Abstract
Background
Metastasis to regional lymph nodes (LNs) through lymphatic vessels is common in cancer progression and is an important prognostic factor in many cancers. Recent evidence suggests that tumour lymphangiogenesis promotes lymphatic metastasis.
Aims
To study the role of lymph vessel density (LVD) in gastric cancer and investigate whether LVD is associated with LN metastasis/prognosis.
Methods
Lymphatics of 117 primary human gastric cancer cases were investigated by quantitative immunohistochemical staining for podoplanin. The relation between LVD and LN metastasis and other established clinicopathological parameters was analysed. The relation between LVD and prognosis was also studied.
Results
Mean LVD of “hot spots” was 11.6/case. LVD significantly correlated with LN and podoplanin positive lymphatic invasion. High LVD was associated with worse overall survival. In multivariate analysis, positive LVD was a significant independent predictor of overall survival, depth of invasion, and TNM stage. LVD significantly correlated with LN metastasis at surgery and podoplanin positive lymphatic invasion. In multivariate analysis, positive LVD was an independent significant predictor of LN metastasis.
Conclusions
Increased podoplanin expression is significantly associated with LN metastasis, and may play an important role in detecting LN metastasis in gastric cancer. Furthermore, LVD may be a significant prognostic factor in gastric cancer at any stage. In addition, LVD and lymph vessel invasion detected by podoplanin immunohistochemistry are associated with LN metastasis in T1 early gastric cancer. LVD assessment by podoplanin immunohistochemistry may become a useful predictor of LN metastasis in T1 early gastric cancer and may influence the decision making process for additional surgery.
Keywords: stomach, cancer, lymph vessel density, lymph node, metastasis, prognosis
The absence of specific markers for lymphatics in cancer has made the identification of tumour lymphatics difficult. For pathological diagnosis in cases with small vessels invaded by malignant cells, it is important to distinguish between lymphatic vessels and blood capillaries by light microscopy. However, both of these microvessels are so similar on haematoxylin and eosin (HE) staining that it is often difficult to distinguish between them accurately. The recent discovery that podoplanin, an approximately 38 kDa membrane mucoprotein originally detected on the surface of rat podocytes,1 is a specific marker for lymphatic endothelium2 has had a major impact on lymphatic studies. Podoplanin has become a valuable marker for detecting lymph vessels, for assessing lymph vessel density (LVD), and for identifying lymph vessel invasion (LVI) by tumour cells.3,4,5,6
Recent evidence suggests that tumour lymphangiogenesis—the growth of tumour associated lymphatic vessels—promotes lymphatic metastasis.7,8,9,10 In gastric cancer, increased LVD detected by immunohistochemistry (IHC) is associated with lymph node metastasis,11 and is also correlated with an unfavourable prognosis in other types of cancer.3,4,5,12,13 Furthermore, in some types of cancer, LVI is known to be associated with lymph node metastasis.6,12,13
“Podoplanin has become a valuable marker for detecting lymph vessels, for assessing lymph vessel density, and for identifying lymph vessel invasion by tumour cells”
The likelihood of lymph node metastasis in early gastric carcinoma with intramucosal carcinoma or submucosal invasion is about 0–4% and 15%, respectively.14,15 When the endoscopic mucosal resection (EMR) technique is applied to patients with early gastric cancer, recurrences with lymph node metastasis may occur, especially in those with submucosal invasion. The absence of useful indicators for lymph node metastasis in early gastric cancer has made the prediction of lymph node metastasis difficult. Thus, a reliable marker for lymph node metastasis that could be applied to EMR specimens would be very helpful.
To evaluate whether podoplanin positive lymph vessels can identify tumour lymphangiogenesis in gastric cancer, we investigated the relation between lymph node metastasis and LVD or LVI. We also examined whether LVD or LVI had any value or relevance with respect to predicting disease outcome, and in predicting lymph node metastasis in cases with T1 (referred to as UICC TNM classification) early gastric cancer.
Materials and methods
Patients and tumour samples
We studied paraffin wax embedded archival specimens from 117 patients with primary gastric carcinoma, who were diagnosed and treated in the Osaka Police Hospital, Japan. The cases were randomly selected from the database in the period 1992–6 and are summarised in table 1. The patients had undergone the following types of gastrectomies: partial, five cases; subtotal, 90 cases; and total, 22 cases. All patients had undergone lymph node dissection. Forty four (38%) of the patients had lymph node metastasis at the time of surgery. Seventy three of the patients had early gastric cancer (T1 tumour), in which the tumour was confined to the mucosa or submucosa. Histological type and lymphatic or venous invasion of the tumours were evaluated according to the criteria of the Japanese classification of gastric carcinoma.16 Histological stage grouping, referred to as UICC TNM classification, was confirmed by histological examination. All of the patients were followed up clinically; the mean follow up time was 42 months and ranged from one to 84 months. Twenty three patients died of gastric carcinoma. Patients who died of other diseases were excluded from our study. Twenty four patients had relapsed by the time of the last follow up. Seventeen patients had peritoneal dissemination, 14 had liver metastasis, three had lymph node metastasis, and four had lung metastasis.
Table 1 The association between LVD and other parameters in 117 patients with gastric cancer.
| Factor | N | Mean (SD) LVD/field | p Value |
|---|---|---|---|
| Sex | 0.5542 | ||
| Male | 84 | 11.48 (7.89) | |
| Female | 33 | 11.85 (7.10) | |
| Depth of invasion | 0.0026 | ||
| pT1 | 73 | 11.10 (7.45) | |
| pT2 | 11 | 14.28 (10.00) | |
| pT3–4 | 33 | 11.76 (7.26) | |
| Lymph node metastasis | <0.001 | ||
| Negative | 73 | 10.11 (6.78) | |
| Positive | 44 | 14.02 (8.42) | |
| Stage | 0.1259 | ||
| I | 78 | 10.63 (7.00) | |
| II | 17 | 11.65 (8.60) | |
| III | 15 | 15.13 (9.22) | |
| IV | 7 | 14.43 (7.28) | |
| Venous invasion | 0.1563 | ||
| Negative | 100 | 11.99 (7.83) | |
| Positive | 17 | 9.18 (6.05) | |
| LVI‐HE | 0.0111 | ||
| Negative | 88 | 10.60 (7.20) | |
| Positive | 29 | 14.59 (8.27) | |
| LVI‐IHC | <0.001 | ||
| Negative | 75 | 8.97 (5.77) | |
| Positive | 42 | 16.24 (8.40) |
LVD, podoplanin positive lymph vessel density; LVI‐HE, lymph vessel invasion detected by haematoxylin and eosin staining; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry.
Immunohistochemistry
For podoplanin immunostaining, 4 μm thick paraffin wax embedded sections were dewaxed. They were then placed in a solution of 97% methanol and 3% hydrogen peroxide for five minutes, followed by autoclaving for antigen retrieval. After washing in phosphate buffered saline, the slides were treated for 20 minutes with protein block serum free (Dako Co, Carpinteria, California, USA). This was followed by overnight incubation at 4°C in a humidified chamber with a 1/200 dilution of antihuman podoplanin mouse antibody (AngioBio Co, Del Mar, California, USA), the specificity of which was confirmed by western blot analysis (data not shown). After the overnight treatment, to avoid a non‐specific biotin reaction, Histofine simple stain MAX PO (Nichirei, Tokyo, Japan) was used as the second antibody for 60 minutes, according to the manufacturer's instructions. Colour was developed using diaminobenzidine with 0.01% hydrogen peroxide. Haematoxylin was used as a counterstain. For the negative control, all reagents except for the primary antibody were used.
LVD and LVI assessment
Determination of LVD was performed according to Weidner et al.17 The immunostained sections were scanned by light microscopy at low magnification (×4) and the areas of tissue with the greatest numbers of distinctly highlighted lymph vessels (“hot spots”) were selected. LVD was then determined by counting all immunostained vessels at a total magnification of ×200 from five areas for each case. The mean number of lymph vessels in each case was determined and considered as the LVD. LVD assessed in this manner may be not equivalent to LVD seen in multiple areas of multiple samples of the stomach; however, it is well accepted that these hot spots, not arbitrary areas, are representative of the entire tumour.17 Although there was some variability in the distribution of lymph vessels within the layers of the gastric wall, the five “hot spot” areas were chosen to obtain an objective assessment and to avoid observer variation. LVI‐IHC was considered to be present if at least one tumour cell cluster was clearly visible inside the podoplanin positive lymphatic space,18 and cases with at least one area of LVI‐IHC were considered to be LVD‐IHC positive. Scoring and counting were performed blindly by three investigators who had no clinical knowledge of the patients, and there was significant correlation (p = 0.001) between the individual LVD and LVI assessments.
Statistics
The Mann‐Whitney test was used to examine the association of LVD with LVI‐HE, LVI‐IHC, lymph node metastasis, and venous invasion. The correlation of LVD with depth of invasion or TNM stage was investigated by the Kruskal‐Wallis test. Fisher's exact test or the χ2 test was used to examine the association between lymph node metastasis, LVI‐HE, and LVI‐IHC. A multivariate model using multiple logistic regression analysis was used to evaluate the statistical strength of independent associations between covariates and lymph node metastasis. Overall survival (OS) curves were obtained using the Kaplan–Meier method and compared using the log rank test. A multivariate model using Cox stepwise regression analysis was used to evaluate the statistical strength of independent associations between covariates and OS. A p value less than 0.05 was considered significant. A computer program package (JMP IN 5.1.1J; SAS Institute Inc) was used for all statistical testing and management of the database.
Results
Podoplanin expression in gastric cancer
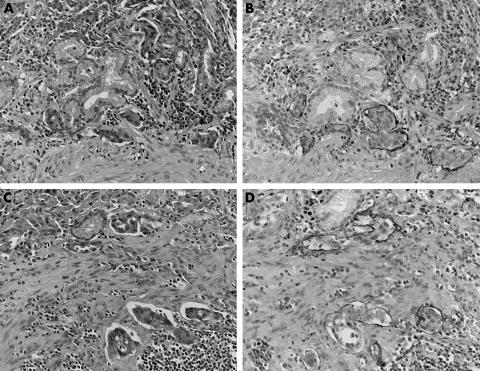
Podoplanin positive lymph vessels were present in all gastric carcinomas. All of the stained vessels were typically thin walled and devoid of red blood cells. Lymph vessels were restricted to the endothelium not only in peritumorous lesions but also in intratumorous lesions. Lymphatic vessels were found in all layers of the gastric wall. Lymphatic vessels from the muscularis mucosae to the superficial submucosa were slightly higher in number, and the occasional accumulation of lymphatic vessels was noted in both the muscularis mucosae and the superficial submucosa. Occasional invasion of the carcinoma cells into the lymph vessels was seen (fig 1). Invasion of carcinoma cells into the lymph vessels was seen mostly in the muscularis mucosae and the submucosa. In some cases, lymphatic invasion was noted in the mucosa, the muscularis propria, or the subserosa. LVD was determined independently by three observers. The mean LVD was considered as the LVD number for each case. The mean (SD) LVD of all cases was 11.6 (7.6)/case.
Figure 1 Immunohistochemical analysis for podoplanin in gastric cancer. Podoplanin positive lymph vessels can be seen from the muscularis mucosae to the superficial submucosa. (A–D) Invasion of the carcinoma cells into the lymph vessels was seen mostly in the muscularis mucosae and the submucosa. In some cases (A, B), lymphatic invasion was noted in the mucosa. Although it is difficult to identify lymph vessel invasion by haematoxylin and eosin staining (A, C), podoplanin immunohistochemistry made it easy to identify lymph vessel invasion (B, D).
LVD is correlated with lymph node metastasis and LVI
As shown in table 1, LVD was correlated with lymph node metastasis (p = 0.001), LVI‐IHC (p < 0.001), LVI‐HE (p = 0.0111), and depth of invasion (p = 0.0026). A significant correlation was also noted for both LVI‐IHC and LVI‐HE and lymph node metastasis (p < 0.001 for each; table 2). There was no significant correlation between LVD and the sex of the patients, venous invasion, or TNM stage.
Table 2 Lymph node metastasis according to LVI.
| Factors | Lymph node metastasis | ||
|---|---|---|---|
| Negative | Positive | p Value | |
| LVI‐HE | |||
| Negative | 70 | 18 | <0.001 |
| Positive | 3 | 26 | |
| LVI‐IHC | <0.001 | ||
| Negative | 59 | 16 | |
| Positive | 14 | 28 | |
LVD, podoplanin positive lymph vessel density; LVI‐HE, lymph vessel invasion detected by haematoxylin and eosin staining; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry.
LVD and LVI are correlated with patients' survival
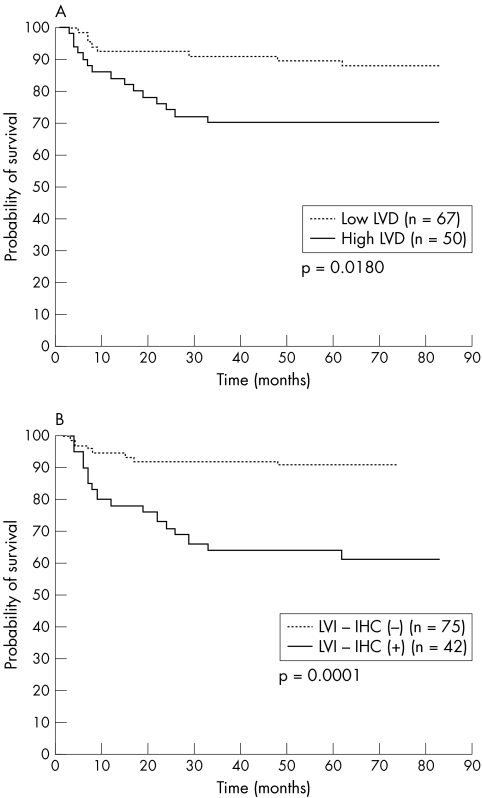
To examine the correlation between LVD and patient's prognosis, the patients were divided into two groups: those with less than (low LVD) and those with more than (high LVD) 12 lymph vessels/case, according to the mean LVD of all cases (mean, 11.6; SD, 7.6/case). Survival analysis was performed on 117 patients and the following variables were examined: LVD, LVI‐IHC, LVI‐HE, sex of patients, depth of invasion, lymph node metastasis, TNM stage, and venous invasion. Univariate survival analysis showed that LVD, LVI‐IHC, LVI‐HE, depth of invasion, lymph node metastasis, TNM stage, and venous invasion were of significant prognostic value for OS (table 3; fig 2). As shown in table 4, multivariate Cox regression analysis of the depth of invasion, lymph node metastasis, TNM stage, LVD, and LVI‐IHC focusing on OS identified the following as independent significant prognostic factors: LVD (p = 0.0422), depth of invasion (p = 0.0118), and TNM stage (p = 0.0492). The odds ratio for LVD was 2.49.
Table 3 Univariate analysis of OS in relation to clinicopathological factors in 117 patients with gastric cancer.
| Factor | N | OS | |
|---|---|---|---|
| N (%) | p Value | ||
| Sex | |||
| Male | 84 | 19 (22.6) | 0.1837 |
| Female | 33 | 4 (12.1) | |
| Depth of invasion | <0.001 | ||
| pT1–2 | 84 | 3 (3.6) | |
| pT3–4 | 33 | 20 (60.1) | |
| Lymph node metastasis | <0.001 | ||
| Negative | 73 | 2 (2.7) | |
| Positive | 44 | 21 (47.7) | |
| TNM stage | <0.001 | ||
| I | 78 | 1 (1.3) | |
| II–IV | 39 | 22 (56.4) | |
| Venous invasion | <0.001 | ||
| Negative | 100 | 14 (14.0) | |
| Positive | 17 | 9 (52.9) | |
| LVI‐HE | <0.001 | ||
| Negative | 88 | 6 (6.8) | |
| Positive | 29 | 17 (58.6) | |
| LVI‐IHC | 0.0001 | ||
| Negative | 75 | 7 (9.3) | |
| Positive | 42 | 16 (38.1) | |
| LVD (mean LVD/field) | 0.0180 | ||
| <12 | 67 | 8 (11.9) | |
| ⩾12 | 50 | 15 (30.0) | |
LVD, podoplanin positive lymph vessel density; LVI‐HE, lymph vessel invasion detected by haematoxylin and eosin staining; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry; OS, overall survival.
Figure 2 Association of LVD (podoplanin positive lymph vessel density) and LVI‐IHC (lymph vessel invasion detected by podoplanin immunohistochemistry) with patients' prognosis in gastric cancer (Kaplan–Meier method and log rank test). High (A) LVD (p = 0.0180) or (B) LVI‐IHC (p = 0.0001) was significantly related to death.
Table 4 Results of multivariate Cox regression analysis for OS in 117 patients with gastric cancer.
| Variable | Odds ratio (95% CI) | p Value |
|---|---|---|
| Depth of invasion | 0.0118 | |
| PT1–2 | 1.0 (referent) | |
| PT3–4 | 5.836 (1.478 to 23.044) | |
| Lymph node metastasis | 0.8056 | |
| Negative | 1.0 (referent) | |
| Positive | 1.261 ( 0.199 to 7.978) | |
| TNM stage | 0.0492 | |
| I | 1.0 (referent) | |
| II–IV | 15.824 (1.010 to 248.021) | |
| LVD (mean LVD/field) | 0.0422 | |
| <12 | 1.0 (referent) | |
| ⩾12 | 2.487 (1.032 to 5.993) | |
| LVI‐IHC | 0.5257 | |
| Negative | 1.0 (referent) | |
| Positive | 1.350 (0.534 to 3.414) |
LVD, podoplanin positive lymph vessel density; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry; OS, overall survival.
Correlation between LVD or LVI and lymph node metastasis in T1 early gastric cancer
To assess whether LVD or LVI had any value or relevance with respect to predicting lymph node metastasis in EMR specimens, we examined the correlation between LVD or LVI and lymph node metastasis in 73 patients with T1 early gastric cancer, in which the tumour was confined to the mucosa (32 cases) or submucosa (41 cases). The mean (SD) LVD of 73 cases with T1 early gastric cancer was 11.1 (7.4)/case. As shown in table 5, LVD correlated with depth of invasion (p = 0.0008), lymph node metastasis (p = 0.0094), LVI‐HE (p = 0.0223), and LVI–IHC (p < 0.001). A significant correlation between LVI–IHC and lymph node metastasis was also noted (p = 0.0398). LVI‐HE was identified in only two of 73 cases, and LVI‐IHC was found in 14 cases. In HE staining, no association was detected between lymphatic invasion and lymph node metastasis (p = 0.2329; table 6). As shown in table 7, multivariate logistic regression analysis of the depth of invasion, LVD, and LVI‐IHC focusing on lymph node metastasis identified LVD as an independent significant predictor of lymph node metastasis (p = 0.0414). The odds ratio for LVD was 6.24.
Table 5 The relation between LVD and other parameters in 73 patients with T1 gastric cancer.
| Factor | N | Mean (SD) LVD/field | p Value |
|---|---|---|---|
| Sex | 0.2967 | ||
| Male | 52 | 10.50 (7.16) | |
| Female | 21 | 12.57 (8.06) | |
| Depth of invasion | 0.0008 | ||
| Mucosa | 32 | 8.06 (6.02) | |
| Submucosa | 41 | 13.6 (7.64) | |
| Lymph node metastasis | 0.0094 | ||
| Negative | 64 | 10.25 (7.09) | |
| Positive | 9 | 17.11 (7.54) | |
| Venous invasion | 0.3299 | ||
| Negative | 72 | 11.18 (7.46) | |
| Positive | 1 | 5 | |
| LVI‐HE | 0.0223 | ||
| Negative | 71 | 10.62 (6.97) | |
| Positive | 2 | 28 (2.83) | |
| LVI‐IHC | <0.001 | ||
| Negative | 59 | 9.21 (6.17) | |
| Positive | 14 | 18.40 (7.64) |
LVD, podoplanin positive lymph vessel density; LVI‐HE, lymph vessel invasion detected by haematoxylin and eosin staining; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry.
Table 6 The relation between lymph node metastasis and LVI in 73 patients with T1 gastric cancer.
| Factors | Lymph node metastasis | ||
|---|---|---|---|
| Negative | Positive | p Value | |
| LVI‐HE | 0.2329 | ||
| Negative | 63 | 8 | |
| Positive | 1 | 1 | |
| LVI‐IHC | 0.0398 | ||
| Negative | 54 | 5 | |
| Positive | 10 | 4 | |
LVD, podoplanin positive lymph vessel density; LVI‐HE, lymph vessel invasion detected by haematoxylin and eosin staining; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry.
Table 7 Results of multivariate logistic regression analysis for lymph node metastasis in 73 patients with T1 gastric cancer.
| Variable | Odds ratio (95% CI) | p Value |
|---|---|---|
| Depth of invasion | 0.8717 | |
| Mucosa | 1.0 (referent) | |
| Submucosa | 1.179 (0.158 to 10.459) | |
| LVD (mean LVD/field) | 0.0414 | |
| <12 | 1.0 (referent) | |
| ⩾12 | 6.237 (1.204 to 47.745) | |
| LVI‐IHC | 0.3292 | |
| Negative | 1.0 (referent) | |
| Positive | 2.496 (0.390 to 16.845) |
LVD, podoplanin positive lymph vessel density; LVI‐IHC, lymph vessel invasion detected by podoplanin immunohistochemistry.
Discussion
Tumour metastasis may depend on the capacity of tumour cells to induce angiogenesis and/or lymphangiogenesis. For tumours to spread to regional lymph nodes, cancer cells must first invade the lymphatic system. In various human cancers, it is not known whether this is achieved through the formation and invasion of newly induced lymphatics from within the tumour (tumour lymphangiogenesis), or by expansion and invasion of pre‐existing lymphatics at the tumour periphery.19 This issue remains unsolved because of the absence of specific markers for lymphatics and the lack of detailed knowledge concerning the molecular mechanisms of lymphangiogenesis.
Progress in finding markers for the identification of lymphatic vessels came with the discovery of podoplanin.1 Immunohistochemical staining with antibodies to purified podoplanin revealed its expression in the small lymphatic vessels of normal skin and kidney.2 The function of podoplanin is currently unknown. Podoplanin is a potentially useful marker for lymphatic endothelium and further characterisation of its expression in normal and tumour associated lymphatics is needed.
“Our findings suggest that lymphatic metastasis may be achieved through the formation and invasion of newly induced lymphatics both within the tumour and in the tumour periphery”
In our study, podoplanin immunohistochemistry revealed that lymph vessels were present in all of the gastric carcinomas examined. Lymph vessels were restricted to the endothelium not only in peritumorous lesions but also in intratumorous lesions. LVD assessment demonstrated that it was significantly correlated with lymph node metastasis. Furthermore, LVI‐IHC also correlated with lymph node metastasis. Our findings suggest that lymphatic metastasis may be achieved through the formation and invasion of newly induced lymphatics both within the tumour and in the tumour periphery. In addition, survival curves determined by the Kaplan–Meier method and univariate analysis demonstrated that high LVD and LVI‐IHC were negatively associated with OS. In addition, based on multivariate Cox regression analysis, LVD was identified as an independent prognostic factor.
Recent advances have enabled the early detection of gastric carcinoma by endoscopy and treatment by means of EMR. However, even when the carcinoma is completely resected by EMR, additional surgery is necessary when lymph node metastasis appears likely, or when cancer cells have invaded the submucosa.20,21 Risk factors for nodal metastasis in T1 early gastric cancer include submucosal invasion, tumour size, and histological type.21 However, the absence of useful indicators for lymph node metastasis in T1 early gastric cancer has made the prediction of lymph node metastasis difficult. Thus, a reliable marker for lymph node metastasis that could be evaluated in EMR specimens would be very useful. In our study, LVD was correlated with lymph node metastasis and LVI‐IHC. A significant correlation between LVI‐IHC and lymph node metastasis was also noted in T1 early gastric cancer. In these cases, lymphatic invasion was clearly identified using podoplanin IHC. Furthermore, there was a significant association between lymphatic invasion and lymph node metastasis detected by podoplanin IHC but not by HE staining. Our results showed that LVD assessment may be a useful predictor of lymph node metastasis, or may become a decision making factor for additional surgery in T1 early gastric cancer.
Take home messages
Increased podoplanin expression, a measure of lymph vessel density (LVD), is significantly associated with lymph node metastasis, and may play an important role in detecting lymph node metastasis in gastric cancer
LVD may be a significant prognostic factor in gastric cancer at any stage
LVD and lymph vessel invasion detected by podoplanin immunohistochemistry are associated with lymph node metastasis in T1 early gastric cancer
LVD assessment by podoplanin immunohistochemistry may become a useful predictor of lymph metastasis in T1 early gastric cancer and may influence the decision making process for additional surgery
Acknowledgements
We thank A Tsukiyama, Department of Pathology, Osaka Police Hospital, Osaka, Japan; and E Taniguchi, Department of Pathology, Wakayama Medical University, Wakayama, Japan, for expert technical assistance.
Abbreviations
EMR - endoscopic mucosal resection
HE - haematoxylin and eosin
IHC - immunohistochemistry
LVD - podoplanin positive lymph vessel density
LVI‐HE - lymph vessel invasion detected by haematoxylin and eosin staining
LVI‐IHC - lymph vessel invasion detected by podoplanin immunohistochemistry
OS - overall survival
References
- 1.Breiteneder‐Geleff S, Matsui K, Soleiman A.et al Podoplanin, novel 43‐kd membrane protein of glomerular epithelial cells, is down‐regulated in puromycin nephrosis. Am J Pathol 19971511141–1152. [PMC free article] [PubMed] [Google Scholar]
- 2.Breiteneder‐Geleff S, Soleiman A, Kowalski H.et al Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999154385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birner P, Schindl M, Obermair A.et al Lymphatic microvessel density in epithelial ovarian cancer: its impact on prognosis. Anticancer Res 2000202981–2985. [PubMed] [Google Scholar]
- 4.Birner P, Obermair A, Schindl M.et al Selective immunohistochemical staining of blood and lymphatic vessels reveals independent prognostic influence of blood and lymphatic vessel invasion in early‐stage cervical cancer. Clin Cancer Res 2001793–97. [PubMed] [Google Scholar]
- 5.Birner P, Schindl M, Obermair A.et al Lymphatic microvessel density as a novel prognostic factor in early‐stage invasive cervical cancer. Int J Cancer 20019529–33. [DOI] [PubMed] [Google Scholar]
- 6.Schoppmann S F, Birner P, Studer P.et al Lymphatic microvessel density and lymphovascular invasion assessed by anti‐podoplanin immunostaining in human breast cancer. Anticancer Res 2001212351–2355. [PubMed] [Google Scholar]
- 7.Karpanen T, Egeblad M, Karkkainen M J.et al Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001611786–1790. [PubMed] [Google Scholar]
- 8.Mandriota S J, Jussila L, Jeltsch M.et al Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumour metastasis. EMBO J 200120672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacker S A, Caesar C, Baldwin M E.et al VEGF‐D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 20017186–191. [DOI] [PubMed] [Google Scholar]
- 10.Skobe M, Hawighorst T, Jackson D G.et al Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 20017192–198. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu K, Kubo H, Yamaguchi K.et al Suppression of VEGFR‐3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci 200495328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoppmann S F, Bayer G, Aumayr K.et al Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 2004240306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Yasuoka H, Tsujimoto M.et al Lymph vessel density correlates with nodal status, VEGF‐C expression, and prognosis in breast cancer. Breast Cancer Res Treat 200591125–132. [DOI] [PubMed] [Google Scholar]
- 14.Hirota T, Ming S C. Early gastric carcinoma. In: GH Ming SC, ed. Pathology of the gastrointestinal tract. Philadelphia: WB Saunders, 1992570–583.
- 15.Hirota T, Ming S C, Itabashi M. Pathology of early gastric cancer. In: Nishi M, Ichikawa H, Nakajima T, Maruyama K, et al, eds. Gastric cancer. Tokyo: Springer‐Verlag, 199366–87.
- 16.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma. 13th ed. Tokyo: Kanehara Publishing, 1999 [DOI] [PubMed]
- 17.Weidner N, Semple J P, Welch W R.et al Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 19913241–8. [DOI] [PubMed] [Google Scholar]
- 18.Heimburg S, Oehler M K, Papadopoulos T.et al Prognostic relevance of the endothelial marker CD 34 in ovarian cancer. Anticancer Res 1999192527–2529. [PubMed] [Google Scholar]
- 19.Pepper M S. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res 20017462–468. [PubMed] [Google Scholar]
- 20.Ono H, Kondo H, Gotoda T.et al Endoscopic mucosal resection for treatment of early gastric cancer. Gut 200148225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folli S, Morgagni P, Roviello F.et al Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian research group for gastric cancer (IRGGC). Jpn J Clin Oncol 200131495–499. [DOI] [PubMed] [Google Scholar]