Abstract
Background
Haemoglobin E is a variant haemoglobin that can lead to considerable morbidity in compound heterozygous states with β thalassaemia. Therefore, its detection is important because it permits antenatal counselling. The parts of the world where haemoglobin E is prevalent are resource poor and detection can therefore be problematical. A simple visual test using 2,6‐dichlorophenolindophenol (DCIP) has been developed in Thailand, but its use has not become widespread. This test has now become available in kit form.
Aims/Methods
To evaluate the new DCIP test kit for the detection of haemoglobin E.
Results
Seventeen of 18 samples from individuals with haemoglobin E gave positive results and one gave an equivocal result. False positive or equivocal results were seen in three of 21 individuals with other disorders of globin chain synthesis but were not seen in normal subjects.
Conclusions
This study evaluated the sensitivity, specificity, and reproducibility of the kit and confirmed the usefulness of the DCIP test as a screening test for haemoglobin E. In countries with limited health resources, its use would reduce the number of samples requiring referral to a central laboratory for definitive tests.
Keywords: haemoglobin E, haemoglobinopathy, variant haemoglobin, DCIP, under‐resourced laboratories
Haemoglobin E is the most common variant haemoglobin worldwide, being very prevalent in an area stretching from northeastern India and Bangladesh through Burma (Myamur), Laos, Kampuchea (Cambodia), Thailand, Vietnam, Malaysia, the Philippines, and Indonesia.1 It is also found in Pakistan, Nepal, Sri Lanka, and Turkey. The detection of haemoglobin E is important for the antenatal diagnosis of disorders of globin chain synthesis because its interaction with β thalassaemia produces a compound heterozygous state that varies in severity from thalassaemia minor to, more often, thalassaemia intermedia or thalassaemia major. Many of the countries in which haemoglobin E is prevalent have under‐resourced laboratories and expensive and relatively difficult techniques such as high performance liquid chromatography (HPLC) and haemoglobin electrophoresis are available only in major centres. Nevertheless, the detection of haemoglobin E is important if the social and economic burden of β thalassaemia intermedia and major is to be avoided.
“Many of the countries in which haemoglobin E is prevalent have under‐resourced laboratories and expensive and relatively difficult techniques such as high performance liquid chromatography and haemoglobin electrophoresis are available only in major centres”
A simple visual test for the detection of haemoglobin E has been proposed, based on a serendipitous observation when a blue dye, 2,6‐dichlorophenolindophenol (DCIP) was used in testing for red cell enzyme deficiencies.2,3 It was noted that solutions containing haemoglobin E (from patients with either haemoglobin E heterozygosity or homozygosity) became turbid when exposed to this dye. This test has been used in Thailand for antenatal screening in under‐resourced areas,4,5 but there are other countries with a high prevalence of haemoglobin E where routine antenatal screening for this variant haemoglobin is not carried out. The test as initially described has been modified and is now available in kit form.6 We have evaluated the sensitivity and specificity of this kit to establish whether it could be recommended for screening whenever more complex and expensive tests are not readily available.
Materials and methods
The blood samples used in our study were venous samples anticoagulated with EDTA, which had been submitted to our haemoglobinopathy laboratory for investigation of suspected thalassaemia or haemoglobinopathy. Normal samples and samples from individuals with β thalassaemia trait or known to include haemoglobins S, C, and D were also studied to establish whether either thalassaemia trait or other variant haemoglobins gave false positive results. Haemoglobins S and D occur in some parts of the Indian subcontinent and thus may be present in populations tested for haemoglobin E. Similarly, haemoglobin C occurs sporadically in India and in Thailand. All samples were also studied by HPLC (Bio‐Rad variant II β Thal Short programme; Bio‐Rad, Hemel Hempstead, Berkshire, UK) with the nature of any abnormality found being confirmed by haemoglobin electrophoresis and other relevant techniques, according to our usual laboratory protocols.
Tests were performed using a kit purchased from PCL Holding Co Ltd, Sirinthorn Road, Bangplad, Bangkok, Thailand. A 20 μl sample of blood was added to 2 ml of a modified DCIP reagent and the tube was incubated at 37°C for 15 minutes before adding 20 μl of a second reagent to stop the reaction. The tube was then read visually. We found that tests were difficult to read, the turbidity of samples with haemoglobin E being less than that of samples with haemoglobin S in a sickle solubility test. Therefore, in addition to a light box, we also used a glass slide with three parallel lines, which was placed on the light box behind the test sample (fig 1).
Figure 1 A test being performed in our laboratory. The sample on the right is from a patient with heterozygosity for haemoglobin E, whereas the sample on the left is normal.
Tests were read without knowledge of ethnic origin or results of other laboratory investigations. Tests were graded as definitely positive, equivocal, or negative. Both definitely positive and equivocal results were classified as positive, from the point of view of whether further investigation to confirm the presence of haemoglobin E was needed. To assess reproducibility, 37 tests were read in duplicate by two observers, each blinded to the assessment of the other observer. The first observer had three years' experience of laboratory haematology and the second observer had 25 years' experience. Neither had previously used this kit.
Results
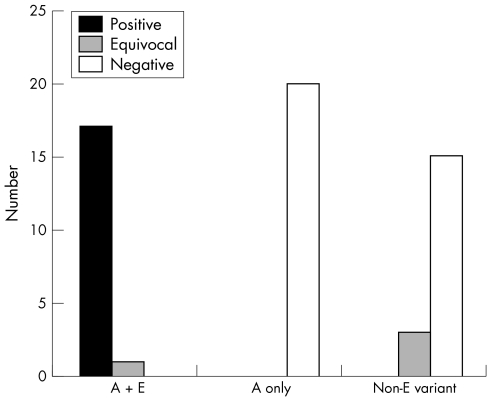
In total, 56 samples were tested, of which 18 were from patients with haemoglobin E trait and 18 contained one or more variant haemoglobin other than haemoglobin E (haemoglobin C, haemoglobin S, haemoglobin D‐Iran, haemoglobin D‐Punjab/Los Angeles). Table 1 and fig 2 summarise the results. Sensitivity was 100%, specificity was 92%, positive predictive value was 85.7%, and negative predictive value was 100%. All samples with haemoglobin E gave either a definitely positive (17) or an equivocal result (one). The single equivocal result was during the first week that the kit was used when we did not realise how subtle the changes in turbidity could be; this sample was graded as definitely positive by the second observer. Equivocal results were obtained with samples containing haemoglobins S plus C (two of three) and S plus F (one of four). No definitely positive results were obtained in samples that did not contain haemoglobin E.
Table 1 Results of assessment of normal and abnormal samples by the first observer.
| Nature of sample | Number of samples | Definitely positive | Equivocal | Negative |
|---|---|---|---|---|
| A+E | 18 | 17 | 1 | 0 |
| A+S | 1 | 0 | 0 | 1 |
| A+C | 6 | 0 | 0 | 6 |
| A+D | 4 | 0 | 0 | 4 |
| S+C | 3 | 0 | 2 | 1 |
| S+F | 4 | 0 | 1 | 3 |
| β Thalassaemia trait | 3 | 0 | 0 | 3 |
| Normal | 17 | 0 | 0 | 17 |
Figure 2 Histogram showing the number of definitely positive, equivocal, and negative tests for subjects with haemoglobin E trait (A + E), subjects with haemoglobin A only (A only), and subjects with a variant haemoglobin other than haemoglobin E (non‐E variant).
Table 2 shows the results of duplicate observations by two blinded observers on 37 samples.
Table 2 Results of assessment of results by two observers, each blinded to the assessment of the other.
| Grading by observer 2 | Grading by observer 1 | ||
|---|---|---|---|
| Definitely positive | Equivocal | Negative | |
| Definitely positive | 11 | 3 | |
| Equivocal | 1 | 3 | |
| Negative | 1 | 18 | |
Discussion
Although the DCIP test was more difficult to read than a sickle solubility test, positive (definitely positive or equivocal) results were obtained with all 18 samples containing haemoglobin E. No positive results were obtained with samples not containing a variant haemoglobin, whereas three of 18 samples from patients with variant haemoglobins gave false positive results. We found no false positive results in simple heterozygotes for variant haemoglobins other than haemoglobin E, and Sanchaisuriya et al also obtained negative results in 12 patients who were heterozygous for haemoglobin C.7 Equivocally positive results in individuals with sickle cell anaemia are not a practical problem because: (1) sickle cell anaemia is uncommon in most parts of the world where haemoglobin E is prevalent; and (2) its detection is desirable, so that a false positive screening test leading to the performance of a definitive test would be an advantage, rather than a disadvantage.
“The kit would be improved if a positive and negative control were provided”
The detection of the presence of haemoglobin E with sufficient accuracy to permit antenatal counselling of pregnant women who are at risk of carrying a fetus with haemoglobin E/β thalassaemia compound heterozygosity requires a combination of two methods, usually cellulose acetate electrophoresis at alkaline pH and either citrate agar or agarose gel electrophoresis at acid pH or HPLC. These methods require economic resources—specifically, a well equipped laboratory and staff with considerable technical skills—which, in developing countries are likely to be found only in major centres. The DCIP test provides a useful screening test to indicate which samples need to be referred from a peripheral hospital or clinic to a central hospital for more definitive tests. If combined with an osmotic fragility screening test for α0 and β thalassaemia,4,6,8 the total number of samples requiring more definite tests would be considerably reduced and the service would become more affordable. However, it should be noted that the test is not easy to read and careful training of staff and a quality assurance programme would be needed in field conditions. Quality control could be achieved by the testing of samples sent out from a central laboratory as part of an external quality assurance scheme. In addition, the kit would be improved if a positive and negative control were provided.
Take home messages
We evaluated a new DCIP test kit for the detection of haemoglobin E, a variant haemoglobin that can lead to considerable morbidity in compound heterozygous states with β thalassaemia
All 18 samples from individuals with haemoglobin E gave positive (definitely positive or equivocal) results and false positive/equivocal results were seen in three of 21 individuals with other disorders of globin chain synthesis, but were not seen in normal subjects
In countries with limited health resources, the use of this test would reduce the number of samples requiring referral to a central laboratory for definitive tests
The test is not easy to read and careful training of staff and a quality assurance programme would be needed in field conditions
We do not recommend the use of this screening test in countries where HPLC or haemoglobin electrophoresis on all relevant samples is affordable.
Acknowledgements
We are grateful for the help and advice of Professor Suthat Fucharoen and Professor Supon Fucharoen, Thailand, and Mr D Roper, London
Abbreviations
DCIP - 2,6‐dichlorophenolindophenol
HPLC - high performance liquid chromatography
References
- 1.Bain B J.Haemoglobinopathy diagnosis. 2nd ed. Oxford: Blackwell Publishing, 2005
- 2.Frischer H, Carson P E, Bowman J E.et al Visual test for erythrocytic glucose‐6‐phosphate dehydrogenase, 6‐phosphogluconic dehydrogenase, and glutathione reductase deficiencies. J Lab Clin Med 197381613–624. [PubMed] [Google Scholar]
- 3.Frischer H, Bowman J, Hemoglobin E. An oxidatively unstable mutation. J Lab Clin Med 197585531–539. [PubMed] [Google Scholar]
- 4.Wiwanitkit V, Suwansaksri J, Paritpokee N. Combined one‐tube osmotic fragility test and dichlorophenol‐indolphenol test screening for hemoglobin disorders, an experience in 213 Thai pregnant women. Clin Lab 200248525–528. [PubMed] [Google Scholar]
- 5.Winichagoon P, Thitivichianlert A, Lebnak T.et al Screening for the carriers of thalassemias and abnormal hemoglobins at the community level. Southeast Asian J Trop Med Public Health 200233(suppl)145–150. [PubMed] [Google Scholar]
- 6.Fucharoen G, Sanchaisuriya K, Sae‐ung N.et al A simplified screening strategy for thalassaemia and haemoglobin E in rural communities in south‐east Asia. Bull World Health Organ 200482364–372. [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchaisuriya K, Fucharoen G, Sae‐ung N.et al Molecular characterization of hemoglobin C in Thailand. Am J Hematol 200167189–193. [DOI] [PubMed] [Google Scholar]
- 8.Chow J, Phelan L, Bain B J. Evaluation of single‐tube osmotic fragility as a screening test for thalassemia. Am J Hematol 200579198–201. [DOI] [PubMed] [Google Scholar]