80.Van Leeuwen R, Ikram M K, Vingerling J R.et al Blood pressure, atherosclerosis, and the incidence of age‐related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003443771–3777. [DOI] [PubMed] [Google Scholar]
- 81.Klein R, Klein B E, Wong T Y.et al The association of cataract and cataract surgery with the long‐term incidence of age‐related maculopathy: the Beaver Dam eye study. Arch Ophthalmol 20021201551–1558. [DOI] [PubMed] [Google Scholar]
- 82.Wang J J, Klein R, Smith W.et al Cataract surgery and the 5‐year incidence of late‐stage age‐related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology 20031101960–1967. [DOI] [PubMed] [Google Scholar]
- 83.Freeman E E, Munoz B, West S K.et al Is there an association between cataract surgery and age‐related macular degeneration? Data from three population‐based studies. Am J Ophthalmol 2003135849–856. [DOI] [PubMed] [Google Scholar]
- 84.Ferris F L., 3rd iscussion of a model of spectral filtering to reduce photochemical damage in age‐related macular degeneration. Trans Am Ophthalmol Soc 200410295. [PMC free article] [PubMed] [Google Scholar]
- 85.Martin D F, Gensler G, Klein B E K.et al The effect of cataract surgery on progression to advanced AMD (abstract number 1907). In: ARVO annual meeting: May 7, 2002 2002; Fort Lauderdale, Florida, 200276
- 86.Jordan D R, Valberg J D. Dyschromatopsia following cataract surgery. Can J Ophthalmol 198621140–143. [PubMed] [Google Scholar]
- 87.Bennett L W. Pseudophakic erythropsia. J Am Optom Assoc 199465273–276. [PubMed] [Google Scholar]
- 88.Werner J S, Steele V G, Pfoff D S. Loss of human photoreceptor sensitivity associated with chronic exposure to ultraviolet radiation. Ophthalmology 1989961552–1558. [DOI] [PubMed] [Google Scholar]
- 89.Miyake K, Ichihashi S, Shibuya Y.et al Blood‐retinal barrier and autofluorescence of the posterior polar retina in long‐standing pseudophakia. J Cataract Refract Surg 199925891–897. [DOI] [PubMed] [Google Scholar]
- 90.Kraff M C, Sanders D R, Jampol L M.et al Effect of an ultraviolet‐filtering intraocular lens on cystoid macular edema. Ophthalmology 198592366–369. [DOI] [PubMed] [Google Scholar]
- 91.Komatsu M, Kanagami S, Shimizu K. Ultraviolet‐absorbing intraocular lens versus non‐UV‐absorbing intraocular lens: comparison of angiographic cystoid macular edema. J Cataract Refract Surg 198915654–657. [DOI] [PubMed] [Google Scholar]
- 92.Van der Schaft T L, Mooy C M, de Bruijn W C.et al Increased prevalence of disciform macular degeneration after cataract extraction with implantation of an intraocular lens. Br J Ophthalmol 199478441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sparrow J, Miller A S, Zhou J. Blue light‐absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg 200430873–878. [DOI] [PubMed] [Google Scholar]
- 94.Wenzel A C G, Reme C E. Protective effect of the AcrySof Natural IOL on retinal damage induced by acute blue light in mice. Symposium on Cataract, IOL and Refractive Surgery, Abstract 515 2004131
- 95.Evans J R. Risk factors for age‐related macular degeneration. Prog Retin Eye Res 200120227–253. [DOI] [PubMed] [Google Scholar]
- 96.Ernest P H. Light‐transmission‐spectrum comparison of foldable intraocular lenses. J Cataract Refract Surg 2004301755–1758. [DOI] [PubMed] [Google Scholar]
- 97.Curcio C A, Sloan K R, Kalina R E.et al Human photoreceptor topography. J Comp Neurol 1990292497–523. [DOI] [PubMed] [Google Scholar]
- 98.Curcio C A, Owsley C, Jackson G R. Spare the rods, save the cones in aging and age‐related maculopathy. Invest Ophthalmol Vis Sci 2000412015–2018. [PubMed] [Google Scholar]
- 99.Naarendorp F, Denny N, Frumkes T E. Rod light and dark adaptation influence cone‐mediated spatial acuity. Vis Res 19882867–74. [PubMed] [Google Scholar]
- 100.Stabell B, Stabell U. Rod suppression of cone‐mediated information about colour and form during dark adaptation. Scand J Psychol 199031139–148. [DOI] [PubMed] [Google Scholar]
- 101.Naarendorp F, Frumkes T. The influence of short‐term adaptation of human rods and cones on cone‐mediated grating visibility. J Physiol 1991432521–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohand‐Said S, Hicks D, Leveillard T.et al Rod‐cone interactions: developmental and clinical significance. Prog Retin Eye Res 200120451–467. [DOI] [PubMed] [Google Scholar]
- 103.Stabell B, Stabell U. Effects of rod activity on color perception with light adaptation. J Opt Soc Am A Opt Image Sci Vis 2002191249–1258. [DOI] [PubMed] [Google Scholar]
- 104.Buck S L. Rod‐cone interactions in human vision. In: Chalupa LM, Werner JS, eds. The visual neurosciences. Vol 1. Cambridge, MA: The MIT Press, 2003863–878.
- 105.Curcio C A, Millican C L, Allen K A.et al Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci 1993343278–3296. [PubMed] [Google Scholar]
- 106.Jackson G R, Owsley C, McGwin G., Jr Aging and dark adaptation. Vis Res 1999393975–3982. [DOI] [PubMed] [Google Scholar]
- 107.Schefrin B E, Tregear S J, Harvey L O., Jret al Senescent changes in scotopic contrast sensitivity. Vis Res 1999393728–3736. [DOI] [PubMed] [Google Scholar]
- 108.Jackson G R, Owsley C. Scotopic sensitivity during adulthood. Vis Res 2000402467–2473. [DOI] [PubMed] [Google Scholar]
- 109.Kadlecova V, Peleska M, Vasko A. Dependence on age of the diameter of the pupil in the dark. Nature 19581821520–1521. [DOI] [PubMed] [Google Scholar]
- 110.Koch D D, Samuelson S W, Haft E A.et al Pupillary size and responsiveness. Implications for selection of a bifocal intraocular lens. Ophthalmology 1991981030–1035. [PubMed] [Google Scholar]
- 111.Hennelly M L, Barbur J L, Edgar D F.et al The effect of age on the light scattering characteristics of the eye. Ophthalmic Physiol Opt 199818197–203. [PubMed] [Google Scholar]
- 112.Gegenfurtner K R, Mayser H, Sharpe L T. Seeing movement in the dark. Nature 1999398475–476. [DOI] [PubMed] [Google Scholar]
- 113.Gegenfurtner K R, Mayser H M, Sharpe L T. Motion perception at scotopic light levels. J Opt Soc Am A Opt Image Sci Vis 2000171505–1515. [DOI] [PubMed] [Google Scholar]
- 114.Aarnisalo E A. Effects of yellow filter glasses on the results of photopic and scotopic photometry. Am J Ophthalmol 1988105408–411. [DOI] [PubMed] [Google Scholar]
- 115.Reeves A. Visual adaptation. In: Chalupa LM, Werner JS, eds. The visual neurosciences. Vol. 1. Cambridge, MA: The MIT Press, 2003851–862.
- 116.Werner J S. Night vision in the elderly: consequences for seeing through a “blue filtering” intraocular lens. Br J Ophthalmol 2005891518–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown B, Brabyn L, Welch L.et al Contribution of vision variables to mobility in age‐related maculopathy patients. Am J Optom Physiol Opt 198663733–739. [DOI] [PubMed] [Google Scholar]
- 118.Sunness J S, Rubin G S, Applegate C A.et al Visual function abnormalities and prognosis in eyes with age‐related geographic atrophy of the macula and good visual acuity. Ophthalmology 19971041677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Owsley C, Jackson G R, Cideciyan A V.et al Psychophysical evidence for rod vulnerability in age‐related macular degeneration. Invest Ophthalmol Vis Sci 200041267–273. [PubMed] [Google Scholar]
- 120.Owsley C, Jackson G R, White M.et al Delays in rod‐mediated dark adaptation in early age‐related maculopathy. Ophthalmology 20011081196–1202. [DOI] [PubMed] [Google Scholar]
- 121.Greenstein V C, Thomas S R, Blaustein H.et al Effects of early diabetic retinopathy on rod system sensitivity. Optom Vis Sci 19937018–23. [DOI] [PubMed] [Google Scholar]
- 122.Kline D W. Light, ageing and visual performance. In: Marshall J, ed. The susceptible visual apparatus. Vol 16. London: Macmillan Press, 1991150–161.
- 123.Charman W N. Vision and driving‐‐a literature review and commentary. Ophthalmic Physiol Opt 199717371–391. [PubMed] [Google Scholar]
- 124.Owsley C, McGwin G., Jr Vision impairment and driving. Surv Ophthalmol 199943535–550. [DOI] [PubMed] [Google Scholar]
- 125.Klein B E, Klein R, Lee K E.et al Associations of performance‐based and self‐reported measures of visual function. The Beaver Dam Eye Study. Ophthalmic Epidemiol 1999649–60. [DOI] [PubMed] [Google Scholar]
- 126.Mainster M A, Timberlake G T. Why HID headlights bother older drivers. Br J Ophthalmol 200387113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Owsley C, Stalvey B T, Phillips J M. The efficacy of an educational intervention in promoting self‐regulation among high‐risk older drivers. Accid Anal Prev 200335393–400. [DOI] [PubMed] [Google Scholar]
- 128.McMurdo M E, Gaskell A. Dark adaptation and falls in the elderly. Gerontology 199137221–224. [DOI] [PubMed] [Google Scholar]
- 129.Hausdorff J M, Rios D A, Edelberg H K. Gait variability and fall risk in community‐living older adults: a 1‐year prospective study. Arch Phys Med Rehabil 2001821050–1056. [DOI] [PubMed] [Google Scholar]
- 130.Donald I P, Bulpitt C J. The prognosis of falls in elderly people living at home. Age Ageing 199928121–125. [DOI] [PubMed] [Google Scholar]
- 131.Jackson G R. Pilot study on the effect of a blue‐light‐blocking IOL on rod‐mediated (scotopic) vision. In: American Society for Cataract and Refractive Surgery: April 15–20, 2005 2005; Washington, DC, USA, 200581
- 132.Scilley K, Jackson G R, Cideciyan A V.et al Early age‐related maculopathy and self‐reported visual difficulty in daily life. Ophthalmology 20021091235–1242. [DOI] [PubMed] [Google Scholar]
- 133.Skene D J. Optimization of light and melatonin to phase‐shift human circadian rhythms. J Neuroendocrinol 200315438–441. [DOI] [PubMed] [Google Scholar]
- 134.Abbott A. Restless nights, listless days. Nature 2003425896–898. [DOI] [PubMed] [Google Scholar]
- 135.Menaker M. Circadian rhythms. Circadian photoreception. Science 2003299213–214. [DOI] [PubMed] [Google Scholar]
- 136.Foster R G. Neurobiology: bright blue times. Nature 2005433698–699. [DOI] [PubMed] [Google Scholar]
- 137.Newman L A, Walker M T, Brown R L.et al Melanopsin forms a functional short‐wavelength photopigment. Biochemistry 20034212734–12738. [DOI] [PubMed] [Google Scholar]
- 138.Van Gelder R N. Blue light and the circadian clock. Br J Ophthalmol 2004881353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wee R, Van Gelder R N. Sleep disturbances in young subjects with visual dysfunction. Ophthalmology 2004111297–303. [DOI] [PubMed] [Google Scholar]
- 140.Cajochen C, Munch M, Kobialka S.et al High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab 2005901311–1316. [DOI] [PubMed] [Google Scholar]
- 141.Melyan Z, Tarttelin E E, Bellingham J.et al Addition of human melanopsin renders mammalian cells photoresponsive. Nature 2005433741–745. [DOI] [PubMed] [Google Scholar]
- 142.Panda S, Nayak S K, Campo B.et al Illumination of the melanopsin signaling pathway. Science 2005307600–604. [DOI] [PubMed] [Google Scholar]
- 143.Brown R L, Robinson P R. Melanopsin—shedding light on the elusive circadian photopigment. Chronobiol Int 200421189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lerchl A. Biological rhythms in the context of light at night (LAN). Neuro Endocrinol Lett 200223(Suppl 2)23–27. [PubMed] [Google Scholar]
- 145.Lambert G W, Reid C, Kaye D M.et al Effect of sunlight and season on serotonin turnover in the brain. Lancet 20023601840–1842. [DOI] [PubMed] [Google Scholar]
- 146.Yannielli P, Harrington M E. Let there be “more” light: enhancement of light actions on the circadian system through non‐photic pathways. Prog Neurobiol 20047459–76. [DOI] [PubMed] [Google Scholar]
- 147.Espana R A, Scammell T E. Sleep neurobiology for the clinician. Sleep 200427811–820. [PubMed] [Google Scholar]
- 148.Flory J D, Manuck S B, Matthews K A.et al Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Res 200412911–19. [DOI] [PubMed] [Google Scholar]
- 149.Gulcin I, Buyukokuroglu M E, Oktay M.et al On the in vitro antioxidative properties of melatonin. J Pineal Res 200233167–171. [DOI] [PubMed] [Google Scholar]
- 150.Tunez I, del Carmen Munoz M, Feijoo M.et al Melatonin effect on renal oxidative stress under constant light exposure. Cell Biochem Funct 20032135–40. [DOI] [PubMed] [Google Scholar]
- 151.Rodriguez C, Mayo J C, Sainz R M.et al Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004361–9. [DOI] [PubMed] [Google Scholar]
- 152.Marchiafava P L, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res 199926184–189. [DOI] [PubMed] [Google Scholar]
- 153.Liang F Q, Aleman T S, Zaixin Yang.et al Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport 2001121011–1014. [DOI] [PubMed] [Google Scholar]
- 154.Liang F Q, Green L, Wang C.et al Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res 2004781069–1075. [DOI] [PubMed] [Google Scholar]
- 155.Reiter R J. Mechanisms of cancer inhibition by melatonin. J Pineal Res 200437213–214. [DOI] [PubMed] [Google Scholar]
- 156.Leon‐Blanco M M, Guerrero J M, Reiter R J.et al Melatonin inhibits telomerase activity in the MCF‐7 tumor cell line both in vivo and in vitro. J Pineal Res 200335204–211. [DOI] [PubMed] [Google Scholar]
- 157.Leon‐Blanco M M, Guerrero J M, Reiter R J.et al RNA expression of human telomerase subunits TR and TERT is differentially affected by melatonin receptor agonists in the MCF‐7 tumor cell line. Cancer Lett 200421673–80. [DOI] [PubMed] [Google Scholar]
- 158.Cevik H, Erkanli G, Ercan F.et al Exposure to continuous darkness ameliorates gastric and colonic inflammation in the rat: both receptor and non‐receptor‐mediated processes. J Gastroenterol Hepatol 200520294–303. [DOI] [PubMed] [Google Scholar]
- 159.Mayo J C, Sainz R M, Tan D X.et al Anti‐inflammatory actions of melatonin and its metabolites, N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine (AFMK) and N1‐acetyl‐5‐methoxykynuramine (AMK), in macrophages. J Neuroimmunol 2005165139–149. [DOI] [PubMed] [Google Scholar]
- 160.Reiter R J, Tan D X, Manchester L C.et al Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann N Y Acad Sci 2002959238–250. [DOI] [PubMed] [Google Scholar]
- 161.Haimov I, Laudon M, Zisapel N.et al Sleep disorders and melatonin rhythms in elderly people. BMJ 1994309167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Terman M, Terman J S. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr 200510647–63 quiz 672. [DOI] [PubMed] [Google Scholar]
- 163.Jones S H. Circadian rhythms, multilevel models of emotion and bipolar disorder‐‐an initial step towards integration? Clin Psychol Rev 2001211193–1209. [DOI] [PubMed] [Google Scholar]
- 164.Yaprak M, Altun A, Vardar A.et al Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int J Cardiol 200389103–107. [DOI] [PubMed] [Google Scholar]
- 165.Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Garcia M.et al Light/dark patterns of interleukin‐6 in relation to the pineal hormone melatonin in patients with acute myocardial infarction. Cytokine 20042689–93. [DOI] [PubMed] [Google Scholar]
- 166.Fei G H, Liu R Y, Zhang Z H.et al Alterations in circadian rhythms of melatonin and cortisol in patients with bronchial asthma. Acta Pharmacol Sin 200425651–656. [PubMed] [Google Scholar]
- 167.Schernhammer E S, Hankinson S E. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 2005971084–1087. [DOI] [PubMed] [Google Scholar]
- 168.Karasek M, Kowalski A J, Suzin J.et al Serum melatonin circadian profiles in women suffering from cervical cancer. J Pineal Res 20053973–76. [DOI] [PubMed] [Google Scholar]
- 169.Schernhammer E S, Laden F, Speizer F E.et al Night‐shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst 200395825–828. [DOI] [PubMed] [Google Scholar]
- 170.Skene D J, Swaab D F. Melatonin rhythmicity: effect of age and Alzheimer's disease. Exp Gerontol 200338199–206. [DOI] [PubMed] [Google Scholar]
- 171.Reiter R J, Cabrera J, Sainz R M.et al Melatonin as a pharmacological agent against neuronal loss in experimental models of Huntington's disease, Alzheimer's disease and parkinsonism. Ann N Y Acad Sci 1999890471–485. [DOI] [PubMed] [Google Scholar]
- 172.Wu Y H, Feenstra M G, Zhou J N.et al Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab 2003885898–5906. [DOI] [PubMed] [Google Scholar]
- 173.Reiter R J, Tan D X, Pappolla M A. Melatonin Relieves the Neural Oxidative Burden that Contributes to Dementias. Ann N Y Acad Sci 20041035179–196. [DOI] [PubMed] [Google Scholar]
- 174.Armstrong S M, Redman J R. Melatonin: a chronobiotic with anti‐aging properties? Med Hypotheses 199134300–309. [DOI] [PubMed] [Google Scholar]
- 175.Erren T C, Reiter R J, Piekarski C. Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften 200390485–494. [DOI] [PubMed] [Google Scholar]
- 176.Magri F, Sarra S, Cinchetti W.et al Qualitative and quantitative changes of melatonin levels in physiological and pathological aging and in centenarians. J Pineal Res 200436256–261. [DOI] [PubMed] [Google Scholar]
- 177.Pauley S M. Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med Hypotheses 200463588–596. [DOI] [PubMed] [Google Scholar]
- 178.Herljevic M, Middleton B, Thapan K.et al Light‐induced melatonin suppression: age‐related reduction in response to short wavelength light. Exp Gerontol 200540237–242. [DOI] [PubMed] [Google Scholar]
- 179.Mishima K, Okawa M, Shimizu T.et al Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J Clin Endocrinol Metab 200186129–134. [DOI] [PubMed] [Google Scholar]
- 180.Asplund R, Lindblad B E. Sleep and sleepiness 1 and 9 months after cataract surgery. Arch Gerontol Geriatr 20043869–75. [DOI] [PubMed] [Google Scholar]
- 181.Asplund R, Ejdervik Lindblad B. The development of sleep in persons undergoing cataract surgery. Arch Gerontol Geriatr 200235179–187. [DOI] [PubMed] [Google Scholar]
- 182.Gegenfurtner K R, Sharpe L T.Color vision: from genes to perception. Cambridge, UK: Cambridge University Press, 1999
- 183.Land E H. The retinex theory of color vision. Sci Am 1977237108–128. [DOI] [PubMed] [Google Scholar]
- 184.Valberg A, Lange‐Malecki B. “Colour constancy” in Mondrian patterns: a partial cancellation of physical chromaticity shifts by simultaneous contrast. Vis Res 199030371–380. [DOI] [PubMed] [Google Scholar]
- 185.Verriest G.The spectral curve of relative luminous efficiency in different age groups of aphakic eyes. Vol 13. Colour vision deficiencies II. International Symposium, Edinburgh, 1973. Basel, Switzerland: Karger, 1974 [PubMed]
- 186.Delahunt P B, Webster M A, Ma L.et al Long‐term renormalization of chromatic mechanisms following cataract surgery. Vis Neurosci 200421301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shah S.Explantation of a visible‐blue‐filtering‐fitering lens because of subjective color disparity. In: American Society for Cataract and Refractive Surgery, 15–20 April 2005; Washington, DC 2005
- 188.Lucas R J, Douglas R H, Foster R G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 20014621–626. [DOI] [PubMed] [Google Scholar]