Abstract
Background/aim
Subvisible micropulse diode laser photocoagulation localises retinal laser damage because brief micropulses allow little time for heat conduction to spread temperature rise from the retinal pigment epithelium to the neural retina. Treatment power is often chosen as a multiple of that needed for visible continuous wave lesions. The authors measured clinical laser powers needed for visible end point micropulse and continuous wave diode laser retinal photocoagulation.
Methods
Six parallel rows of 10 diode laser (810 nm) burns were made in the superior peripheral retina of six consecutive patients undergoing their initial frequency doubled Nd:YAG (532 nm) panretinal photocoagulation for proliferative or severe non‐proliferative diabetic retinopathy. All photocoagulation exposures were 125 µm in retinal diameter and 0.2 seconds in duration. Micropulse exposures were performed with 500 Hz, 0.3 ms micropulses. The minimal power needed (1) for visible continuous wave diode photocoagulation was determined from two adjacent rows of laser lesions and (2) for visible micropulse diode photocoagulation from four additional adjacent rows of laser lesions. Fluorescein angiograms and red‐free fundus photographs were obtained immediately and 6 days after laser photocoagulation in each patient. Calculations were performed to determine the extent to which clinical parameters exceeded ANSI Z136.1‐2000 maximal permissible exposure (MPE) levels for laser exposure.
Results
Continuous wave and micropulse lesions typically required 300 mW (60 mJ) and 1800 mW (54 mJ), respectively. Visible continuous wave and micropulse lesions exceeded MPE levels by multiples of 36× and 133×, respectively. Laser energies were similar for visible continuous wave and micropulse lesions.
Conclusion
Visible micropulse lesions require 6× more power but roughly the same energy as visible continuous wave lesions. No significant difference was demonstrable in the minimal power needed for photographically and angiographically apparent diode micropulse lesions. MPE levels are designed to provide a 10× safety margin. This safety margin was 3.7× greater for micropulse than continuous wave diode laser photocoagulation.
Keywords: retinal photocoagulation, age related macular degeneration, diabetes, laser
Conventional continuous wave photocoagulators produce laser pulses of uniform power. Repetitively pulsed (micropulse) photocoagulators produce laser pulses (pulse “envelopes”) that contain a series (“pulse train”) of very brief micropulses.1 Each micropulse in the envelope has the same duration and power. The pulse train of micropulses has a characteristic frequency (repetition rate in Hz) and duty factor (the percentage of time that the laser is “on” during the pulse envelope).
Laser radiation is absorbed primarily by melanin in the RPE and choroid during retinal photocoagulation. Absorption converts laser energy into heat, increasing the temperature of pigmented tissues. Heat conduction spreads this temperature increase from pigmented to adjacent non‐pigmented or unexposed tissues.1,2,3 Brief micropulse therapy can localise laser effects primarily to pigmented tissues.1,4,5,6,7
The magnitude and duration of a tissue's temperature rise history determines the severity of its thermal injury.1,8 Standard visible end point laser photocoagulation protocols produce 40–60°C temperature rises, far above the threshold for barely visible threshold retinal lesions. Burns become visible when the neural retina is damaged by heat conduction, loses its transparency, and scatters white ophthalmoscopy light back at the observer.3
A retinal laser threshold is the exposure needed for a 50% probability of a retinal effect of some type.1,9 The ANSI Z136.1‐2000 standard provides formulas for calculating maximum permissible exposures (MPEs) based on decades of experimental threshold studies.10,11,12,13,14 MPEs are designed to offer a 10× safety margin, so biological effects are anticipated at exposure multiples 10× greater than MPE levels.9,15 Clinical retinal photocoagulation exposures exceed MPEs by differing multiples depending on laser pulse duration. For example, laser parameters are roughly 10× MPE for subvisible choroidal neovascularisation transpupillary thermotherapy and 40× MPE for conventional visible end point argon photocoagulation.15
Subvisible photocoagulation potentially localises and decreases chorioretinal thermal injury.1,4,5,6,16,17,18,19 Power in subvisible retinal procedures is often selected as a fraction of that needed to produce visible lesions at peripheral retinal sites.18,20,21 We determined the minimal powers needed for visible continuous wave and micropulse diode laser lesions and calculated their MPE multiples from the ANSI Z136.1‐2000 standard.
Patients and methods
Six consecutive patients requiring panretinal photocoagulation for proliferative or severe non‐proliferative diabetic retinopathy were included in this study. All patients had preoperative fluorescein angiograms 3 weeks or less before study enrolment. Individuals with previous retinal laser therapy or significant media opacities were excluded from the study. Each patient received and signed a full informed consent. The study protocol was approved by the institutional ethics committee, Lambersart, France.
Before conventional continuous wave (CW) frequency doubled Nd:YAG (532 nm) panretinal photocoagulation, repetitively pulse diode photocoagulation (Iris Medical OcuLight SLx photocoagulator, Iridex Corporation, Mountain View, CA, USA) was performed in a 3–4 mm region of each patient's superior retinal periphery. Six parallel rows of 10 diode laser burns were made. Each diode laser exposure was 125 µm in retinal diameter and 0.2 seconds in duration. Repetitively pulse diode photocoagulation was performed with 500 Hz, 0.3 ms micropulses (15% duty factor) in 0.2 second exposures.
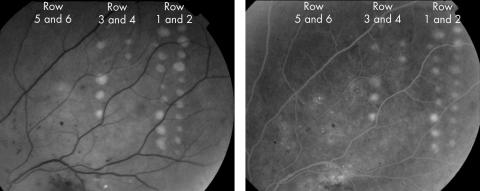
The minimal power needed for visible continuous wave diode laser photocoagulation was determined from two adjacent rows of laser lesions. The minimal power needed for visible diode micropulse photocoagulation was determined from four additional adjacent rows of laser lesions. Figure 1 shows postoperative red‐free and fluorescein angiographic retinal images of patient 2. Micropulse powers 1×, 2×, 4×, and 6× greater than the power needed for a visible CW diode laser burn were used in the four micropulse laser lesion columns, as shown in table 1. Red‐free photographs and fluorescein angiograms were obtained immediately and 6 days after laser therapy to classify lesions as photographically or angiographically “visible” or “subvisible.” Calculations determined the factor by which clinical powers exceeded ANSI Z136.1‐2000 MPE levels.
Figure 1 Retinal images taken immediately after diode laser photocoagulation of patient 2. (A) Red‐free fundus photograph. Two rows of continuous wave lesions are shown on the right. Micropulse lesions are shown in rows 3–6, as described in table 1. Haemorrhages and exudates were present preoperatively and are of diabetic origin. Only continuous wave and higher power micropulse lesions are visible. (B) A late phase fluorescein angiogram frame showing laser lesions and leakage from diabetic microaneurysms and telangiectatic vessels. All angiographically apparent lesions were also apparent on red‐free photographs.
Table 1 Power selection regimen and percentage of visible lesions in each treatment row†.
| Row number | CW or MP | Power* | Energy† | Percentage of lesions visible out of 10 laser exposures | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | ||||
| 1 and 2 | CW | Pvis | Evis = Pvis × 0.2 s | 100 | 100 | 100 | 100 | 100 | 100 |
| 3 | MP | Pvis | 15% of Evis | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | MP | 2 × Pvis | 30% of Evis | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | MP | 4 × Pvis | 60% of Evis | 0 | 20 | 0 | 0 | 0 | 0 |
| 6 | MP | 6 × Pvis | 90% of Evis | 10 | 30 | 0 | 40 | 30 | 20 |
*Abbreviations CW and MP connote continuous wave and micropulse exposures, respectively.
†Pvis and Evis are the minimal powers and energies needed for visible lesions, respectively.
Results
Tables 1 and 2 summarise study results. Table 1 shows that visible diode laser lesions could be obtained in all patients with CW photocoagulation and five of six patients with micropulse treatment. Powers needed to produce visible lesions are shown in table 2, along with the multiplication factors by which they exceed ANSI Z136.1‐2000 MPEs. Preoperative angiograms permitted discrimination of laser from pre‐existing diabetic lesions.
Table 2 Treatment powers and MPE multiplication factors*.
| Patient number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Power needed for a visible CW lesion | 300 mW | 300 mW | 250 mW | 300 mW | 300 mW | 300 mW |
| Energy needed for a visible CW lesion | 60 mJ | 60 mJ | 50 mJ | 60 mJ | 60 mJ | 60 mJ |
| CW MPE multiplication factor† | 36× | 36× | 30× | 36× | 36× | 36× |
| Power needed for a visible MP lesion | 1800 mW | 1800 mW | ¶ | 1800 mW | 1800 mW | 1800 mW |
| Energy needed for a visible MP lesion | 54 mJ‡ | 54 mJ | ¶ | 54 mJ | 54 mJ | 54 mJ |
| MP MPE multiplication factor† | 133× | 133× | ¶ | 133× | 133× | 133× |
*Abbreviations CW and MP in this table connote continuous wave and micropulse exposures, respectively.
†The number of times that delivered corneal energy exceeds ANSI Z136.1‐2000 maximum permissible exposure (MPE)13 for corneal energy. For a 125 μm retinal laser spot size diameter, 810 nm, 0.2 second envelope, 500 Hz exposure of 100, 0.3 ms micropulses, CA = 1.660 and CE = 4.902. CA is the correction factor used to increase MPE values in the near infrared (IR‐A) due to decreased RPE absorption in IR‐A.13 CE is the correction factor used to calculate the ocular extended source MPE from the small source MPE when the laser source subtends a visual angle greater than αmin (the apparent visual angle which divides small source from extended source viewing).13
‡(power)×(duration)×(duty factor) = (1800 mW)×(0.2 s)×(0.15) = 54 mJ
¶As noted in table 1, micropulse lesions were not demonstrable in this patient even with power settings 6× MPE.
Minimal powers for CW and micropulse lesions were typically 300 mW and 1800 mW, respectively. Thus, the ratio of micropulse to CW power for a 125 μm, 0.2 second laser exposure was 6× (1800/300), compared to the MPE ratio of 3.7× (133/36). Roughly the same laser energy was needed for visible CW and micropulse lesions, as shown in tables 1 and 2. There was no significant difference in the power required to produce photographically and angiographically visible lesions.
Variability in fundus pigmentation and laser lens alignment produced some variability in the appearance of laser lesions at identical photocoagulator settings. Micropulse lesions were apparent postoperatively only at the highest power settings in four of the five patients, although they could be detected in patient 2 at 60% of the laser energy needed for continuous wave lesions (table 1). Micropulse lesions were not observable in patient 3, possibly because the lower minimal power for a CW lesion required per protocol use of subvisible maximal micropulse power.
Red‐free and angiographic results were similar in all patients, as shown in figure 1. Angiographic findings were more apparent in some patients 6 days after rather than immediately after treatment. Our six study patients were followed for 2 years after treatment and all had satisfactory responses to panretinal photocoagulation.
Discussion
Repetitively pulsed laser photocoagulation potentially localises thermal effects to the retinal pigment epithelium (RPE) because very brief micropulses provide little time for heat conduction to spread temperature rise from pigmented absorbers to the neural retina.1,4,6 Some denaturation of intracellular proteins occurs at exposures 1/10th to 1/100th of single pulse thresholds.5,22,23 Each micropulse damages a small percentage of target tissue molecules, and repetitive micropulses combine to produce visible or subvisible effects according to the semiempirical n−1/4 law.1,5,10,13,14,22,23
Our study was limited by its small size. We estimated the minimal power needed for visible micropulse and CW lesions, but did not determine a formal “threshold” for 50% lesion probability. Our finding that the same total energy is needed for visible micropulse and CW lesions is consistent with thermal modelling predictions.2,8 The additional finding that 6× more power is needed for micropulse than CW clinical diode lesions corresponds roughly to the experimental 4× power factor found in Dutch belted rabbit experiments.24 In those experiments, histological damage was confined to the RPE when subvisible micropulse power was 25% or less than visible lesion settings.24 We observed no visible photographic or angiographic damage with laser micropulse powers less than half of those necessary for visible lesions (row 3 and 4 lesions).
The ANSI Z136.1‐2000 standard can be used to compute MPEs for clinical laser parameters based on their exposure duration, retinal spot size, wavelength, micropulse duration, and pulse repetition frequency.15 In terms of laser energy delivered to the cornea, clinical parameters exceed MPE by 1.4× for verteporfin photodynamic therapy,25 9.3× MPE for diode laser transpupillary thermotherapy for CNV,26,27 37× MPE for argon or FD‐YAG green photocoagulation (300 mW, 0.2 second, 200 μm spot diameter), and over 200× MPE for 0.8 μs FD:YAG green repetitive pulse photocoagulation.19 Since MPEs represent exposures 1/10th of those likely to produce laser effects, higher MPE multiples suggest greater likelihood of tissue damage.
MPEs were not designed to be used for clinical parameterisation, but clinical laser procedures of similar duration have similar MPE multiples. For example, our 30–36× MPE multiples for CW diode photocoagulation (table 2) are similar to the 37× MPE multiple for conventional argon or frequency doubled Nd:YAG green CW photocoagulation. Thresholds for visible laser effects should be roughly twice those of subvisible histological effects.9 Our 133× MPE multiples for visible micropulse diode lesions are 1.3–2.4× greater than those used for treating diabetic macular oedema with subthreshold micropulse diode laser photocoagulation.21,28 Micropulsing provides a multiplicity of potential combinations of spot size, pulse repetition frequency, and micropulse and pulse envelope durations. MPEs provide a self consistent method for estimating the relative effects of different clinical micropulse exposure parameters.15
We found no significant difference in the laser powers needed for photographically and angiographically apparent lesions with 0.3 ms repetitively pulsed photocoagulation. As shown in figure 1, angiographically apparent lesions were also apparent on red‐free photographs. This differs from 0.8 μs subvisible frequency doubled Nd:YAG green repetitively pulsed photocoagulation in which angiographic lesions are apparent at lower irradiances than ophthalmoscopic visible ones.29,30 Submillisecond (133× MPE) and submicrosecond (>200× MPE) repetitive pulse photocoagulation differ in their probable localisation of laser effects to RPE cells or their melanin granules, respectively. Localised melanin granule heating may be the origin of angiographic detectable outer blood‐retinal barrier defects demonstrable at irradiances lower than visible lesions in submicrosecond repetitively pulsed photocoagulation.
Abbreviations
CW - continuous wave
MPE - maximal permissible exposure
RPE - retinal pigment epithelium
References
- 1.Mainster M A. Decreasing retinal photocoagulation damage: principles and techniques. Sem Ophthalmol 199914200–209. [DOI] [PubMed] [Google Scholar]
- 2.Mainster M A, White T J, Tips J H.et al Retinal‐temperature increases produced by intense light sources. J Opt Soc Am 197060264–270. [DOI] [PubMed] [Google Scholar]
- 3.Mainster M A. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology 198693952–958. [DOI] [PubMed] [Google Scholar]
- 4.Pankratov M M. Pulsed delivery of laser energy in experimental thermal retinal photocoagulation. Proc Soc Photo‐Optical Instrum Eng 19901202205–213. [Google Scholar]
- 5.Sliney D H, Marshall J. Tissue specific damage to the retinal pigment epithelium: mechanisms and therapeutic implications. Lasers Light Ophthalmol 1992517–28. [Google Scholar]
- 6.Roider J, Hillenkamp F, Flotte T.et al Microphotocoagulation: selective effects of repetitive short laser pulses. Proc Natl Acad Sci USA 1993908643–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roider J, Lindemann C, el‐Hifnawi el S.et al Therapeutic range of repetitive nanosecond laser exposures in selective RPE photocoagulation. Graefes Arch Clin Exp Ophthalmol 1998236213–219. [DOI] [PubMed] [Google Scholar]
- 8.Birngruber R. Thermal modeling in biological tissues. In: Hillenkamp F, Pratesi R, Sacchi CA, eds. Lasers in medicine and biology. New York: Plenum Publishing, 198077–97.
- 9.Sliney D H, Mellerio J, Gabel V P.et al What is the meaning of threshold in laser injury experiments? Implications for human exposure limits. Health Phys 200282335–347. [DOI] [PubMed] [Google Scholar]
- 10.Sliney D H, Wolbarsht M L.Safety with lasers and other optical sources: a comprehensive handbook. New York: Plenum Press, 1980
- 11. Guidelines on limits of exposure to broad‐band incoherent optical radiation (0.38 to 3 microM). International Commission on Non‐Ionizing Radiation Protection. Health Phys 199773539–554. [PubMed] [Google Scholar]
- 12.American Conference of Governmental Industrial Hygienists Threshold limit values for chemical substances physical agents: biological exposure indices. Cincinnati: American Conference of Governmental Industrial Hygienists, 1997
- 13.American National Standards Institute American National Standard for the Safe Use of Lasers, ANSI Z136.1‐2000. Washington, DC: American National Standards Institute, 2000
- 14.International Electrotechnical Commission Safety of laser products. Part 1: Equipment classification requirements and user's guide, IEC 60825‐1. Geneva, Switzerland: International Electrotechnical Commission, 2001
- 15.Mainster M A, Turner P L. Retinal injuries from light: mechanisms, hazards and prevention. In: Ryan SJ, Hinton DR, Schachat AP, et al, eds. Retina. Vol 2, eds. London: Elsevier Publishers 20061857–1870.
- 16.Friberg T R, Karatza E C. The treatment of macular disease using a micropulsed and continuous wave 810‐nm diode laser. Ophthalmology 19971042030–2038. [DOI] [PubMed] [Google Scholar]
- 17.Roider J, Brinkmann R, Wirbelauer C.et al Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch Ophthalmol 19991171028–1034. [DOI] [PubMed] [Google Scholar]
- 18.Moorman C M, Hamilton A M. Clinical applications of the MicroPulse diode laser. Eye 199913145–150. [DOI] [PubMed] [Google Scholar]
- 19.Framme C, Brinkmann R, Birngruber R.et al Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br J Ophthalmol 2002861099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanga P E, Reck A C, Hamilton A M P. Micropulse laser in the treatment of diabetic macular edema. Semin Ophthalmol 199914210–213. [DOI] [PubMed] [Google Scholar]
- 21.Laursen M L, Moeller F, Sander B.et al Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 2004881173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillenkamp F. Interaction between laser radiation and biological systems. In: Hillenkamp F, Pratesi R, Sacchi CA, eds. Lasers in biology and medicine. New York: Plenum Publishing, 198037–68.
- 23.Ham W T, Jr, Mueller H A, Wolbarsht M L.et al Evaluation of retinal exposures from repetitively pulsed and scanning lasers. Health Phys 198854337–344. [DOI] [PubMed] [Google Scholar]
- 24.Kim S Y, Sanislo S R, Dalal R.et al The selective effect of micropulse diode laser upon the retina (ARVO abstract 3584). Invest Ophthalmol Vis Sci 199637S779 [Google Scholar]
- 25.Treatment of Age‐Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age‐ related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials—TAP report. Arch Ophthalmol 19991171329–1345. [PubMed] [Google Scholar]
- 26.Reichel E, Berrocal A M, Ip M.et al Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age‐related macular degeneration. Ophthalmology 19991061908–1914. [DOI] [PubMed] [Google Scholar]
- 27.Mainster M A, Reichel E. Transpupillary thermotherapy for age‐related macular degeneration: long‐ pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Lasers 200031359–373. [PubMed] [Google Scholar]
- 28.Luttrull J K, Musch D C, Mainster M A. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol 20058974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roider J, Brinkmann R, Wirbelauer C.et al Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol 20008440–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roider J, Brinkmann R, Wirbelauer C.et al Variability of RPE reaction in two cases after selective RPE laser effects in prophylactic treatment of drusen. Graefes Arch Clin Exp Ophthalmol 199923745–50. [DOI] [PubMed] [Google Scholar]