Abstract
Large cell neuroendocrine carcinoma (LCNEC) is a high grade type of neuroendocrine tumour with an aggressive clinical course. This report describes the first case of LCNEC combined with an adenocarcinoma component in the common bile duct. A 68 year old man presented with jaundice. Severe stenosis of the bile duct was revealed by endoscopic retrograde cholangiography, and adenocarcinoma cells were detected by brush cytology. Pancreaticoduodenectomy was performed, and the patient died of disease three months after surgery. A tumour measuring 2.0 cm in diameter was located in the intrapancreatic portion of the bile duct. Histologically, the tumour consisted of a LCNEC component and a well differentiated adenocarcinoma component. There were transitional areas between the two components. Immunohistochemically, LCNEC cells were reactive for neuroendocrine markers, but no specific hormonal expression was found. Chromogranin A positive cells were found in some areas of the adenocarcinoma component. These findings are consistent with the theory that both of the carcinoma components originated from a common pluripotent stem cell.
Keywords: large cell neuroendocrine carcinoma, adenocarcinoma, common bile duct, c‐kit (CD117)
Large cell neuroendocrine carcinoma (LCNEC) is a high grade type of neuroendocrine tumour, first defined in the lungs by Travis et al.1 Many cases of LCNEC have been reported in the extrapulmonary regions but, to our knowledge, LCNEC arising in the common bile duct has not been described previously in the literature. Here we report the first case of LCNEC, composite with adenocarcinoma, arising in the common bile duct.
Case report
A 68 year old man presented with jaundice. Endoscopic cholangiography showed severe stenosis of the common bile duct and brush cytology detected adenocarcinoma cells. Abdominal computed tomography scans before surgery found no evidence of metastasis to the liver or lymph nodes. The patient was diagnosed as having obstructive jaundice caused by bile duct carcinoma, and underwent pancreaticoduodenectomy with lymph node dissection and cholecystectomy. Computed tomography scans two months after surgery demonstrated massive liver metastasis, and he died of liver failure five months after the initial diagnosis, despite the transient beneficial effect of chemotherapy. No necropsy was performed.
Pathology
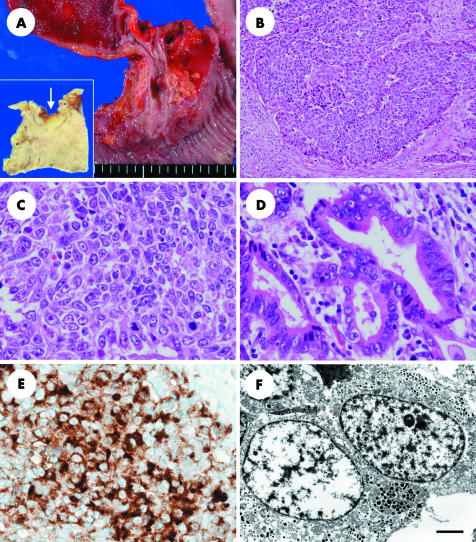
Macroscopically, the surgical specimen showed an infiltrative nodular tumour measuring 2.0 × 0.9 cm located in the intrapancreatic portion of the common bile duct (fig 1A). There were no gallstones in either the gallbladder or bile ducts. Microscopically, the tumour comprised LCNEC and well differentiated adenocarcinoma. The LCNEC component made up approximately 80% of the tumour. The tumour proliferated in a nesting pattern with rosette‐like structures (fig 1B). The tumour cells had nuclei with coarsely granular chromatin and prominent nucleoli, eosinophilic cytoplasm, and polygonal features (fig 1C). The mitotic ratio was high, averaging 43/10 high power fields. There were extensive necrotic foci. The Grimelius stain identified argyrophilic granules in the cytoplasm of the tumour cells, but the Fontana‐Masson argentaffin reaction was negative. The carcinoma permeated the bile duct wall and invaded the pancreas. Massive vascular infiltrations were evident. The adenocarcinoma component occupied a small area of the tumour in the superficial mucosal portion. The carcinoma cells were columnar and had nuclear features similar to LCNEC cells (fig 1D). There were transitional areas between both components. There was no evidence of metastasis in the dissected lymph nodes.
Figure 1 (A) The surgical specimen shows that the tumour is located in the intrapancreatic segment of the common bile duct and measures 2.0 × 0.9 cm. The cut surface of the tumour (arrow) is shown in the inset. (B) Large cell neuroendocrine carcinoma cells proliferate in a nesting pattern with rosette‐like structures; original magnification, ×100. (C) Large cell neuroendocrine carcinoma cells have nuclei with coarsely granular chromatin and prominent nucleoli, eosinophilic cytoplasm, and frequent mitotic figures; original magnification, ×400. (D) Well differentiated adenocarcinoma cells show similar nuclear figures to large cell neuroendocrine carcinoma, but with fewer mitotic figures; original magnification, ×400. (E) Immunohistochemistry for chromogranin A shows positive cytoplasmic staining in large cell neuroendocrine carcinoma cells; original magnification, ×400. (F) Ultrastructure of the large cell neuroendocrine carcinoma cells reveals dense core neurosecretory granules in the cytoplasm. Scale bar, 2 μm.
Immunohistochemically, the LCNEC cells stained positively for chromogranin A (fig 1E), neurone specific enolase, CD56, and cytokeratin (CAM 5.2), but were negative for synaptophysin and CD117 (c‐kit). No specific hormonal expression was seen with immunohistochemistry for adrenocorticotrophic hormone, calcitonin, gastrin, glucagon, growth hormone, human chorionic gonadotrophin, insulin, prolactin, secretin, serotonin, somatostatin, and pancreatic polypeptide. Adenocarcinoma cells were mostly negative for neuroendocrine markers, but there were some areas in which chromogranin A positive cells were identified. In the LCNEC area, 67.5% and 71.4% of cells were positive for p53 and Ki‐67 (MIB‐1), respectively, and in the adenocarcinoma area these figures were 40.0% and 23.6%.
Ultrastructural analysis revealed dense core neurosecretory granules in the cytoplasm (fig 1F).
Discussion
The most common malignancy of the extrahepatic bile ducts is adenocarcinoma, which accounts for more than 90% of tumours in this location.2 Neuroendocrine tumours of the extrahepatic bile ducts are extremely rare and represent less than 0.4%.2 Composite glandular–endocrine cell carcinoma is composed of adenocarcinoma and endocrine cell carcinoma, and endocrine cells form a large proportion of the tumour—at least 30–50%.3 There have been reports of more than 16 cases of pure neuroendocrine carcinoma4 and seven cases of composite glandular–endocrine cell carcinoma of the extrahepatic bile ducts.5,6,7,8,9,10,11 All of the neuroendocrine carcinoma components in these cases were small cell carcinoma (SCC). To the best of our knowledge, only one case of LCNEC of the gallbladder12 and two cases in the ampulla of Vater have been reported,13,14 but none has been described in the common bile duct. In this report, we describe the first case of LCNEC of the common bile duct.
“Large cell neuroendocrine carcinomas (LCNECs) seem to have a highly aggressive nature, and composite glandular–endocrine carcinomas with an LCNEC component have an unfavourable outcome”
There are two possible explanations for a tumour consisting of two different components—neuroendocrine carcinoma and adenocarcinoma. One is a composite glandular–endocrine cell carcinoma, which originates in a common precursor cell, differentiates in two directions, and usually has a transitional zone.3 The other is a collision tumour, in which two separate tumours coincidentally arise next to one another and are closely located but not intermingled.3 In our case, there was a gradual transitional zone between the LCNEC and the adenocarcinoma components. Carcinoma cells immunohistochemically stained for chromogranin A were also found in some adenocarcinoma areas. Therefore, the tumour in our present case is probably a composite tumour arising from multipotential stem cells in accordance with previous reports. Approximately 80% of the present tumour was found to be LCNEC and the remainder was adenocarcinoma. Ki‐67 positivity was much higher in the LCNEC area than in the adenocarcinoma area. Thus, the difference in the area occupied by the two components might be explained by the higher proliferation activity of the LCNEC component.
Take home messages
To the best of our knowledge, this is the first report of a large cell neuroendocrine carcinoma (LCNEC) combined with an adenocarcinoma in the common bile duct.
Histologically, the tumour consisted of a LCNEC component and a well differentiated adenocarcinoma component
Both of the carcinoma components appeared to originate from a common pluripotent stem cell
This tumour type appears to have a poor prognosis
Table 1 summarises the clinicopathological features of composite glandular–endocrine cell carcinoma cases of the common bile duct. Cavazza et al reported a case of LCNEC of the ampulla of Vater, where the tumour measured 3 cm and the patient died of disease eight months after surgery,13 and Cheng et al described a similar tumour that measured 1.8 cm in length and the patient died of disease six months after initial diagnosis.14 LCNEC of the lungs has a remarkably poor prognosis even if the tumour is at an early clinical stage. The survival rate for LCNEC is not significantly different from that seen for SCC in the lungs.1 LCNECs seem to have a highly aggressive nature, and composite glandular–endocrine carcinomas with an LCNEC component have an unfavourable outcome, as in our patient, similar to those with SCC, also in the biliary tract.
Table 1 Clinicopathological features of composite glandular–endocrine cell carcinoma cases of the common bile duct.
| Case | First author | Age/sex | Tumour size (cm) | Tumour histology | Treatment | Prognosis |
|---|---|---|---|---|---|---|
| 1 | Motojima5 | 67/M | 2.5 | SCC>>AC | PD, radiation, mitomycin | DOD, 10 months AS |
| 2 | Tanaka9 | 68/M | 6.0 | SCC+AC | Surgery | Alive, 6 years AS |
| 3 | Kim10 | 64/M | 3 | SCC>AC | PD | Discharged, 30 days AS |
| 4 | Edakuni11 | 82/F | 6 | SCC>AC | PD+CC | Alive, 45 months AS |
| 5 | Present case | 68/M | 2.0 | LCNEC>>AC | PD+CC, cisplatin, etoposide | DOD, 3 months AS |
AC, adenocarcinoma; AS, after surgery; CC, cholecystectomy; DOD, died of disease; F, female; LCNEC, large cell neuroendocrine carcinoma; M, male; PD, pancreaticoduodenectomy; SCC, small cell carcinoma.
Recently, c‐kit protein (CD117) overexpression has been reported in pulmonary LCNEC cells,15 but the LCNEC cells in our present case showed no immunoreactivity for c‐kit. C‐kit is a transmembrane receptor with tyrosine kinase activity, the overexpression of which is related to tumour cell proliferation. There have been reports that overexpression of c‐kit in itself does not correlate with the survival rate. In contrast, Casali et al reported that CD117 protein expression was significantly correlated with poor outcome and recurrence.15 Although it is possible that treatment aimed at specifically inhibiting c‐kit kinase activity will be effective in LCNEC as well as in SCC, if given together with intensive chemotherapy, other therapeutic targets should be selected.
Acknowledgements
The authors thank F Serikawa for electron micrographs and K Okazaki, H Ninomiya, and Y Yanaida for the immunohistochemistry.
Abbreviations
LCNEC - large cell neuroendocrine carcinoma
SCC - small cell carcinoma
Footnotes
Accepted for publication 11 April 2005
References
- 1.Travis W D, Linnoila R I, Tsokos M G.et al Neuroendocrine tumors of the lung with proposed criteria for large‐cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 199115529–553. [DOI] [PubMed] [Google Scholar]
- 2.Carriaga M T, Henson D E. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 199575(suppl 1)171–190. [DOI] [PubMed] [Google Scholar]
- 3.Lewin K. Carcinoid tumors and the mixed (composite) glandular–endocrine cell carcinomas. Am J Surg Pathol 198711(suppl 1)71–86. [DOI] [PubMed] [Google Scholar]
- 4.El Rassi Z S, Mohsine R M, Berger F.et al Endocrine tumors of the extrahepatic bile ducts. Pathological and clinical aspects, surgical management and outcome. Hepatogastroenterology 2004511295–1300. [PubMed] [Google Scholar]
- 5.Motojima K, Furui J, Terada M.et al Small cell carcinoma of the pancreas and biliary tract. J Surg Oncol 199045164–168. [DOI] [PubMed] [Google Scholar]
- 6.Ducla‐Soares J, Ferreira M, Campos C.et al Composite tumor of the main bile duct producing several regulatory peptides. Am J Gastroenterol 199287668–671. [PubMed] [Google Scholar]
- 7.Nishihara K, Tsuneyoshi M, Niiyama H.et al Composite glandular–endocrine cell carcinoma of the extrahepatic bile duct: immunohistochemical study. Pathology 19932590–94. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto J, Abe Y, Nishihara K.et al Composite glandular–neuroendocrine carcinoma of the hilar bile duct: report of a case. Surg Today 199828758–762. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Shibui S, Nomura K.et al Brain metastases from adenoendocrine carcinoma of the common bile duct: a case report. Jpn J Clin Oncol 199929252–255. [DOI] [PubMed] [Google Scholar]
- 10.Kim S H, Park Y N, Yoon D S.et al Composite neuroendocrine and adenocarcinoma of the common bile duct associated with Clonorchis sinensis: a case report. Hepatogastroenterology 200047942–944. [PubMed] [Google Scholar]
- 11.Edakuni G, Sasatomi E, Satoh T.et al Composite glandular–endocrine cell carcinoma of the common bile duct. Pathol Int 200151487–490. [DOI] [PubMed] [Google Scholar]
- 12.Papotti M, Cassoni P, Sapino A.et al Large cell neuroendocrine carcinoma of the gallbladder. Am J Surg Pathol 2000241424–1428. [DOI] [PubMed] [Google Scholar]
- 13.Cavazza A, Gallo M, Valcavi R.et al Large cell neuroendocrine carcinoma of the ampulla of Vater. Arch Pathol Lab Med 2003127221–223. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S P, Yang T L, Chang K M.et al Large cell neuroendocrine carcinoma of the ampulla of Vater with glandular differentiation. J Clin Pathol 2004571098–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casali C, Stefani A, Rossi G.et al The prognostic role of c‐kit protein expression in resected large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg 200477247–252. [DOI] [PubMed] [Google Scholar]