Abstract
Background
Myopericytoma (MPC) is a recently proposed term to describe a group of tumours that originate from perivascular myoid cells and show a range of histological growth patterns. Only a small number of series describing MPC have been reported. MPC is frequently misdiagnosed as a sarcoma.
Aims
To document the clinical and histopathological findings of a series of MPCs, to describe the range of growth patterns and morphological spectrum, and to compare MPC with myofibroma (MF).
Patients/Methods
Fourteen patients with features of MPC and/or MF were identified from the archival files of the department of anatomical pathology, Royal Prince Alfred Hospital, Sydney, Australia.
Results
There were six female and eight male patients. The mean and median patient ages were 37 and 35.5 years, respectively. The tumours were located in the skin, subcutis, or superficial soft tissues of the distal extremities (13 patients) or the head and neck region (one patient), and showed a spectrum of morphological appearances. They were divided into two groups based upon the predominant growth pattern corresponding to MPC (seven cases) and MF (seven cases). The feature most suggestive of MPC was the presence of a concentric perivascular arrangement of plump spindle shaped cells. The presence of a zonation/biphasic appearance was most characteristic of MF.
Conclusions
MPC exhibits a spectrum of growth patterns that overlap with MF. Tumours can be designated as MPC or MF depending on the predominant growth pattern.
Keywords: haemangiopericytoma, myofibroma, myopericytoma, perivascular myoma, soft tissue
In 1942, Stout1 described a tumour that he termed haemangiopericytoma (HPC), which characteristically occurred on the extremities, and consisted of plump spindle shaped cells arranged around prominent, thin walled, branched, “stag horn” blood vessels. Stout believed that HPC was a morphologically heterogeneous group of tumours, and recognised both myoid differentiation and an overlap with glomus tumours. Subsequently, Enzinger expanded the term HPC to describe any tumour characterised by the presence of a prominent, branching, thin walled vascular pattern aligning it with vascular tumours.2 More recently it has been recognised that this vascular pattern is non‐specific and may be seen in a variety of other benign and malignant tumours, including benign fibrous histiocytoma, synovial sarcoma, mesenchymal chondrosarcoma, solitary fibrous tumour, leiomyosarcoma, endometrial stromal sarcoma, and infantile fibrosarcoma.3 Thus, it is clearly inappropriate to classify tumours solely on the basis of their vascular pattern. The use of immunohistochemistry has assisted the appropriate classification of many tumours displaying this vascular pattern. However, a small subset remains, probably representing examples of myopericytoma (MPC). Thus, we seem to have gone full circle in the past 50 years or so, back to what Stout originally described as HPC. Because the term HPC has become non‐specific, there has been a general push to make MPC the new term,4 and this has now been endorsed by the World Health Organisation (WHO).5 In 1998, Granter and colleagues4 formally used the term MPC and comprehensively described three morphological appearances that formed a spectrum of tumours seen in the putative MPC category. These patterns/tumour types were that of myofibromatosis, archetypal MPC, and glomangiopericytoma (GPC). In the recent WHO classification of soft tissue tumours5 MPC and myofibroma (MF) are listed as separate entities and GPC appears as a subtype of MPC.
“In our experience, both myopericytoma and myofibroma are frequently misdiagnosed as other tumours, frequently sarcomas”
MPC is recognised, on the basis of both immunohistochemical and ultrastructural studies, as a tumour derived from the perivascular myoid cell, and shares features of both smooth muscle cells and glomus cells. The tumour group of MPC has a spectrum of growth patterns that show some overlap with those of MF. Candidates for the progenitor cell of origin for the myopericyte (and by implication MPC) include the myofibroblast or the pericyte, both of which exhibit properties of modified smooth muscle cells rather than endothelial cells. The term “myofibroblast” is applied to a spindle shaped cell with elongated nucleus and pale eosinophilic cytoplasm that usually shows a desmin negative, actin positive immunohistochemical phenotype. Fibroblasts and myofibroblasts are thought to represent functional variants of a single cell type. The pericyte is defined both by its orientation to blood vessel walls and its immunohistochemical or ultrastructural characteristics. It is a contractile arborising cell arranged ubiquitously along capillaries and venules, with multiple extensions of the cells encircling the vasculature. It is viewed as a pluripotential resting stem cell, capable of differentiating along smooth muscle, pericyte, glomus cell, osseous, fibroblast, and adipocyte cell lines.4,6,7 Indeed, differentiation of pericytes into myofibroblasts and smooth muscle cells has been documented.7 This concept accounts for the spectrum of tumours and may explain the distinctive features of each variety.4,5,6,7,8 The relation to the so called perivascular epithelioid cell is not known. However, because perivascular epithelioid cell tumours show different morphological and immunohistochemical characteristics (epithelioid cells, HMB45 positive staining, and a propensity to involve intra‐abdominal rather than peripheral sites), they appear to be unrelated to MPC.
Only two series of MPC cases and several case reports4,9,10,11,12 have been published since the term gained recognition with the publication of the paper by Granter and colleagues in 1998.4 In our experience, both MPC and MF are frequently misdiagnosed as other tumours, frequently sarcomas. In an attempt to clarify these entities we reviewed 14 cases retrieved from our surgical and consultation/review files that showed features of MPC and/or MF. The aims of our study were to document the clinical and histopathological features of MPC, to describe its range of growth patterns and morphological spectrum, and to compare it with MF.
Materials and methods
The computer based archival records of the department of anatomical pathology of Royal Prince Alfred Hospital, Sydney, Australia were searched for cases that had been coded as MF, MPC, HPC, glomangioma, or glomangiomyoma between 1999 and 2004. Thirty five cases were identified and all available haematoxylin and eosin and immunohistochemically stained sections from each case were retrieved. On review, 14 cases were considered to display features of MPC or MF. These cases included routine surgical (two cases) and consultation/review (12 cases) specimens. All the specimens had been formalin fixed, paraffin wax embedded, and stained with haematoxylin and eosin. Immunohistochemical stains using the avidin–biotin–alkaline phosphatase method with appropriate positive and negative controls were performed on 13 cases. Table 1 lists the antibodies used.
Table 1 Source and dilution of antibodies.
| Antigen | Antibody clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| SMA | 1A4 | 1/100 | None | Dako (Glostrup, Denmark) |
| Desmin | D33 | 1/100 | Microwave (EDTA, Tris, citrate pH 8.0) | Dako (Glostrup, Denmark) |
| S100 protein | Polyclonal | 1/500 | None | Dako (Carpinteria, California, USA) |
| HMB45 | HMB45 | 1/100 | None | Dako (USA) |
| Vimentin | V9 | 1/100 | Microwave (EDTA, Tris, citrate pH 8.0) | Dako (Denmark) |
| CD34 | QBEnd 10 | 1/50 | Microwave (EDTA, Tris, citrate pH 8.0) | Dako (Denmark) |
| CK | AE1/AE3 | 1/100 | Protease digest (proteinase K) | Dako (USA) |
CK, cytokeratin; SMA, smooth muscle actin.
Clinical details and follow up data were obtained from hospital records, referring pathologists, and clinicians, where possible (see acknowledgements).
Results
Clinical features
Table 2 summarises the clinical features of the 14 patients.
Table 2 Clinical features of the 14 patients with myopericytoma or myofibroma.
| Case | Sex/Age | Site | Duration (months) | Size (mm) | Follow up (months) |
|---|---|---|---|---|---|
| 1 | M/30 | Right hand, acral | – | 12 | – |
| 2 | F/13 | Right ankle | 24 | 25 | FOD 6 |
| 3 | F/13 | Left ankle | 5 | 30 | – |
| 4 | F/71 | Left ankle | – | 10 | FOD 1 |
| 5 | M/39 | Sole of foot | 24 | 11 | FOD 2 |
| 6 | M/60 | Left hand, dorsum | – | 8 | – |
| 7 | M/48 | Knee | 144 | 9 | – |
| 8 | F/35 | Left posterior neck | – | 10 | – |
| 9 | F/60 | Right upper arm | 36 | 12 | FOD 10 |
| 10 | M/30 | Left thumb, IP | 6 | 10 | FOD 1 |
| 11 | M/28 | Foot | 2 | 14 | FOD 56 |
| 12 | M/38 | Right ankle | – | 18 | FOD 2 |
| 13 | F/22 | Left finger, MCP | – | 18 | – |
| 14 | M/36 | Left knee | – | 45 | – |
Patient 2 had complete excision of residual tumour with same histological appearances 25 months after the initial biopsy and is free of disease 6 months later.
Patient 5 had complete excision of residual disease 4 months after biopsy and is free of disease two months later.
F, female; FOD, free of disease; IP, interphalangeal joint; M, male; MCP, metacarpophalangeal joint; RD, residual disease; –, unknown.
Myopericytoma
There were seven cases with a predominant MPC‐type growth pattern that were classified as MPC. These patients were aged 13 to 71 years (mean, 39; median, 39), and comprised four males and three females. All the tumours were situated in the distal extremities (hand, knee, ankle, and foot). The duration of the lesion was recorded in only four patients at five, 24, 24, and 144 months. Two patients presented with painful lesions and the remaining five with mass lesions. Six had single lesions, and one with a lesion of the left ankle had a similar lesion excised from the left knee 16 months previously, although this was not available for review. Initial management consisted of incisional biopsy, complete or incomplete excision, followed by either complete excision or close observation. The initial proffered pathological differential diagnoses of the five consultation cases included a proliferating dermal tumour “probably malignant”, liposarcoma, monophasic synovial sarcoma, angiomatoid (malignant) fibrous histiocytoma, glomus tumour, and angioleiomyoma, whereas that of the two review cases was MPC (table 3).
Table 3 Proffered diagnoses and immunohistochemical profile of 14 cases of myopericytoma or myofibroma.
| Case | Diagnosis | Proffered diagnoses | Antigen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SMA | Desmin | S100 | HMB45 | Vimentin | CD34 | CK | |||
| 1 | MPC | Proliferating dermal tumour, probably malignant | 3+ | Negative | Negative | Negative | – | – | – |
| 2 | MPC | Infantile haemangiopericytoma | 3+ | – | – | Negative | – | – | – |
| 3 | MPC | Liposarcoma, monophasic synovial sarcoma, angiomatoid MFH | – | – | – | – | – | – | – |
| 4 | MPC | MPC | Positive | Patchy | Negative | – | – | – | Negative |
| 5 | MPC | Angioleiomyoma | + | – | Negative | – | – | – | – |
| 6 | MPC | MPC | Positive | Weak | Negative | Negative | – | – | – |
| 7 | MPC | Glomus tumour | Positive | Patchy | – | – | – | Negative | – |
| 8 | MF | ? Haemangioma | 1+ | Negative | – | – | – | Negative | – |
| 9 | MF | Solid angioleiomyoma | 3+ | Negative | 1+ (nuclei) | – | – | – | – |
| 10 | MF | Leiomyoma, fibroma, perineurioma | 3+ | Negative | Negative | – | – | – | – |
| 11 | MF | ?Leiomyosarcoma, angiomatoid MFH | Moderate | Occ cell | Negative | Negative | – | Negative | Negative |
| 12 | MF | MF | 3+ | Negative | Negative | Negative | Positive | – | Negative |
| 13 | MF | Giant cell tumour of tendon sheath, reparative giant cell granuloma | Positive | Neg | Negative | Negative | – | – | Negative |
| 14 | MF | ?AV malformation | Positive | Patchy | Negative | – | – | Negative | – |
Positive immunohistochemical staining was graded from weakly positive (1+) to strongly positive (3+).
AV, arteriovenous; CK, cytokeratin; MF, myofibroma; MFH, malignant fibrous histiocytoma; MPC, myopericytoma; SMA, smooth muscle actin; Occ, occasional; –, unknown.
Myofibroma
The remaining seven cases had a predominant MF‐type growth pattern and were classified as MF. These patients were aged 22 to 60 years (mean, 35.6; median, 35) and comprised four men and three women. Six of the tumours were situated in the extremities (upper arm, thumb, finger, knee, ankle, and foot), and two in the head and neck region. The duration of the lesion was recorded in three patients as two, six, and 36 months. All were solitary. One patient presented with a painful lesion and the remaining six with mass lesions. Initial management was either wide excision or incomplete excision followed by re‐excision. The initial proffered pathological differential diagnoses of the seven cases (all were consultation cases) included possible haemangioma, solid angioleiomyoma, fibroma, perineurioma, leiomyosarcoma, angiomatoid (malignant) fibrous histiocytoma, giant cell tumour of tendon sheath, and reparative giant cell granuloma (table 3).
Follow up was available for seven of the 14 patients. All were free of disease at a mean follow up period of 11 months (range, 1–56). One patient (case 2) had residual disease, and although there was no evidence of clinical or radiological progression in the interim, had complete excision of the residual tumour 25 months later. This was similar histologically to the original tumour. She was free of disease six months later. A second patient (case 5) with residual disease after incisional biopsy had subsequent complete excision and is free of disease at short follow up (two months) (table 2).
Pathological features
The tumours could be divided into two groups corresponding to MPC (seven cases) and MF (seven cases). All had an overlapping spectrum of appearances, which included plump spindle shaped cells, some with a myofibroblastic appearance, in sheets and fascicles, focally arranged around blood vessels in a concentric pattern, and included an HPC‐like vascular pattern. This HPC‐like vascular pattern was present in all cases to a variable degree, but was less prominent in cases designated as MF.
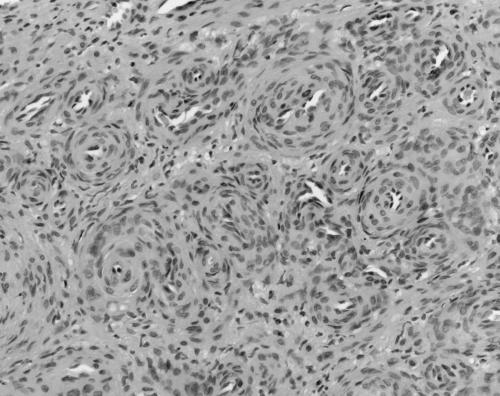
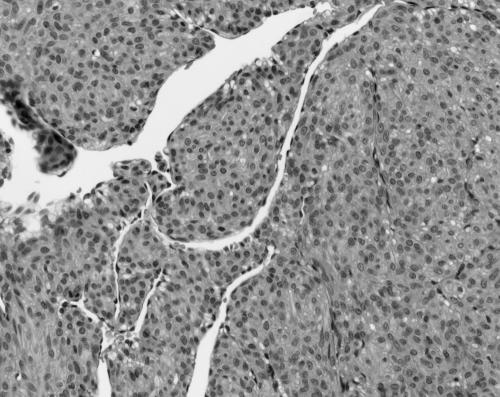
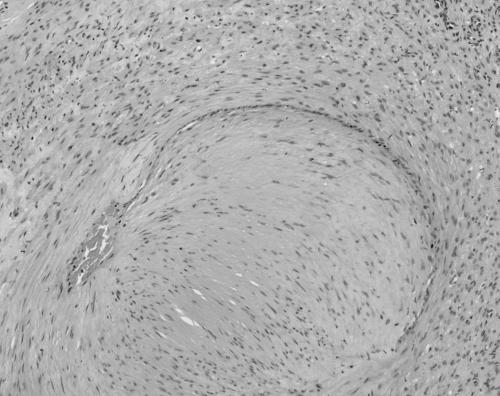
The MPCs ranged in size from 11 to 30 mm. All were present in skin, subcutaneous tissues, and/or superficial soft tissues. MPCs were characterised by a proliferation of bland round to ovoid cells with eosinophilic cytoplasm arranged in a concentric perivascular pattern (figs 1, 2). These cells bore some resemblance to glomus cells, but were distinguished from them by their larger size, more abundant cytoplasm, and lack of a clearly demarcated cell border. Many of the vessels had an HPC‐like vascular pattern. An associated spindle cell component, somewhat resembling smooth muscle differentiation (although with less cytoplasmic eosinophilia or elongated nuclei), was present in most (six of seven) cases (fig 3). Hyalinisation and hyalinised nodules protruding into vascular spaces similar to the pattern seen in myofibromatosis were noted in three of seven cases. Mitoses were infrequent in all instances (less than 1/10 high power fields). Necrosis was not seen. Immunohistochemical staining for smooth muscle actin (SMA) was available in six cases, all of which were positive. Staining for desmin was performed in four cases, and was weakly positive with a patchy distribution in three cases and negative in one. S100 protein (four cases), HMB45 (three cases), CD34 (one case), and cytokeratin (one case) were negative. The feature most suggestive of MPC was the presence of a concentric perivascular arrangement of plump spindle shaped to glomoid cells (table 4).
Figure 1 Case 7: myoperictyoma. Plump cells arranged around blood vessels (haematoxylin and eosin stained; original magnification, ×200).
Figure 2 Case 5: myoperictyoma. Plump cells with a glomoid appearance arranged around haemangiopericytoma‐like blood vessels (haematoxylin and eosin stained; original magnification, ×400).
Figure 3 Case 5: myoperictyoma. Spindle shaped cells resembling smooth muscle (haematoxylin and eosin stained; original magnification, ×200).
Table 4 Microscopic features that distinguish MPC from MF.
| Feature | MPC | MF |
|---|---|---|
| HPC‐like vascular spaces* | ++ | + |
| Concentric perivascular proliferation | +++ | + |
| Zonation/biphasic appearance† | + | +++ |
| Glomus‐like cells‡ | + to +++ | − |
| Hyalinised paucicellular areas | + | +++ |
| Myoid‐like cells§ | + | ++ |
| Primitive cells¶ | + | ++ |
| Bulging or intravascular proliferation | + | ++ |
| Myoid nodules | −/+ | ++ |
| Necrosis | − | + |
*Numerous lesional blood vessels that are variable in size, ectatic, with thin walls and a branching “staghorn” arrangement; †architectural arrangement of myoid‐like cells and primitive cells in separate areas, usually the myoid cells peripherally and the primitive cells centrally; ‡cuboidal shaped cells, with distinct cell borders, clear to eosinophilic cytoplasm, and central round nuclei; §oval/spindle shaped cells with eosinophilic cytoplasm; ¶round/polygonal cells with a small amount amphophilic cytoplasm and indistinct cell borders, typically surrounding blood vessels in HPC‐like areas.
HPC, haemangiopericytoma; MF, myofibroma; MPC, myopericytoma.
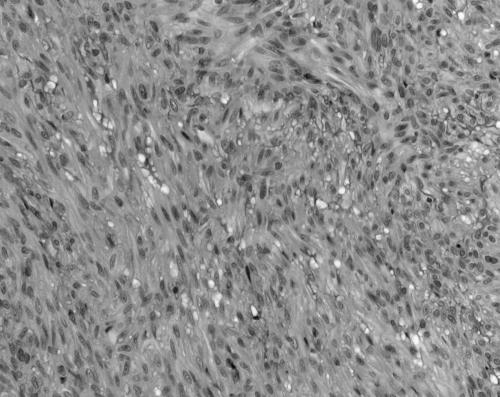
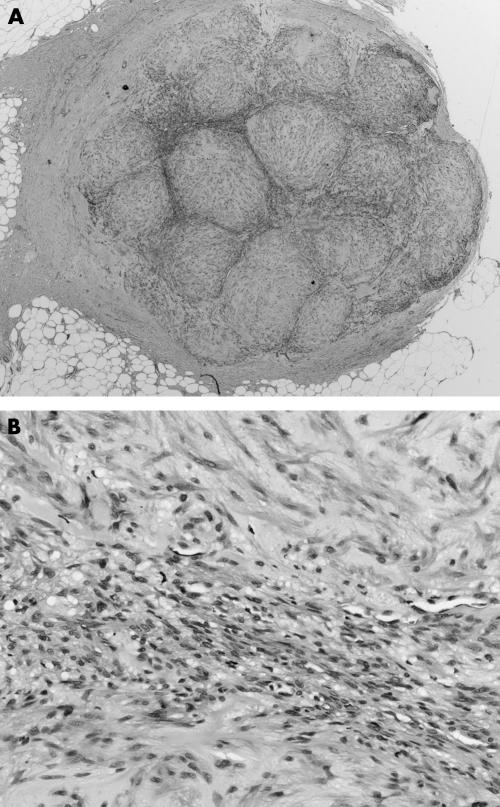
The MFs ranged in size from 10 to 45 mm. All were present superficially in skin and/or subcutaneous tissue. They were characterised in all instances by a biphasic zonation pattern composed of fascicles of spindle shaped cells with abundant eosinophilic cytoplasm that resemble smooth muscle in the peripheral zones of the lesion (fig 4). In the central areas of six of the seven cases there was a population of more primitive round to polygonal or small spindle shaped cells, with small amounts of amphophilic cytoplasm, slightly pleomorphic hyperchromatic nuclei, vesicular chromatin, and indistinct cell borders. These cells were typically arranged in an HPC‐like vascular pattern. Hyalinised paucicellular areas were present in all seven cases. Nodules of cells bulging into vascular lumens were identified in three of seven cases (fig 5). Immunohistochemical staining for SMA was positive in all cases. Staining for desmin, performed on all seven cases, was negative in five and showed patchy positive staining in two. Staining for S100 protein was negative (four cases) and an occasional nucleus showed weak positive staining in one case. HMB45 (three cases), cytokeratin (three cases), and CD34 (three cases) were negative. The feature most suggestive of MF was the presence of a zonation/biphasic appearance with fascicles of spindle shaped cells with elongated blunt ended nuclei and pale eosinophilic cytoplasm (table 4).
Figure 4 Case 9: myofibroma. (A) Profile view of lesion showing zonation (haematoxylin and eosin stained; original magnification, ×20). (B) View showing biphasic cell types (haematoxylin and eosin stained; original magnification, ×200).
Figure 5 Case 7: myofibroma. Hyalinised nodule bulging into a blood vessel (haematoxylin and eosin stained; original magnification, ×100).
Discussion
The classification and terminology of tumours showing a so called HPC‐type vascular pattern has been confusing and continues to evolve. We believe that the move away from the traditional HPC terminology, and the designation of MPC as a distinct entity, as endorsed by the WHO,5 are useful steps forward in this evolution. Although the clinical and pathological features of several series of MPC cases have been reported,4,5,10 these tumours continue to be misdiagnosed. In our series, two of seven cases of MPC were initially considered to be malignant, potentially resulting in inappropriate treatment, unnecessary patient anxiety, and medicolegal consequences. It is hoped that by continued emphasis on the clinical and pathological features of this entity, misdiagnosis will be less frequent.
Our series confirms the previously reported observations that MPC occurs mainly in childhood to mid‐adult years, with a male predominance.5,13 Our MPC and MF groups both had a slightly younger age than that documented by both Granter and colleagues4 (median, 39 v 40 years for MPC and 35 v 37 years for MF) and Requena and colleagues,7 whose group of patients with cutaneous MF had an age range of 6 to 83 years (mean, 45; median, 44). Our findings support the view that MPC is a benign, usually subcutaneous tumour, with a predilection for the distal extremities. However, as noted previously, it can arise at other sites, including proximal extremities, head, and neck. Occasional cases have also been documented in skeletal muscle, bones (skull, vertebrae, ribs, femur, and tibia are most often affected), and other visceral organs.14 Some skin lesions have a purplish hue (mimicking a haemangioma, as in our case 5), whereas others present as white nodules or fixed masses. Larger skin lesions can cause ulceration. In our series, a considerable proportion presented with pain or tenderness (four of seven cases), although they have been reported to be generally painless (unless squeezed).3 They can be slow growing (the tumour in patient 7 was present for 12 years), and sometimes arise in childhood (patients 2 and 3, each aged 13 years). They are usually solitary (in adults), but multiple lesions are not infrequent in children (patient 3, age 13, with two similar lesions on the left leg 16 months apart) and typically within a defined anatomical region.5,14 Although recurrence has been reported to occur in 10–20% of patients, it was not seen in our series, albeit with limited follow up. Recurrence has been hypothesised to occur because of the extension of cords of tumour beyond the main lesion, or as a reflection of multifocal disease.15
The dermal and subcutaneous nodules of MPC are more circumscribed than deeper nodules, which can be infiltrative. They are rubbery or firm and scar‐like in consistency, typically with a white grey or pink cut surface. In our series, the tumours ranged in size from 5 to 45 mm, similar to other reports, where tumour size varied from a few millimetres to 5 cm, with an average of 0.5–1.5 cm in largest diameter.5,13,14
Table 4 summarises the microscopic features that we found useful for categorising MPC and MF.
Our series confirms the observations that MPCs are circumscribed but unencapsulated tumours composed of relatively monomorphic oval to spindle shaped myoid‐like cells with cytoplasm that may be eosinophilic to amphophilic, and which share overlapping morphological features with MF. Three growth patterns have been described (MPC, MF, and GPC).4,5 Features of all three may be present in a lesion, although one or other pattern may predominate. In our view, cases with a predominant MF pattern should be classified as MF. We found no cases with a predominant GPC‐type pattern (as described by Granter and colleagues4). Although we noted cases with focal areas of glomoid cells (cases 1 and 5), we felt that these lesions resembled MPC in most other respects. It is our view that because the MPC‐like and GPC‐like patterns share so many features they are best regarded as a single entity. All the cases in our series had low mitotic rates, less than 1/10 high power fields, in accordance with other reports.5 Necrosis was not seen in our series and is not typically present, although the hyalinised paucicellular nodules seen in the MF type may resemble ischaemic necrosis.
We confirmed the presence of a clinical and morphological overlap among these groups, showing the growth patterns described as MPC like and MF like. The concept of a spectrum of tumours with a common histogenetic precursor cell is supported by our findings.
“It is our view that because the myopericytoma‐like and glomangiopericytoma‐like patterns share so many features they are best regarded as a single entity”
Myofibroblasts and pericytes (and MPC) are uniformly positive (sometimes focally) for SMA and vimentin, and are sometimes weakly positive for desmin, as is reflected in our series. The cases in our series showed negative staining for HMB45, epithelial membrane antigen, CD34, CD68, and cytokeratins. Other series confirm that the lesional cells are usually negative for S100 protein (we had one case with weak positive nuclear staining) and neurone specific enolase.5,13,16
Ultrastructural studies have been performed in nine cases reported in the literature.7,8,10,11,16,17 Ultrastructurally, the myopericyte has a folded nucleus,7 prominent rough endoplasmic reticulum, thin cytoplasmic filaments with focal dense bodies (myofilaments), subplasmalemmal densities, micropinocytotic vesicles, and elongated cell processes partly covered by an external basal lamina and occasional intercellular attachment sites. These features are suggestive of myoid differentiation.3,13
Based on our observations that no tumours recurred or metastasised, we recommend local and complete surgical excision with continued observation as the treatment of MPC and MF.
The follow up of our series supports the benign nature of MPC, with no evidence of disease recurrence in the seven patients with follow up details available. Most MPCs do not recur after excision. It has been reported that some even regress spontaneously after a diagnostic biopsy, although this was not seen in the follow up of the two patients where the tumour was incompletely excised in our series. Recurrences may be related to incomplete excision resulting from poor circumscription of the lesion. This characteristically occurs in deep soft tissue lesions where the cells extend around blood vessels beyond the edge of the main tumour mass. Therefore, superficial tumours tend to follow a benign course, with few recurring after complete excision, whereas deeper seated tumours, multinodular tumours in childhood, and multiple visceral lesions may pursue a more aggressive course.6,13
Malignancy in MPC appears to be very rare; only one paper has documented its occurrence.10 Histological features reported to be associated with malignant behaviour included high mitotic rate, high cellularity, pleomorphism, and necrosis.5,10
Several previous studies have noted a relation between MF and MPC, some noting a morphological spectrum of appearances. Smith and colleagues17 and Guitart and colleagues16 noted that cutaneous MF in adults and infantile myofibromatosis share many properties. Mentzel et al described an association between infantile HPC and infantile myofibromatosis, forming a continuous spectrum of a single entity.18 Variend et al added congenital fibrosarcoma to the same spectrum.19 It is now recognised that congenital fibrosarcoma is also related to cellular mesoblastic nephroma, sharing a common t(12;15)(p13;q25) chromosomal translocation.20 In 1996, Requena et al described four morphological patterns of myofibroma (table 5),7 and suggested that they represent different stages of evolution of the same lesion. In addition, they hypothesised that the common cell of origin was the myopericyte (based on immunohistochemical and ultrastructural studies) and suggested the term MPC. In 1998, Granter and colleagues4 adopted the term MPC and described three morphological appearances forming a spectrum of tumours in the MPC category (table 5). Their description of the three types of tumour matches well with that described elsewhere in the literature, albeit using different nomenclature.6,13,14,15 At the same time, the term and concept of perivascular myoma, in lieu of the term MPC, was proposed by Kutzner.9 He suggested this term after describing various types of cutaneous adult myofibroma (table 5).
Table 5 Nomenclature for myopericyte tumours used in various publications (modified from Mikami and colleagues11).
| Publication | Myopericytoma type | Myofibroma type | Glomangiopericytoma |
|---|---|---|---|
| WHO (2002)5 | Myopericytoma | Myofibroma | Glomangiopericytoma |
| Requena et al (1996)7 | Cutaneous adult myofibroma: | ||
| Vascular type | |||
| Nodular or cellular type | |||
| Multinodular or biphasic type | |||
| Leiomyoma like or fascicular type | |||
| Granter et al (1998)4 | Myopericytoma | Myofibromatosis “infantile type” | Glomangiopericytoma |
| Kutzner (1998)9 | Myopericytoma | Sclerotic myofibroma | Glomangiopericytoma |
| Vascular | Classic biphasic myofibroma | ||
| Solid |
The differential diagnosis of MPC and MF varies with the predominant pattern/type of tumour. For tumours of the MPC type, biopsies from the central areas of the lesion may resemble various types of sarcoma, especially those composed of small round cells arranged in an HPC‐like vascular pattern. The list of possible tumours includes Ewing's sarcoma, which stains positively for CD99; poorly differentiated synovial sarcoma, which may have a biphasic growth pattern, and stains positively for epithelial membrane antigen, cytokeratin, bcl‐2, and CD99; mesenchymal chondrosarcoma, which has islands of cartilage and stains positively for S100; phosphaturic mesenchymal tumour, which has a variety of patterns and is often associated with calcification and osteoclast‐like giant cells21; and angiomatoid fibrous histiocytoma, which has angiomatoid spaces, hypercellularity as a result of proliferation of short spindle and/or ovoid cells, and a lymphoid infiltrate.11,13,15 For tumours of the MF type the differential diagnosis includes smooth muscle tumour, which has fascicles of spindle shaped cells, cigar shaped nuclei, and is positive for SMA and desmin; inflammatory myofibroblastic tumour, which has a characteristic plasma cell infiltrate; nodular fasciitis, which typically involves a fascial plane, has a more prominent myxoid matrix, scattered chronic inflammatory cells, and extravasated erythrocytes; benign fibrous histiocytoma, which has a more pronounced storiform growth pattern, focal positive staining for SMA, and is usually positive for factor XIIIa; and neurofibroma, which is positive for S100; all lack an HPC pattern.13,14 For tumours that include glomoid cells the main differential diagnosis is glomus tumour, including the glomangioma subtype. These have round to oval cells arranged in an eccentric fashion around vessels, with abundant eosinophilic cytoplasm, and in contrast to MPC have a distinct cell border and lack an HPC‐like vascular pattern.11
Take home messages
We support the use of the term myopericytoma (MPC) to describe a spectrum of tumours typified by a haemangiopericytoma‐like vascular architectural pattern with features of perivascular myoid (myopericytic) differentiation
MPC shows a spectrum of growth patterns that overlap with myofibroma (MF)
Tumours can be categorised as MPC or MF depending on the predominant growth pattern: the presence of a concentric perivascular arrangement of plump spindle shaped cells in MPC and a zonation/biphasic appearance in MF
We suggest that the glomangiopericytoma‐like pattern should be included within the MPC group
Conclusions
We support the use of the term MPC to describe a spectrum of tumours typified by an HPC‐like vascular architectural pattern and showing features of perivascular myoid (myopericytic) differentiation. Our review of the clinical details and histological features of 14 cases of MPC and MF provides further support for a spectrum of growth patterns/tumour types as described by Granter et al.4 We suggest that the GPC‐like pattern should be included within the MPC group.
Acknowledgements
We are very grateful to the following pathologists and clinicians who kindly contributed case material and provided clinical information. Dr T Bowen, Fairfield Heights, NSW, Australia; Dr S McKenzie, Wollongong, NSW, Australia; Dr K Singh, Auburn, NSW, Australia; Dr Q‐H Fan, Nanjing, PR China; Dr S McGahan, Lismore, NSW, Australia; Dr G Chu, North Sydney, NSW, Australia; Dr R Levingston, Macquarie Park, NSW, Australia; Dr A Meyer, Lismore, NSW, Australia; Dr R Juska, Clayton, Victoria, Australia; Dr D Townsend, Brisbane, Qld, Australia.
Abbreviations
GPC - glomangiopericytoma
HPC - haemangiopericytoma
MF - myofibroma
MPC - myopericytoma
SMA - smooth muscle actin
WHO - World Health Organisation
References
- 1.Stout A P, Murray M R. Hemangiopericytoma. A vascular tumour featuring Zimmerman's pericytes. Ann Surg 194211626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzinger F M, Smith B H. Hemangiopericytoma. An analysis of 106 cases. Hum Pathol 1976761–82. [DOI] [PubMed] [Google Scholar]
- 3.Kempson R L, Fletcher C DM, Evans H L.et al, eds. Perivascular tumours. In: Atlas of tumour pathology. Tumours of the soft tissues. Fascicle 30, 3rd Series. Washington: AFIP, 2001371–383.
- 4.Granter S R, Badizadegan K, Fletcher C D. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am J Surg Pathol 199822513–525. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher C DM, Unni K K, Mertens F. eds. Pericytic (perivascular) tumours. In: World Health Organisation classification of tumours. Pathology and genetics. Tumours of soft tissue and bone. Lyon: IARC Press, 2002135–139.
- 6.Weiss S W, Goldblum J R. Haemangiopericytoma and solitary fibrous tumor. In: Enzinger and Weiss's soft tissue tumors. 4th ed. St Louis, Missouri: Mosby 20011001–1021.
- 7.Requena L, Kutzner H, Hugel H.et al Cutaneous adult myofibroma: a vascular neoplasm. J Cutan Pathol 199623445–447. [DOI] [PubMed] [Google Scholar]
- 8.Dictor M, Elner A, Andersson T.et al Myofibromatosis‐like hemangiopericytoma metastasizing as differentiated vascular smooth‐muscle and myosarcoma. Myopericytes as a subset of “myofibroblasts”. Am J Surg Pathol 1992161239–1247. [DOI] [PubMed] [Google Scholar]
- 9.Kutzner H. Perivascular myoma: a new concept for “myofibroblastic” tumors with perivascular myoid differentiation. [In German.] Verh Dtsch Ges Pathol 199882301–308. [PubMed] [Google Scholar]
- 10.McMenamin M E, Fletcher C D. Malignant myopericytoma: expanding the spectrum of tumours with myopericytic differentiation. Histopathology 200241450–460. [DOI] [PubMed] [Google Scholar]
- 11.Mikami Y, Shiomi T, Manabe T. Perivascular myoma: case report with immunohistochemical and ultrastructural studies. Pathol Int 20025269–74. [DOI] [PubMed] [Google Scholar]
- 12.Cox D P, Giltman L. Myopericytoma of the thoracic spine: a case report. Spine 200328E30–E32. [DOI] [PubMed] [Google Scholar]
- 13.Weiss S W, Goldblum J R. Myofibroma and myofibromatosis. In: Enzinger and Weiss's soft tissue tumors. 4th ed. St Louis, Missouri: Mosby 2001357–363.
- 14.Kempson R L, Fletcher C DM, Evans H L.et al, eds. Myofibroma, solitary and multicentric (myofibromatosis). In: Atlas of tumour pathology. Tumours of the soft tissues. Fascicle 30; 3rd Series. Washington: AFIP, 200164–66.
- 15.Fletcher C D. ed. Hemangiopericytoma. In: Diagnostic histopathology of tumours. 2nd ed. Edinburgh: Churchill Livingstone, 200077–85.
- 16.Guitart J, Ritter J H, Wick M R. Solitary cutaneous myofibromas in adults: report of six cases and discussion of differential diagnosis. J Cutan Pathol 199623437–444. [DOI] [PubMed] [Google Scholar]
- 17.Smith K J, Skelton H G, Barrett T L.et al Cutaneous myofibroma. Mod Pathol 19892603–609. [PubMed] [Google Scholar]
- 18.Mentzel T, Calonje E, Nascimento A G.et al Infantile hemangiopericytoma versus infantile myofibromatosis. Study of a series suggesting a continuous spectrum of infantile myofibroblastic lesions. Am J Surg Pathol 199418922–930. [DOI] [PubMed] [Google Scholar]
- 19.Variend S, Bax N M A, Van Gorp J. Are congenital myofibromatosis, congenital fibrosarcoma and congenital haemangiopericytoma histogenetically related? Histopathology 19952657–62. [DOI] [PubMed] [Google Scholar]
- 20.Knezevich S R, Garnett M J, Pysher T J.et al ETV6–NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res 1998585046–5048. [PubMed] [Google Scholar]
- 21.Folpe A L, Fanburg‐Smith J C, Billings S D.et al Most osteomalacia‐associated mesenchymal tumors are a single histopathologic entity. An analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 2004281–30. [DOI] [PubMed] [Google Scholar]