Abstract
Aims
To analyse the entity specific incidence and disease specific survival (DSS) of non‐Hodgkin lymphomas (NHLs) in Tyrol/Austria, 1991–2000.
Methods
Data from 1307 NHLs (excluding primary cutaneous lymphomas and monoclonal gammopathies of undetermined significance) were obtained. Current status was available for all patients. Except for 29 cases of small lymphocytic (CLL/SLL), lymphoblastic leukaemia (ALL), and myeloma (MM), which were diagnosed cytologically, diagnoses were reclassified on paraffin wax embedded archival material according to new World Health Organisation criteria. Sex specific age adjusted standardised incidence rates were computed using Segi's population weighting. Annual incidence changes were calculated by weighted least square regression analysis. Survival was estimated by the Kaplan–Meier method and compared by log rank test.
Results
NHL more frequently affected men (male/female ratio, 1.52). Mean age of occurrence was 61 and 66 years for men and women, respectively. The incidence rate of 14.3 remained constant. There was a significant increase in diffuse large B cell lymphoma (DLBCL) and decrease in CLL/SLL in men, and a decrease in MM in women. Overall DSS was 64% during the mean follow up (43 months). Age, T‐NHL, λ light chain restriction in MM, and male sex in CLL/SLL were associated with poor prognosis. In B‐NHL, DSS decreased in the following order: hairy cell leukaemia, marginal zone lymphoma, follicular lymphoma, Burkitt lymphoma, ALL, DLBCL, CLL, MM, and mantle cell lymphoma.
Conclusions
The incidence of NHL in Tyrol has changed in the past decade, with a significant increase in DLBCL, decrease in CLL/SLL in men, and decrease in MM in women.
Keywords: epidemiology, non‐Hodgkin lymphoma, prognosis
Non‐Hodgkin lymphomas (NHLs) are lymphopoietic malignancies of B cell, T cell (B‐NHL and T‐NHL, respectively), or natural killer cell origin.1 Epidemiological data on the incidence of NHL are multifaceted. Most studies have shown a rising incidence in the highly aggressive subtypes during the past few decades.2,3,4,5,6,7,8,9 However, increased exposure to risk factors10—such as immunosuppressive drugs,11 herbicides and solvents,12,13,14,15,16 viral and Helicobacter pylori infections,11,17,18,19,20,21,22 tobacco abuse and fat intake,23,24,25 hair dyes,26,27 and certain antibiotics28—can only partly explain the observed epidemiological changes. The increased survival of patients treated with cytotoxic regimens for neoplastic diseases, the improved prognosis for patients with inborn immunodeficiency syndromes,29,30,31 and the rising incidence of autoimmune disorders32,33 may also play a role in the altering incidence of NHL. Disturbed cellular interactions and communications between B and T cells, as frequently seen in older individuals,34 is probably also linked to NHL. Indeed, age appears to be an important risk factor for the development of NHL.2,3,4,5,6,7,8
“Most studies have shown a rising incidence in the highly aggressive subtypes of non‐Hodgkin lymphoma during the past few decades”
Studies from Sweden described an average 3% annual increase in the incidence of NHL during an observation period between 1971 and 1990, and a decreased incidence from 1991 to 2000.12,13 A population based registry from the Netherlands showed that the incidence of NHL was stable between 1981 and 1989.35 Other European studies showed an annual increase in NHL of 4% between 1973 and 1992.2,4,5,6 In Sardinia3 an average 6% annual increase in the rate of NHL was found between 1974 and 1993. In the USA, overall age adjusted incidence rates (http://seer.cancer.gov/) have stabilised after a steady annual increase of 3–4% between 1970 and 1980.7 Groves et al showed high grade NHL to have the most rapidly growing incidence, particularly among men, for the observation period 1978–1995.8 However, patients with multiple myeloma (MM), acute lymphoblastic leukaemia (ALL), Burkitt lymphoma (BL), and chronic lymphocytic leukaemia (CLL), recognised by the current World Health Organisation (WHO) classification as NHL,1 were not considered in these studies. Moreover, NHL classification inconsistencies as a result of a variety of systems used, such as Kiel (1974), Lukes/Collins (1974), WHO (1976), Working Formulation (1982), REAL (1994), and WHO (1999),1,36 make direct comparisons between the studies difficult or impossible.
In our present study, we analysed the epidemiology of NHL in Tyrol, reclassifying all cases according to current WHO criteria.1 We considered all nodal and extranodal NHLs diagnosed between 1991 and 2000 in the demographically well documented, previously unreported Tyrolean population (http://www.tirol.gv.at/themen/zahlenundfakten/statistik).
Materials and methods
Patients
Incidence data in this cross sectional study were obtained from the population based Cancer Registry of Tyrol and compared with data from the database of the Institute of Pathology at the Innsbruck Medical University, Austria. In Tyrol, all biopsy NHL diagnoses (except primary cutaneous NHL, isolated ALL, and monoclonal gammopathies of undetermined significance (MGUS)) are performed at the Institute of Pathology (Innsbruck Medical University) and are encoded as “neoplastic diseases of the lymphopoietic system”. All lymphopoietic malignancies (except MGUS and primary cutaneous NHL) with corresponding current patient status data, such as date of first diagnosis, level of diagnostic evidence (cytology, histology, molecular biology), address (postal code), sex, and life status, including cause of death as annually reported by the registry offices in Tyrol, are listed at the population based cancer registry, provided the patients are Tyrolean citizens or inhabitants, but the exact NHL subtypes are not documented. Thus, comparison of both databases allows accurate determination of the specific incidence of different NHL entities.
Diagnostic tissue samples
With the exception of 29 (2%) patients with ALL, CLL, and MM, in whom the diagnosis was established by cytological examination and fluorescent activated cell sorter analysis (FACS) only, all other 1278 diagnoses were histologically established by morphological, immunohistochemical, and molecular methods. All available 1278 NHL biopsy specimens were revised morphologically by one of us (AT) and reclassified according to the current WHO criteria.1 The 29 primary cytological or FACS ALL, CLL, and MM diagnoses were not revised. In cases where the first diagnosis matched the reviewer's diagnosis morphologically, no further diagnostic methods were applied. In equivocal cases, ancillary immunohistochemical stains were performed as recommended.1,37,38
Statistics
Current status data from all patients, including cause of death, were available at the Cancer Registry of Tyrol. Incidence was calculated as sex specific and age adjusted standardised incidence rate using Segi's population weighting39 for the Tyrolean population, which is reported annually by the Tyrolean government (http://www.tirol.gv.at/themen/zahlenundfakten/statistik). Statistical analysis was performed using Statistical Software Package of Social Sciences 10.0, applying descriptive methods, Pearson, χ2, and Mann–Whitney U tests. Time trend curves were constructed using the curve estimation option. For investigation of the annual incidence changes a least square regression analysis of the standardised incidence rate weighted by the absolute number of cases was applied. Disease specific mortality was defined as patient deaths caused by or with symptomatic lymphoma. Relative five year survival was defined as percentage of patients with NHL who did not die as a result of NHL after an observation period of five years. Survival was estimated by the Kaplan–Meier method and compared by the log rank test; p values < 0.05 were considered significant.
Results
Patients, lymphoma diagnoses, and equivocal diagnoses
During the observation period 1 January 1991 to 31 December 2000, 1612 new NHLs were diagnosed at the Institute of Pathology (Innsbruck Medical University, Austria), 1278 (79%) of them in Tyrolean citizens or inhabitants as assessed by the patients' home postal codes. Including the 29 cases of Tyrolean citizens diagnosed with NHL by cytology alone (ALL, CLL, MM), our present study encompassed a total of 1307 NHLs. Of these, 173 were primary extranodal lymphomas and 603 lymphomas diagnosed on trephine bone marrow biopsies (table 1). Except for 23 (2%) cases, all the other 1255 primary and revised diagnoses matched substantially (data not shown in detail).
Table 1 Age adjusted standardised NHL incidence rate (Tyrol 1991–2000).
| NHL entity | M/F ratio | Sex | Standardised NHL incidence rate | N | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | p Value | ||||
| B‐ALL | 0.90 | F | 1.01 | 1.25 | 1.15 | 1.21 | 0.67 | 2.76 | 1.57 | 0.60 | 0.86 | 0.72 | 0.6933 | 28 |
| M | 1.00 | 0.53 | 0.42 | 1.05 | 1.48 | 1.00 | 0.69 | 2.03 | 1.90 | 0.51 | 0.3031 | 27 | ||
| B‐CLL | 2.10 | F | 2.14 | 3.46 | 3.28 | 3.23 | 3.21 | 2.57 | 2.72 | 2.46 | 1.08 | 2.42 | 0.1171 | 176 |
| M | 5.86 | 8.54 | 7.49 | 5.66 | 5.38 | 5.39 | 4.37 | 3.80 | 5.58 | 3.63 | 0.0111 | 232 | ||
| LPL | 1.13 | F | 0.54 | 0.29 | 0.12 | 0.14 | 0 | 0 | 0.07 | 0.08 | 0.20 | 0.15 | –* | 9 |
| M | 0 | 0.19 | 0.53 | 0 | 0.51 | 0.30 | 0 | 0.27 | 0 | 0 | –* | 7 | ||
| MCL | 3.97 | F | 0.18 | 0.09 | 0 | 0 | 0.06 | 0.32 | 0 | 0.36 | 0 | 0.08 | –* | 8 |
| M | 0 | 1.19 | 0.26 | 0 | 0 | 0.67 | 1.62 | 0.38 | 0.21 | 0 | 0.6604 | 18 | ||
| FL | 0.99 | F | 1.05 | 1.46 | 1.17 | 1.38 | 1.00 | 2.02 | 1.49 | 1.06 | 1.91 | 1.06 | 0.5871 | 67 |
| M | 1.19 | 0.88 | 1.16 | 1.56 | 1.13 | 1.91 | 1.98 | 0.65 | 0.90 | 2.16 | 0.4093 | 56 | ||
| Nodal MZL | 0.53 | F | 0 | 0 | 0 | 0 | 0 | 0.57 | 0 | 0 | 0 | 0 | –* | 2 |
| M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.30 | 0 | 0 | –* | 1 | ||
| MZL of MALT | 1.36 | F | 0.95 | 0.92 | 0.36 | 0.29 | 0.27 | 0.28 | 0.16 | 0.16 | 0.54 | 0.62 | 0.2042 | 25 |
| M | 0.82 | 0.58 | 0.52 | 0.98 | 0.37 | 0.55 | 0.29 | 0.75 | 0.69 | 0.64 | 0.6889 | 26 | ||
| HCL | 5.92 | F | 0 | 0.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0.47 | 0.29 | –* | 4 |
| M | 0.28 | 1.14 | 1.17 | 1.07 | 0.49 | 0.75 | 0.29 | 0.17 | 0.21 | 0.41 | 0.0835 | 23 | ||
| MM | 1.34 | F | 2.61 | 2.69 | 2.07 | 1.97 | 3.17 | 2.34 | 1.66 | 1.56 | 1.81 | 1.26 | 0.0220 | 123 |
| M | 3.24 | 4.71 | 3.72 | 1.81 | 3.59 | 1.36 | 1.94 | 3.23 | 2.66 | 1.98 | 0.1206 | 118 | ||
| DLBCL | 1.39 | F | 2.05 | 2.56 | 1.51 | 2.05 | 1.39 | 2.35 | 2.32 | 2.66 | 2.45 | 2.89 | 0.1330 | 131 |
| M | 2.13 | 1.89 | 3.08 | 1.53 | 3.46 | 4.40 | 3.50 | 3.32 | 3.35 | 4.14 | 0.0215 | 130 | ||
| Mediastinal DLBCL | 0.12 | F | 0.18 | 0.2 | 0.46 | 0.6 | 0.08 | 0.2 | 0 | 0.29 | 0.21 | 0 | –* | 10 |
| M | 0 | 0 | 0 | 0 | 0 | 0.26 | 0 | 0 | 0 | 0 | –* | 1 | ||
| BL | 2.37 | F | 0 | 0.55 | 0 | 0.12 | 0.30 | 0 | 0 | 0.44 | 0 | 0.46 | –* | 7 |
| M | 0 | 0.27 | 0.21 | 0 | 0.67 | 1.16 | 0.47 | 0.57 | 0.38 | 0.71 | 0.7940 | 12 | ||
| T‐ALL | 2.59 | F | 0.41 | 0.32 | 0.31 | 0.70 | 0.23 | 0 | 0 | 0 | 0.30 | 0 | 0.7269 | 9 |
| M | 0.79 | 0.70 | 0.76 | 0.25 | 0 | 0.95 | 1.00 | 0.78 | 0.46 | 0.20 | 0.4329 | 17 | ||
| Mature T‐NHL | 1.53 | F | 0 | 0.47 | 0.27 | 0.26 | 0 | 0.26 | 0.07 | 0.08 | 0.08 | 0.29 | –* | 9 |
| M | 0 | 0.32 | 0 | 0.74 | 0.75 | 0.45 | 0 | 0 | 0.21 | 0.26 | 0.3031 | 11 | ||
| NK lymphoma/leukaemia | 0.50 | F | 0 | 0.32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | –* | 2 |
| M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.20 | –* | 1 | ||
| Nodal ALCL | 1.28 | F | 0 | 0 | 0.15 | 0.14 | 0 | 0 | 0.65 | 0 | 0.21 | 0.64 | –* | 9 |
| M | 0.3 | 0 | 0.29 | 0 | 0.26 | 0.29 | 0.29 | 0.65 | 0 | 0.21 | –* | 8 | ||
| All NHL | 1.52 | F | 11.14 | 14.83 | 9.92 | 12.08 | 10.37 | 13.68 | 10.70 | 9.75 | 10.12 | 10.96 | 0.2607 | 619 |
| M | 15.63 | 19.95 | 19.61 | 14.65 | 18.08 | 18.43 | 16.44 | 17.91 | 16.53 | 15.03 | 0.3704 | 688 | ||
| Total number | 107 | 133 | 167 | 112 | 132 | 142 | 131 | 128 | 130 | 125 | 1307 | |||
*Weighted least square regression analysis not performed because of too few cases.
ALCL, anaplastic large cell lymphoma; ALL, acute lymphoblastic leukaemia/lymphoma; B‐/T‐, B/T cell; BL, Burkitt lymphoma/leukaemia; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HCL, hairy cell leukaemia; LPL, lymphoplasmacytic lymphoma; MALT, mucosa associated lymphoid tissue; MCL, mantle cell lymphoma MM, multiple myeloma; MZL, marginal zone lymphoma; NHL, non‐Hodgkin lymphoma; NK, natural killer cell.
Cumulative proportions of NHL entities and incidence
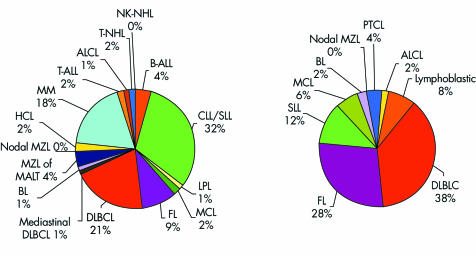
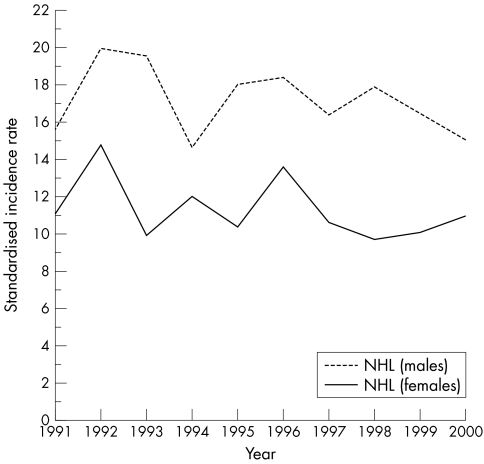
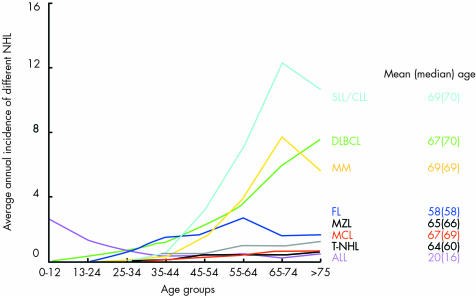
Figure 1 and table 1 show the relative proportions and absolute numbers, in addition to the standardised incidence, of all NHL entities diagnosed between 1991 and 2000 in Tyrolean citizens. During the observation period, the Tyrolean population increased from 630 145 to 672 209, mainly as a result of immigration (average annual increase, 0.72%; http://www.tirol.gv.at/themen/zahlenundfakten/statistik); however, no significant change was seen in the incidence or sex specificity of all NHLs (fig 2). NHL affected men more frequently, and at an earlier age, than women (men: n = 688; mean age, 61 years; median, 65; women: n = 619 (47%); mean age, 66 years; median, 70; p < 0.0001). Fifty seven per cent of all NHLs were detected in individuals older than 65 years (52% in men and 63% in women). Taking into consideration the standard population and the standardised incidence rates, the male to female ratio for NHL in Tyrol was 1.52, with hairy cell leukaemia (HCL; p = 0.017), mantle cell lymphoma (MCL), T‐ALL, BL, and B‐CLL/small lymphocytic lymphoma (CLL/SLL) most commonly affecting male patients and sclerosing (mediastinal) diffuse large B cell lymphoma (DLBCL; p = 0.007), B‐ALL, and follicular lymphoma (FL) more commonly affecting female patients (the sex specific distribution differences of entities, for which p values are not indicated were not significant). The mean age at diagnosis of NHL was specific for the different disease entities (fig 3), but did not change from 1991 to 2000.
Figure 1 Overall proportions of the different NHL entities (left) and of nodal NHLs only (right) in Tyrol, pooled data 1991–2000; ALCL, anaplastic large cell lymphoma; ALL, pooled lymphoblastic lymphomas and acute lymphoblastic (pooled B and T cell) leukaemias; BL, Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HCL, hairy cell leukaemia; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MALT, mucosa associated lymphoid tissue; MM, multiple myeloma; MZL, marginal zone lymphoma; NHL, non‐Hodgkin lymphoma; NK, natural killer cell; SLL, small lymphocytic lymphoma.
Figure 2 Incidence of non‐Hodgkin lymphoma (NHL) in male and female patients in Tyrol 1991–2000.
Figure 3 Age adjusted incidence of selected non‐Hodgkin lymphomas (NHLs) in Tyrol; pooled data 1991–2000. ALL, acute lymphoblastic leukaemia; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; PTCL, peripheral T cell lymphoma; SLL, small lymphocytic lymphoma; T‐NHL, T cell non‐Hodgkin lymphoma.
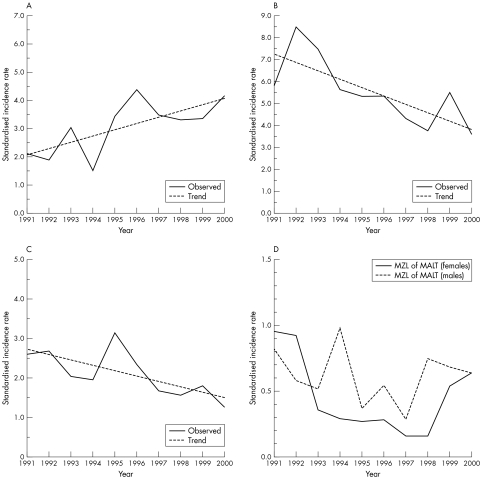
However, significant frequency changes were seen within the specific NHL entities. The incidence of primary DLBCL increased in both male and female patients; the increase reaching significance in men (p = 0.0215, fig 4A). A significant decline was noted in CLL/SLL in male patients (p = 0.0111; fig 4B) and MM in female patients (p = 0.0220; fig 4C). The incidence of HCL decreased in male patients, but this was not significant (p = 0.0835). The incidence of marginal zone lymphomas (MZLs) of the mucosa associated lymphoid tissue (MALT) type decreased significantly in female patients (p = 0.009) between 1991 and 1997, and increased (p = 0.066; fig 4D) between 1998 and 2000, primarily as a result of the increased incidence of non‐gastric MZL of MALT after 1997 (data not shown in detail).
Figure 4 (A) Increasing incidence of primary diffuse large B cell lymphoma in male Tyroleans 1991–2000; p = 0.0215. (B) Declining incidence of B chronic lymphocytic leukaemia/small lymphocytic lymphoma in male Tyroleans 1991–2000; p = 0.0111. (C) Declining incidence of multiple myeloma in female Tyroleans 1991–2000; p = 0.0220. (D) Changes in marginal zone lymphoma (MZL) of mucosa associated lymphoid tissue (MALT) type incidence in Tyrol 1991–2000; male and female patients.
Survival analysis
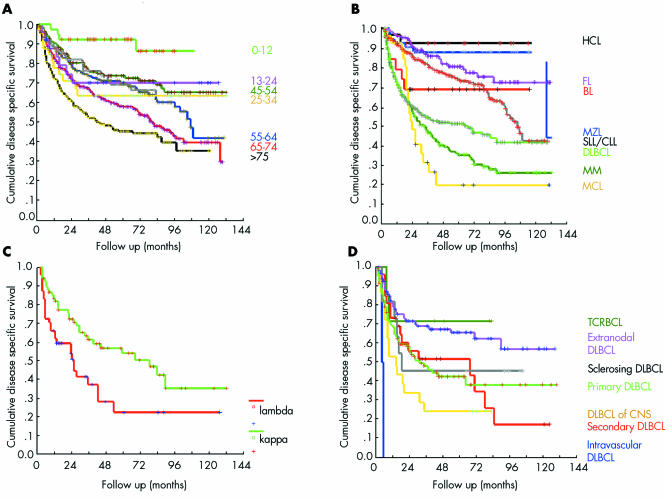
The mean follow up period in our study was 43 months (range, 1–137; median, 33). Table 2 shows the mean, median, relative five year overall (OS), and disease specific survival (DSS). We found significant correlations between age and OS and DSS (p < 0.005 for both; fig 5A), and between B‐NHL versus T‐NHL lineage origin and DSS (64% and 56% OS, respectively; p = 0.0145). Different DSS values were seen for the different B‐NHLs (p < 0.0001; fig 5B). Bone marrow infiltration (corresponding to Ann‐Arbor stage IV disease) was, as expected, a risk factor for worse DSS in DLBCL (p < 0.01). CLL/SLL and true lymphoplasmacytic lymphomas had an identical prognosis and were further analysed together. In these lymphomas, male sex appeared to be a poor prognostic indicator (p = 0.05). In MM, patients expressing the κ light chain had better DSS (p = 0.0217; fig 5C). Within the specific DLBCL subtypes and variants, T cell rich B cell lymphomas (TCRBCLs) had the best DSS, followed by primary extranodal DLBCL (54% gastric DLBCL) and sclerosing (mediastinal) DLBCL; the worst DSS was found in intravascular DLBCL (p = 0.0003, fig 5D).
Table 2 Mean, median, and 5 year overall and disease specific NHL survival in Tyrol 1991–2000.
| NHL entity | Disease specific survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Median (months) | Mean (months) | Relative* | Median (months) | Mean (months) | Relative* | |
| Marginal zone lymphoma of MALT | Not reached | 115 | 88% | 128 | 98 | 78% |
| Follicular lymphoma | Not reached | 105 | 80% | Not reached | 98 | 75% |
| B lymphoblastic leukaemia/lymphoma | Not reached | 88 | 74% | Not reached | 77 | 63% |
| T cell rich B cell lymphoma | Not reached | 62 | 71% | 83 | 62 | 71% |
| Hairy cell leukaemia | Not reached | 111 | 69% | Not reached | 92 | 73% |
| Burkitt lymphoma/leukaemia | Not reached | 82 | 69% | Not reached | 82 | 69% |
| Anaplastic large cell lymphoma | 87 | 68 | 69% | 55 | 57 | 49% |
| B chronic lymphocytic leukaemia | 105 | 90 | 65% | 79 | 73 | 57% |
| Extranodal diffuse large B cell lymphoma | Not reached | 84 | 65% | 60 | 66 | 50% |
| T lymphoblastic leukaemia/lymphoma | Not reached | 83 | 63% | 16 | 63 | 45% |
| Mediastinal diffuse large B cell lymphoma | 18 | 54 | 47% | 18 | 54 | 47% |
| Nodal diffuse large B cell lymphoma | 31 | 59 | 42% | 22 | 41 | 25% |
| Mature T/NK NHLs | 17 | 51 | 36% | 14 | 37 | 25% |
| Multiple myeloma | 33 | 54 | 35% | 24 | 43 | 26% |
| Diffuse large B cell lymphoma of CNS | 14 | 29 | 24% | 9 | 25 | 18% |
| Mantle cell lymphoma | 23 | 44 | 20% | 22 | 42 | 19% |
Mean follow up was 42 months (median, 32; range, 1–132).
*Relative 5 year survival.
CNS, central nervous system; MALT, mucosa associated lymphoid tissue; NHL, non‐Hodgkin lymphoma; NK, natural killer cell.
Figure 5 (A) Disease specific survival in different age groups; p = 0.0011. (B) Disease specific survival in specific non‐Hodgkin lymphomas; p < 0.0001. (C) Disease specific survival in κ and λ expressing myeloma; p = 0.0217. (D) Disease specific survival in different diffuse large B cell lymphoma (DBLC) subtypes and variants; p = 0.0003. BL, Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; CNS, central nervous system; FL, follicular lymphoma; HCL, hairy cell leukaemia; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma; TCRBCL, T cell rich B cell lymphoma.
Considering other causes of death, the highest mortality as a result of secondary malignancies (9–18%) was seen, in declining order, in: HCL, anaplastic large cell lymphoma (ALCL), ALL, CLL, and DLBCL, compared with 0–4% in all other NHLs (p = 0.071). Cardiovascular mortality was most frequently seen, in declining order, in: CLL/SLL, MM, HCL, and DLBCL (p = 0.0049). Increasing age was associated with increased mortality as a result of both secondary malignancies and cardiovascular emergencies.
Discussion
In the study presented here, we considered all NHL entities recognised by the new WHO classification,1 with the exception of primary cutaneous NHL and MGUS, which were diagnosed in Tyrol between 1991 and 2000. We collected our data from two databases (one population based at the Cancer Registry of Tyrol and one case based at the Institute of Pathology) applying only the date of first diagnosis and the patient's postal code as selection conditions, thus keeping selection bias low. All biopsies were revised and reclassified according to the currently used criteria.1 One part of the primary diagnosis was performed before the introduction of the REAL classification in 1994,36 so that direct comparison with the revision diagnoses appeared difficult, but not impossible because of the previous use of the Kiel classification in Austria. The high proportion of over 95% matching or reproducible (Kiel/REAL/WHO) diagnoses indicates the reliability of our present data. Admittedly, our study encompassed a relatively low number of cases compared with others.2,3,4,5,6,7,8 Thus, some observations could be the result of chance and should be interpreted carefully. Nevertheless, this is the first epidemiological study on NHL applying the new WHO classification1 that has been performed on a previously unreported, well documented population.
The average annual standard incidence rate for all NHLs of 14.3 in Tyrol is comparable to that reported in similar populations.1,2,3,4,5,6,7,8 The overall frequency of NHL did not change significantly within the observation period, which might indicate that the introduction of advanced diagnostic technologies, at least between 1991 and 2000 in Tyrol, probably had a minor impact on the observed incidence time trends for the specific NHL entities. As expected, our data are at variance with observation periods before 1990, which showed an increasing incidence of NHL, but agree with data from observation periods after 1990.2,3,4,5,6,7,8,35 Because of the use of different classification systems, a detailed comparison with the cited studies is not possible.
The mean age at diagnosis of NHL in our study was similar to that reported in other European studies.2,3,4,5,6,35 Male Tyrolean patients had a higher risk for NHL (male to female ratio, 1.52), which is in agreement with other reports,1,2,8 and were younger than women at diagnosis. One of several possible explanations for these differences might be that men are more frequently exposed to risk factors such as pesticides, herbicides, and chemical solvents,12,13,14,15 are more likely to smoke cigarettes,23,24 are more frequently infected by human immunodeficiency virus in Europe16 and, particularly in Tyrol, more often infected by hepatitis C virus (ER Bitterlich. Epidemiology of hepatitis C in western Austria. Thesis at the Medical Faculty of the Leopold‐Franzens‐University. Innsbruck, 1999). Nevertheless, considering the low absolute risk of these factors and the small differences in exposure between the two sexes, it seems unlikely that the influence of risk factors is crucial for the observed sex ratios. Differential susceptibility to common infectious agents recognised to be of certain or probable oncogenic potential in both sexes could be another intriguing explanation for the detected sex specific NHL ratios.40
“Within B cell non‐Hodgkin lymphoma, hairy cell leukaemia, followed by marginal zone and follicular lymphoma appeared to be associated with the best prognosis”
We found a significantly rising incidence of DLBCL, particularly in male patients. An increase in the incidence of DLBCL has also been reported in other studies.2,3,4,5,6,7,8,35 Because the median age at diagnosis of DLBCL did not increase within the observation period, we propose that a simple demographical shift within the aging Tyrolean population is probably not the primary reason for this increase. DLBCL is the most frequent disease entity in patients suffering from primary or secondary immunodeficiency,11,17,19,29 and in those exposed to chemical agents.12,13,14,15 We speculate that the increasing frequency of DLBCL could be at least partially associated with the agricultural use of herbicides and pesticides, especially in the rural regions of Tyrol (http://ta1.umweltbundesamt.at/umwelt/landwirtschaft/pflanzenschutz/psm/), and with the rise in iatrogenic immunosuppression,11 human immunodeficiency virus infection,19 and autoimmune disorders.32,33 Improved survival in patients with Hodgkin lymphoma,30,31 the broad application of polychemotherapy regimens for other neoplastic diseases, and the improved prognosis for those with inborn immunodeficiency syndromes may also have an effect. Indeed, in 104 newly diagnosed Tyrolean patients suffering from classic Hodgkin lymphoma between 1991 and 2000, two metachronous DLBCLs were seen (calculated average crude rate 192/100 000 patients with Hodgkin lymphoma/year), and the average DLBCL incidence for this period was 2.7/100 000 individuals/year.
A decreasing incidence of CLL/SLL was found in male patients. Because almost all Tyrolean patients in whom absolute lymphocytosis is detected undergo FACS analysis and, in cases where pathological phenotypes are detected, a trephine bone marrow biopsy, the proportion of underdiagnosed CLL/SLL should be low, so that the observed rate changes probably reflect true incidence modification. Furthermore, changes in diagnostic practice41 and classification systems1,36 are unlikely to be the sole explanation for the observed changes, because the proportion of CLLs/SLLs diagnosed as splenic MZL or MCL and vice versa was low after revision of our collection of samples. There are no well established risk factors for the development of CLL/SLL, so that the reasons for the observed changes are not clear. An association between allogeneic blood transfusion and CLL/SLL is assumed by some authors,42,43 but large studies have failed to support this hypothesis.44 It is possible that the introduction of pre‐transfusion blood irradiation between 1991 and 2000, which destroys all donor nucleated cells,45 might be one reason for the observed decline of CLL/SLL in male patients, but this would not explain the sex specificity.
“The decreasing incidence of marginal zone lymphoma between 1991 and 1994 probably results from the introduction of efficient eradication treatments”
We detected a decreasing incidence of MM in female patients within the observation period, an observation also made in Connecticut (USA) and Vaud (Switzerland) between 1970 and 1987.46,47,48 In our case, one of the reasons for this decrease could be the differentiated diagnostic ascertainment in MM after 1994, taking into consideration all plasmocytosis of bone marrow, presence of M gradients, and free light chains as assessed by immunofixation of blood and urine and radiological examination, which segregate plasma cell dyscrasias into aggressive (MM) and indolent (MGUS, smoldering myeloma) forms. Cases of MGUS were not reported to the Cancer Registry after 1994 but were probably previously considered to be MM because of the bone marrow plasmocytosis.1
The incidence of MZL fluctuated during the observation period; 88% of cases were gastric MZL of MALT. Notably, these variants of MZL are almost always associated with H pylori gastritis and can be cured by H pylori eradication.21,22 Therefore, the decreasing incidence of MZL between 1991 and 1994 probably results from the introduction of efficient eradication treatments.49 The observed rise after 1997 may partially be explained by the increasing resistance of H pylori to antibiotics,50 but is, at least in our small population sample, primarily the result of the rising incidence of non‐gastric MZL of MALT. However, this could be a chance finding as a result of the small number of cases.
As expected,51 age was associated with worse DSS, probably because of higher rates of co‐morbidity that restrict the use of potentially curative treatments in elderly individuals.52,53 Bone marrow involvement was associated with worse DSS in DLBCL, stage IV diseases being a well known risk factor.51 MM with λ light chain restriction had a worse prognosis, probably because these forms of MM are more likely to be associated with amyloidosis and extramedullary spread.1,54 Many studies have shown that T cell lymphomas are more aggressive than B cell ones and that they have decreased treatment response and OS rates,55 which is also consistent with our results. Within B‐NHL, HCL, followed by MZL and FL appeared to be associated with the best prognosis. These findings might be explained by the good response rates to treatment with cladribine56 in HCL, by the benefits of H pylori eradication in gastric MZL,21,22,49 and by the improved treatment options in FL, especially the introduction of rituximab in the past few years.57 MCL was associated with the worst prognosis in our study, consistent with published data.1 MCLs are intermediate growth fraction lymphomas accompanied by the typical translocation t(11;14)(q13;q32); this results in both apoptotic resistance and lost cell cycle control because of overexpression of cyclin D1 and decreased expression of the cyclin dependent kinase inhibitor, p27.58 Within DLBCL, the best DSS was seen in TCRBCL, a disease entity that may be closely related to the indolent nodular lymphocyte predominant Hodgkin lymphoma,59,60 and in sclerosing DLBCL, which is probably related to classic Hodgkin lymphoma.61 Primary extranodal DLBCL in our group (54% located in the stomach) also showed a better prognosis. Indeed, bone marrow involvement was seen in only 5.4% of patients with extranodal DLBCL, whereas the average bone marrow involvement in primary nodal DLBCL was 36% (p = 0.003). As in other studies,62,63 central nervous system and intravascular DLBCL were associated with the worst prognosis. Intravascular DLBCL is characterised by the presence of lymphoma cells in the lumina of small blood vessels as a result of a defect in their homing receptors.1 At the time of presentation, this disorder is typically disseminated to a wide variety of extranodal sites and responds poorly to chemotherapy.63 When we analysed other causes of death, patients suffering from CLL/SLL and HCL had an increased risk for cardiovascular events, probably because of the older age at diagnosis and the long observation periods as a result of intermediate to good DSS, with the attendant risk of increased probability for cumulating events. The multiple lines of treatment given to patients with CLL/SLL may represent an additional cardiovascular risk.64 MM and DLBCL were also associated with higher cardiovascular mortality, both of which are usually treated by anthracyclin based polychemotherapy regimens, which have well known cardiotoxicity and endotheliotoxicity.65
Take home messages
Although there were a limited number of cases, we report the largest single institution study on the incidence and prognosis of non‐Hodgkin lymphoma (NHL) in which all cases were reclassified according to the new World Health Organisation criteria
We found distinct epidemiological changes in NHL
There was an increasing incidence in diffuse large B cell lymphoma and a decreasing incidence in chronic/small cell lymphocytic lymphoma in male patients
There was also a decreasing incidence of multiple myeloma in female patients
Although encompassing a limited number of cases because of the small population of Tyrol, this is the largest single institution study on the incidence and prognosis of NHL in which all cases have been reclassified according to the new WHO classification. Our findings indicate distinct epidemiological changes in NHL, especially an increasing incidence in DLBCL and a decreasing incidence in CLL/SLL in male patients, in addition to a decreasing incidence of MM in female patients.
Abbreviations
ALCL - anaplastic large cell lymphoma
ALL - acute lymphoblastic leukaemia
BL - Burkitt lymphoma
B/T - B/T cell
CLL - chronic lymphocytic leukaemia
DLBCL - diffuse large B cell lymphoma
DSS - disease specific survival
FACS - fluorescent activated cell sorter analysis
FL - follicular lymphoma
HCL - hairy cell leukaemia
LPL - lymphoplasmacytic lymphoma
MCL - mantle cell lymphoma
MGUS - monoclonal gammopathy of undetermined significance
MALT - mucosa associated lymphoid tissue
MM - multiple myeloma
MZL - marginal zone lymphoma
NHL - non‐Hodgkin lymphoma
OS - overall survival
SLL - small lymphocytic lymphoma
TCRBCL - T cell rich B cell lymphoma
WHO - World Health Organisation
References
- 1.Jaffe E S, Harris N L, Stein H, Vardiman J W. eds. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001
- 2.Cartwright R, Brincker H, Carli P M.et al The rise in incidence of lymphomas in Europe 1985–1992. Eur J Cancer 199935627–633. [DOI] [PubMed] [Google Scholar]
- 3.Broccia G, Cocco P, Casula P. Research group on the epidemiology of lymphomas in Sardinia (GELS). Incidence of non‐Hodgkin's lymphoma and Hodgkin's disease in Sardinia, Italy: 1974–1993, Haematologica 20018658–63. [PubMed] [Google Scholar]
- 4.Howe H L, Wingo P A, Thun M J.et al Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst 200193824–842. [DOI] [PubMed] [Google Scholar]
- 5.Pollan M, Lopez‐Abente G, Moreno C.et al Rising incidence of non‐Hodgkin's lymphoma in Spain: analysis of period of diagnosis and cohort effects. Cancer Epidemiol Biomarkers Prev 19987621–625. [PubMed] [Google Scholar]
- 6.Carli P M, Boutron M C, Maynadie M.et al Increase in the incidence of non‐Hodgkin's lymphomas: evidence for a recent sharp increase in France independent of AIDS. Br J Cancer 199470713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devesa S S, Fears T. Non‐Hodgkin's lymphoma time trends: United States and international data. Cancer Res 199252(suppl 19)5432s–40s. [PubMed] [Google Scholar]
- 8.Groves F D, Linet M S, Travis L B.et al Cancer surveillance series: non‐Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 2000921240–1251. [DOI] [PubMed] [Google Scholar]
- 9.Newton R, Ferlay J, Beral V.et al The epidemiology of non‐Hodgkin's lymphoma: comparison of nodal and extra‐nodal sites. Int J Cancer 199772923–930. [DOI] [PubMed] [Google Scholar]
- 10.Tavani A, Pregnolato A, La Vecchia C.et al A case–control study of reproductive factors and risk of lymphomas and myelomas. Leuk Res 199721885–888. [DOI] [PubMed] [Google Scholar]
- 11.Swinnen L J. Overview of posttransplant B‐cell lymphoproliferative disorders. Semin Oncol 199926(5 suppl 14)21–25. [PubMed] [Google Scholar]
- 12.Hardell L, Eriksson M, Degerman A. Exposure to phenoxyacetic acids, chlorophenols, or organic solvents in relation to histopathology, stage, and anatomical localization of non‐Hodgkin's lymphoma. Cancer Res 1994542386–2389. [PubMed] [Google Scholar]
- 13.Hardell L, Eriksson M. Is the decline of the increasing incidence of non‐Hodgkin lymphoma in Sweden and other countries a result of cancer preventive measures? Environ Health Perspect 20031111704–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amadori D, Nanni O, Falcini F.et al Chronic lymphocytic leukaemias and non‐Hodgkin's lymphomas by histological type in farming‐animal breeding workers: a population case control study based on job titles. Occup Environ Med 199552374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatham L, Tolbert P, Kjeldsberg C. Occupational risk factors for subgroups of non‐Hodgkin's lymphoma. Epidemiology 19978551–558. [DOI] [PubMed] [Google Scholar]
- 16.Zheng T, Blair A, Zhang Y.et al Occupation and risk of non‐Hodgkin's lymphoma and chronic lymphocytic leukemia. J Occup Environ Med 200244469–474. [DOI] [PubMed] [Google Scholar]
- 17.Cote T R, Biggar R J, Rosenberg P S.et al Non‐Hodgkin's lymphoma among people with AIDS: incidence, presentation and public health burden. AIDS/cancer study group. Int J Cancer 199773645–650. [DOI] [PubMed] [Google Scholar]
- 18.Mele A, Pulsoni A, Bianco E.et al Hepatitis C virus and B‐cell non‐Hodgkin lymphomas: an Italian multicenter case–control study. Blood 2003102996–999. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi S, Dal Maso L, La Vecchia C. Advances in the epidemiology of HIV‐associated non‐Hodgkin's lymphoma and other lymphoid neoplasms. Int J Cancer 199983481–485. [DOI] [PubMed] [Google Scholar]
- 20.Silvestri F, Pipan C, Barillari G.et al Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood 1996874296–4301. [PubMed] [Google Scholar]
- 21.Wotherspoon A C, Doglioni C, Diss T C.et al Regression of primary low‐grade B‐cell gastric lymphoma of mucosa‐associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993342575–577. [DOI] [PubMed] [Google Scholar]
- 22.Wotherspoon A C, Dogan A, Du M Q. Mucosa‐associated lymphoid tissue lymphoma. Curr Opin Hematol 2002950–55. [DOI] [PubMed] [Google Scholar]
- 23.Freedman D S, Tolbert P E, Coates R.et al Relation of cigarette smoking to non‐Hodgkin's lymphoma among middle‐aged men. Am J Epidemiol 1998148833–841. [DOI] [PubMed] [Google Scholar]
- 24.Adami J, Nyren O, Bergstrom R.et al Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden). Cancer Causes Control 1998949–56. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Hunter D J, Rosner B A.et al Dietary fat and protein in relation to risk of non‐Hodgkin's lymphoma among women. J Natl Cancer Inst 1999911751–1758. [DOI] [PubMed] [Google Scholar]
- 26.Zahm S H, Weisenburger D D, Babbitt P A.et al Use of hair coloring products and the risk of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. Am J Public Health 199282990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altekruse S F, Henley S J, Thun M J. Deaths from hematopoietic and other cancers in relation to permanent hair dye use in a large prospective study (United States). Cancer Causes Control 199910617–625. [DOI] [PubMed] [Google Scholar]
- 28.Kato I, Koenig K L, Baptiste M S.et al History of antibiotic use and risk of non‐Hodgkin's lymphoma (NHL). Int J Cancer 200310799–105. [DOI] [PubMed] [Google Scholar]
- 29.Filipovich A H, Mathur A, Kamat D.et al Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res 199252(suppl 19)5465s–7s. [PubMed] [Google Scholar]
- 30.Jaffe E S, Zarate‐Osorno A, Medeiros J. The interrelationship of Hodgkin's disease and non‐Hodgkin's lymphomas—lessons learned from composite and sequential malignancies. Semin Diagn Pathol 19929297–303. [PubMed] [Google Scholar]
- 31.Greil R, Holzner B, Kemmler G.et al Retrospective assessment of quality of life and treatment outcome in patients with Hodgkin's disease from 1969 to 1994. Eur J Cancer 199935698–706. [DOI] [PubMed] [Google Scholar]
- 32.Cooper G S, Stroehla B C. The epidemiology of autoimmune diseases. Autoimmun Rev 20032119–125. [DOI] [PubMed] [Google Scholar]
- 33.Mellemkjaer L, Linet M S, Gridley G.et al Rheumatoid arthritis and cancer risk. Eur J Cancer 199632A1753–1757. [DOI] [PubMed] [Google Scholar]
- 34.Lazuardi L, Jenewein B, Wolf A M.et al B. Age‐related loss of naïve T cells and dysregulation of T cell/B cell interactions in human lymph nodes. Immunology 200511437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krol A D, le Cessie S, Snijder S.et al Non‐Hodgkin's lymphoma in the Netherlands: results from a population‐based registry. Leuk Lymphoma 200344451–458. [DOI] [PubMed] [Google Scholar]
- 36.Harris N L, Jaffe E S, Stein H.et al A revised European–American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 1994841361–1392. [PubMed] [Google Scholar]
- 37.Oudejans J J, van der Valk P. Immunohistochemical classification of B cell neoplasms. J Clin Pathol 200356193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudejans J J, van der Valk P. Immunohistochemical classification of T cell and NK cell neoplasms. J Clin Pathol 200255892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segi M.Cancer mortality for selected sites in 24 countries (1950–57). Sendai, Japan: Tohoku University of Medicine, 1960
- 40.Cartwright R A, Gurney K A, Moorman A V. Sex ratios and the risks of haematological malignancies. Br J Haematol 20021181071–1077. [DOI] [PubMed] [Google Scholar]
- 41.Banks P M. Changes in diagnosis of non‐Hodgkin's lymphomas over time. Cancer Res 199252(suppl 19)5453s–5s. [PubMed] [Google Scholar]
- 42.Cerhan J R, Wallace R B, Dick F.et al Blood transfusions and risk of non‐Hodgkin's lymphoma subtypes and chronic lymphocytic leukaemia. Cancer Epidemiol Biomarkers Prev 200110361–368. [PubMed] [Google Scholar]
- 43.Vamvakas E C. Allogeneic blood transfusion as a risk factor for the subsequent development of non‐Hodgkin's lymphoma. Transfus Med Rev 200014258–268. [DOI] [PubMed] [Google Scholar]
- 44.Adami J, Nyren O, Bergstrom R.et al Blood transfusion and risk of non‐Hodgkin lymphoma: lack of association. Ann Intern Med 1997127365–371. [DOI] [PubMed] [Google Scholar]
- 45.Moroff G, Leitman S F, Luban N L. Principles of blood irradiation, dose validation, and quality control. Transfusion 1997371084–1092. [DOI] [PubMed] [Google Scholar]
- 46.Zheng T, Mayne S T, Flannery The time trends of multiple myeloma in Connecticut: 1935–1987. Int J Cancer 199250163–164. [DOI] [PubMed] [Google Scholar]
- 47.Levi F, La Vecchia C. Trends in multiple myeloma. Int J Cancer 199046755–756. [DOI] [PubMed] [Google Scholar]
- 48.Morgan G J, Davies F E, Linet M. Myeloma aetiology and epidemiology. Biomed Pharmacother 200256223–234. [DOI] [PubMed] [Google Scholar]
- 49.Bischoff A. Eradikation von Helicobacter pylori. Tripeltherapie in Deutschland zugelassen. Deutsches Ärzteblatt 199693A2845 [Google Scholar]
- 50.Megraud F. Helicobacter pylori resistance to antibiotics: prevalence, mechanism, detection. What's new? Can J Gastroenterol 200317(suppl B)49B–52B. [DOI] [PubMed] [Google Scholar]
- 51.Shipp M A, Harrington D P, Anderson J R.et al A predictive model for aggressive NHL: the international non‐Hodgkin's lymphoma prognostic factors project. N Engl J Med 199329987–994. [DOI] [PubMed] [Google Scholar]
- 52.Coiffier B. Treatment of aggressive non‐Hodgkin's lymphoma. Semin Oncol 199926(5 Suppl 14)12–20. [PubMed] [Google Scholar]
- 53.Mink S A, Armitage J O. High‐dose therapy in lymphomas: a review of the current status of allogeneic and autologous stem cell transplantation in Hodgkin's disease and non‐Hodgkin's lymphoma. Oncologist 20016247–256. [DOI] [PubMed] [Google Scholar]
- 54.Shustik C, Bergsagel D E, Pruzanski W. Kappa and lambda light chain disease: survival rates and clinical manifestations. Blood 19764841–51. [PubMed] [Google Scholar]
- 55.Lopez‐Guillermo A, Cid J, Salar A.et al Peripheral T‐cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. classification. Ann Oncol 19989849–855. [DOI] [PubMed] [Google Scholar]
- 56.Tallman M S, Peterson L C, Hakimian D.et al Treatment of hairy‐cell leukemia: current views. Semin Hematol 199936155–163. [PubMed] [Google Scholar]
- 57.Forstpointner R, Hanel A, Repp R.et al Increased response rate with rituximab in relapsed and refractory follicular and mantle cell lymphomas—results of a prospective randomized study of the German low‐grade lymphoma study group. Dtsch Med Wochenschr 20021272253–2258. [DOI] [PubMed] [Google Scholar]
- 58.Quintanilla‐Martinez L, Davies‐Hill T, Fend F.et al Sequestration of p27Kip1 protein by cyclin D1 in typical and blastic variants of mantle cell lymphoma (MCL): implications for pathogenesis. Blood 20031013181–3187. [DOI] [PubMed] [Google Scholar]
- 59.Rudiger T, Ott G, Ott M M.et al Differential diagnosis between classic Hodgkin's lymphoma, T‐cell‐rich B‐cell lymphoma, and paragranuloma by paraffin immunohistochemistry. Am J Surg Pathol 1998221184–1191. [DOI] [PubMed] [Google Scholar]
- 60.Felgar R E, Stewart K R, Cousar J B.et al T‐cell rich large B‐cell lymphomas contain non‐activated CD8+ cytolytic T cells, show increased tumor cell apoptosis, and have lower bcl‐2 expression than diffuse large B‐cell lymphomas. Am J Pathol 19981531707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calvo K R, Traverse‐Glehen A, Pittaluga S.et al Molecular profiling provides evidence of primary mediastinal large B‐cell lymphoma as a distinct entity related to classic Hodgkin lymphoma: implications for mediastinal gray zone lymphomas as an intermediate form of B‐cell lymphoma. Adv Anat Pathol 200411227–238. [DOI] [PubMed] [Google Scholar]
- 62.Cote T R, Manns A, Hardy C R.et al Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. AIDS/cancer study group. J Natl Cancer Inst 199688675–679. [DOI] [PubMed] [Google Scholar]
- 63.Ferreri A J, Campo E, Ambrosetti A.et al Anthracycline‐based chemotherapy as primary treatment for intravascular lymphoma. Ann Oncol 2004151215–1221. [DOI] [PubMed] [Google Scholar]
- 64.Oscier D, Fegan C, Hillmen P.et al Guidelines Working Group of the UK CLL Forum. British committee for standards in haematology. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol 2004125294–317. [DOI] [PubMed] [Google Scholar]
- 65.Wu S, Ko Y S, Teng M S.et al Adriamycin‐induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol 2002341595–1607. [DOI] [PubMed] [Google Scholar]