Abstract
Background
Recurrent inflammation in chronic pancreatitis (CP) is not well understood.
Aims
To investigate whether decorin, an extracellular matrix (ECM) proteoglycan with macrophage modulating activity, is a pathogenic factor allowing diseased pancreatic stroma to sustain inflammation by affecting the cytokine profile of accumulating inflammatory cells.
Methods
Decorin was examined in 18 donors and 32 patients with CP by quantitative reverse transcription polymerase chain reaction (QRT-PCR), western blotting, and immunohistochemistry of pancreatic specimens. QRT-PCR was used to assess cytokine expression in donor peripheral blood mononuclear cells (PBMC), exposed or not to decorin in vitro, and to compare it with the cytokine profile of circulating and resident mononuclear cells (MNC) of patients with CP.
Results
In CP, desmoplasia is associated with overexpression of decorin in the growing ECM and enlarged pancreatic nerves. In culture, exposure of MNC to decorin stimulated expression of the MNC recruiting chemokine MCP-1. In biopsies, MNC infiltrates in decorin rich CP tissue showed a 300-fold upregulation of MCP-1 compared with decorin free peripheral blood, whereas no difference was found in basal MCP-1 expression in PBMC of patients versus donors. This effect was specific for MCP1—other inflammatory cytokines, such as interleukin 1β and tumour necrosis factor α, were not affected.
Conclusion
Decorin is a molecular marker of desmoplasia in CP, and excessive decorin may allow fibrotic masses to nourish and protract inflammation by deregulating the process of MNC accumulation and activation. These data provide a molecular basis for surgical resection of diseased tissue as a treatment option in CP.
Keywords: extracellular matrix, decorin, MCP1, chronic pancreatitis, chronic inflammation
Despite different aetiologies, the pancreas of patients with chronic pancreatitis (CP) undergoes dramatic tissue remodelling: degrading acini are replaced by grossly enlarged stroma, saturated with hypertrophic nerves, blood vessels, and leucocyte infiltrates.1,2 The inflammatory foci consist mostly of mononuclear cells (MNC), of which half are T cells, one third are macrophages, and the remainder are B cells and plasma cells.3,4 These MNC, especially macrophages, are believed to be involved in the pathogenesis of CP, but the exact mechanism of MNC activation and action in CP is not well understood.5,6
“Overexpression of certain extracellular matrix proteins (such as decorin) during the ongoing desmoplastic reaction in chronic pancreatitis may be the cause of the altered behaviour of mononuclear cells, thus continuously maintaining the inflammatory process”
Certain chemokines have been implicated in the immunopathogenesis of CP and in other chronic inflammatory diseases, such as glomerulonephritis, rheumatoid arthritis, colitis, and Crohn's disease.7 Monocyte chemoattracting protein 1 (MCP-1; CCL2) appears to be one of the most important chemokines involved in CP.8 CP infiltrating MNC, fibroblasts, nerves, and endothelia can produce MCP-1, but the mechanism of its induction remains unclear. Because the recruitment and activation of MNC depend on the microenvironment, changes in pancreatic parenchyma may play an active role in the induction and regulation of the inflammatory process. Recent data indicate that newly formed fibrotic tissue not only serves as scaffolding for the cells and a reservoir for various growth factors and cytokines, but also contains certain “neutral” extracellular matrix (ECM) proteins, which directly interact with MNC and promote inflammatory reactions.9 The most spectacular finding was the ability of EDA containing fibronectin to activate Toll-like receptor 4 on macrophages, thereby adopting a pathway conventionally used by lipopolysaccharide and causing lipopolysaccharide-like responses.10 Another potential MNC regulator appears to be decorin, an ECM proteoglycan, which belongs to the small leucine rich group of proteins. This fibril associated molecule was previously described as an organiser of the ECM and regulator of collagen fibrillogenesis, which has the ability to interfere with transforming growth factor β1 (TGFβ1).11,12,13 In a mouse model, decorin inhibited the binding of autocrine produced TGFβ1 to macrophages, reversing the repressive effect of TGFβ1 on these cells and enhancing the release of interleukin 1β (IL1β), IL6, tumour necrosis factor α (TNFα), inducible nitric oxide synthetase, and the expression of major histocompatibility complex class II genes.14 In addition, decorin inhibits macrophage colony stimulating factor dependent proliferation by upregulation of p27kip1 and p21warf, and induces cell cycle arrest in the G1 phase without affecting cell viability, thereby protecting macrophages from apoptosis and enhancing their survival.15 The ability of decorin to modulate the interaction of other matrix molecules (such as fibronectin) with cells may be an indirect way of macrophage regulation, considering the described activation of Toll-like receptor 4 on macrophages by fibronectin.16 In addition, decorin contributes to the adhesion of macrophages to ECM, through interaction with class A scavenger receptors on macrophages, enabling retention of these cells in atherosclerotic lesions.17 Therefore, it could be hypothesised that overexpression of certain ECM proteins (such as decorin) during the ongoing desmoplastic reaction in CP may be the cause of the altered behaviour of MNC, thus continuously maintaining the inflammatory process. To gain insight into the pathogenesis of CP, we investigated whether decorin represents a link between the excessive desmoplastic reaction and chronic inflammation. Therefore, we studied the expression and distribution of decorin in normal and diseased pancreatic tissues and analysed whether decorin influences the immune pattern in CP.
Patients and methods
Patients
All tissue specimens analysed in our study were obtained according to the institutional review board approved procedures for consent. Human CP tissue samples were obtained from 32 patients (six female, 26 male; median age, 49 years; range, 17–65; 22 cases of alcoholic and 10 of idiopathic aetiology; 15 patients with diabetes) who underwent pancreatic resection as a result of CP. Patients were either from the University Hospital of Bern (Switzerland) or from the University of Heidelberg (Germany). Surgical procedures consisted of a partial pancreaticoduodenectomy, left pancreatic resection, or duodenum preserving pancreatic head resection.
Normal human pancreatic tissue samples were obtained through an organ donor programme from 18 previously healthy individuals (seven female, 11 male; median age, 43 years; range, 17–62). Normal pancreatic tissue was obtained once other organs were taken for transplantation, but a pancreas recipient was not available.
Tissue sampling
Immediately upon surgical removal, tissue samples were either snap frozen in RNAlater and liquid nitrogen (for RNA and protein extraction) or fixed in 10% buffered formalin solution and embedded in paraffin wax 24 hours later (for histological analysis).
Cultures of PBMC
Venous blood of healthy donors was collected in a pyrogen free, heparin containing system (Sarstedt Monovetten, Nuembrecht, Germany). Peripheral blood mononuclear cells (PBMC) were isolated from aliquots (3 ml) of blood, diluted in 3 ml phosphate buffered saline, and then layered on to 3 ml of Ficoll/Hypaque density gradient (Sigma, Poole, Dorset, UK). After centrifugation at 400 ×g for 30 minutes at room temperature, MNC were recovered from the interphase. They were washed twice with complete RPMI-1640 (supplemented with 10% fetal bovine serum, 5μM 2-mercaptoethanol, glutamine, penicillin/streptomycin) and centrifuged at 250 ×g for 10 minutes at room temperature. MNC were resuspended in complete RPMI-1640, adjusted to a concentration of 2 × 106/ml, and incubated with recombinant decorin (EMP Genetech, Denzlingen, Germany) at a concentration of 10 μg/ml for three hours. Contamination of decorin with endotoxin was controlled by LAL assay (CRL, Sülzfeld, Germany). Incubation of cells with the endotoxin blocking agent Polymixin B (Sigma) served as an additional control. After incubation, cells were suspended in MagNA Pure LC lysis buffer (Roche Diagnostics GmbH, Mannheim, Germany) and kept at −80°C for further mRNA preparation and quantitative reverse transcription polymerase chain reaction (QRT-PCR) analysis.
Human pancreatic stellate cells
Small tissue blocks of pancreas (100–150 mg) were obtained during pancreatic surgery (n = 8). The tissue blocks were cut into small pieces (0.5–1 mm3) and placed in uncoated six well plates (3–5 pieces/well) in the presence of 20% fetal bovine serum in a 1/1 (vol/vol) mixture of Dulbecco's modification of Eagle's medium with Ham's F12 medium. L-glutamine (2mM), penicillin/streptomycin, and amphotericin were freshly added. Fine cut tissue blocks were cultured at 37°C in a 5% CO2 saturated humidified atmosphere. Eighteen hours after seeding, the culture medium was changed, and 24 hours later the small tissue blocks were transferred to new culture plates. The pancreatic stellate cells grew out in high number and purity from the tissue blocks one to three days later. The small tissue blocks were removed after two to three weeks. After reaching confluence, monolayers were trypsinised and passaged 1/3. The purity of the cells was assessed by morphology (most cells were stellate-like with long cytoplasmic extensions, some were also spindle shaped) and cytofilament staining of α smooth muscle actin (> 95%), vimentin (100%), and desmin (20–40%). Only cell populations between passages 4 and 6 were used in the subsequent experiments.
Cultures of human pancreatic stellate cells for QRT-PCR analysis
Human pancreatic stellate cells were seeded in 24 well plates in pancreatic stellate cell growth medium at a concentration of 105 cells/ml and allowed to attach for 48 hours. Afterwards, cells were suspended in MagNA Pure LC lysis buffer (Roche Diagnostics GmbH) to analyse gene expression by QRT-PCR, as described below.
Real time quantitative polymerase chain reaction
All reagents and equipment for mRNA/cDNA preparation were purchased from Roche Applied Science (Mannheim, Germany). mRNA was prepared by automated isolation using the MagNA Pure LC instrument and isolation kits I (for cells) and II (for tissues). cDNA was prepared using the First Strand cDNA synthesis kit for RT-PCR (AMV) according to the manufacturer's instructions. Real time PCR was performed with the LightCycler FastStart DNA SYBR green kit, as described previously.18 Decorin, MCP-1, IL1β, TNFα, hypoxanthine phosphoribosyl transferase (HPRT), and cyclophillin B (CPB) primers were obtained from Search-LC (Heidelberg, Germany). The number of specific transcripts was calculated from the standard curve and further normalised to the average expression of two housekeeping genes, CPB and HPRT, as described previously.18 The data from two independent analyses for each sample and parameter were averaged and presented as adjusted transcripts/μl input cDNA.
Western blot analysis
Western blot analysis was performed as described previously.18 Briefly, proteins were extracted from human normal or CP pancreatic tissues and the protein concentration was measured with the micro-BCA protein assay (Pierce, Rockford, Illinois, USA). A 20 μg aliquot of tissue protein lysate was separated by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and blocked with 5% non-fat milk in Tris buffered saline (TBS; 20mM Tris/HCl, 150mM NaCl) with 0.1% Tween 20 for one hour. The membranes were incubated with the primary antibodies (affinity purified polyclonal goat antihuman decorin IgG; R&D Systems Europe Ltd, Abingdon, Oxford, UK) at 4°C overnight, washed with TBS/0.1% Tween 20 and incubated with horseradish peroxidase conjugated rabbit antigoat IgG for one hour at room temperature. Signal detection was performed using an enhanced chemoluminescence reaction (ECL western blotting detection; Amersham Life Science, Amersham, Little Chalfont, Berkshire, UK). The integrity and quantity of the input proteins was confirmed by performing the same western blot procedure using goat antihuman γ tubullin IgG as the primary antibody and horseradish peroxidase labelled donkey antigoat IgG as the secondary antibody (both Santa Cruz Biotechnologies, Heidelberg, Germany).
Immunohistochemistry
Paraffin wax embedded pancreatic tissue sections (4 μm thick) obtained from patients with CP and normal donors were dewaxed with xylene and rehydrated through graded alcohol solutions. After washing with TBS, endogenous peroxidase activity was quenched by incubating the slides in 0.3% hydrogen peroxide in methanol. To block the non-specific activity of secondary antibodies, slides were treated with non-immune normal rabbit serum (Dako, Zurich, Switzerland) for one hour. After overnight incubation at 4°C with anti-decorin IgG (same as for western blot; diluted to 5 μg/ml in normal rabbit serum) slides were washed with TBS/0.05% Tween 20, incubated with 1/200 diluted rabbit antigoat horseradish peroxidase labelled secondary antibodies (Sigma) for one hour, developed using the Dako Envision+™ System (Dako), and lightly counterstained with Mayer's haematoxylin. As a negative control, consecutive sections of all specimens were incubated either in the absence of primary antibody or with normal goat IgG. The slides were analysed by two independent observers blinded to patient status (JK and FdM), with resolution of any differences by joint review and consultation with a third observer (Dr I Esposito, pathologist at the Institute of Pathology, University of Heidelberg, Germany).
Laser capture microdissection of MNC from pancreatic specimens obtained from patients with CP
To determine the cytokine status of MNC in CP in situ, pancreatic inflammatory infiltrates were microscopically localised, laser captured, and microdissected from frozen tissues. Briefly, frozen sections (10 μm) were prepared using a Leica (CM 3050) cryostat and attached to a PEN membrane coated glass slide (Palm Microlaser Technologies, AG, Bernried, Germany). Afterwards, slides were stained in an ice cold solution of 1.5% eosin Y (Merck, Darmstadt, Germany) and 70% ethanol for 30 seconds, followed by dehydration in ice cold 95% and 100% ethanol, each for 20 seconds. Multiple tissue areas (for each patient: 3–4 areas/tissue section ×8 sections ×8 slides) were microdissected using the Palm Robot Microbeam Instrument (Palm Microlaser Technologies). Collected infiltrates were suspended in MagNA Pure lysis buffer and, after mRNA and cDNA preparation, analysed by QRT-PCR.
Statistical analysis
SPSS® statistical software (version 11.0 for Windows) was used for statistical analysis. The Mann-Whitney test was used to estimate the significance of observed differences and the Spearman rank test to establish a correlation with histological and clinical parameters. Significance was set at p < 0.05.
Results
Decorin expression is highly upregulated in patients with CP
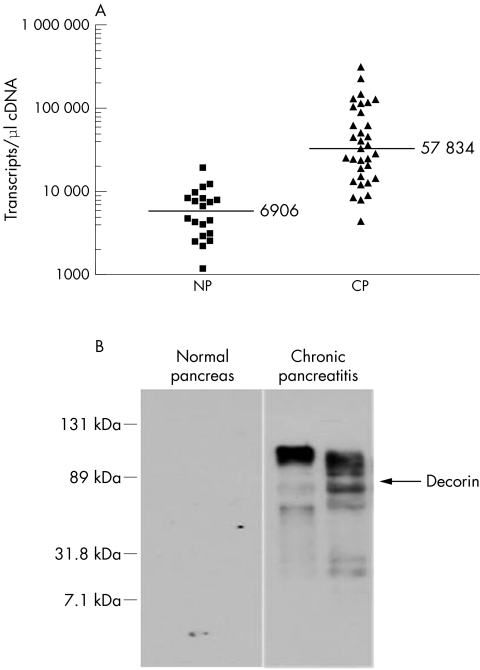
QRT-PCR was performed to evaluate the expression of decorin mRNA in normal and chronically inflamed pancreatic tissue samples. Figure 1A shows an eightfold overexpression of decorin mRNA in tissue in CP (mean, 57 834 (SD, 12 146) transcripts/μl) compared with normal pancreatic tissue (mean, 6906 (SD, 986) transcripts/μl; p < 0.001). However, no correlation was found between the gene expression pattern and the clinical data regarding the aetiology (idiopathic v alcoholic) or the presence of diabetes. Some patient to patient variability in decorin expression was dependent on the degree of the desmoplastic reaction.
Figure 1 Overexpression of decorin in chronic pancreatitis (CP). (A) The expression of decorin mRNA in tissues obtained from the normal pancreas (NP; n = 18) or patients with chronic pancreatitis (n = 32) was analysed by quantitative reverse transcription polymerase chain reaction. Individual data points represent the number of decorin transcripts/μl of input cDNA, normalised to the housekeeping genes hypoxanthine phosphoribosyl transferase and cyclophillin B. (B) The production of decorin protein was analysed in normal pancreatic tissues and CP tissues by western blot analysis. A representative blot showing the accumulation of decorin specific bands and the localisation of recombinant decorin (arrow) is shown. The quantity and quality of the input protein was controlled by probing the stripped blot with γ tubulin (not shown).
The amount of decorin protein in normal pancreatic tissue was below the detection limit of the western blot technique used here. In contrast, a very strong ladder of decorin specific bands (80–120 kDa, depending on individual glycosaminoglycan side chains) was detected in pancreas samples derived from patients with CP (fig 1B).
Localisation of decorin in pancreatic ECM and nerves of patients with CP
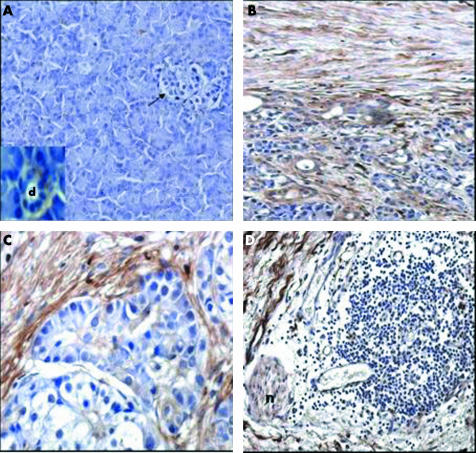
To locate decorin in the tissues, an immunohistochemical analysis was performed in normal (fig 2A) and CP tissue samples (fig 2B–D). In normal pancreatic tissue samples, weak and patchy decorin staining was present in the spare ECM, with a stronger signal around small ducts and vessels (fig 2A and insert). Islets (fig 2A) and acinar cells lacked decorin staining. In contrast, CP tissue samples showed very strong decorin staining in the ECM (fig 2B–D). The strongest decorin staining was seen in ECM localised to the border of residual pancreatic lobules in transition to fibrous tissue. Most interestingly, nerves surrounded by MNC infiltrates showed moderate to strong decorin staining (fig 2D). Analysis of sections under higher magnification indicated that “nerve” decorin was not located in neuronal cells, but in the endonerium, epinerium, and perinerium. Double staining of sections with anti-decorin and anti-smooth muscle actin (a marker of activated pancreatic stellate cells) suggested that pancreatic stellate cells are the main source of decorin (not shown). QRT-PCR analysis of the primary cultures of pancreatic stellate cells confirmed this hypothesis because it revealed high expression of decorin cDNA transcripts by these cells (mean, 140 999 (SD, 27 660) transcripts/μl; n = 7).
Figure 2 Immunohistochemical analysis of decorin in pancreatic tissue sections. (A) Normal pancreas; the arrow indicates an islet; d, duct. (B–D) Chronic pancreatitis; n, nerve. Original magnification, ×100 (A, B, D); ×200 (C).
Influence of decorin on MCP-1 expression in mononuclear cells
To determine whether the overexpressed decorin contributes to the continuous pancreatic MNC recruitment and activation in CP, we analysed the effect of exogenous decorin on MCP-1 production by MNC in vitro and compared these data with the pattern of MCP-1 expression by MNC in the decorin rich environment of CP tissue.
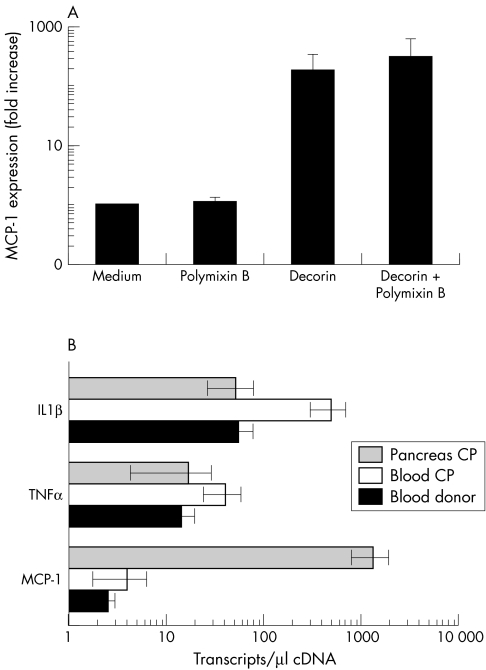
Incubation of PBMC with 10 μg/ml of recombinant decorin in vitro for three hours induced strong expression of the MCP-1 gene compared with medium treated controls (p < 0.05; fig 3A). The endotoxin inhibiting agent Polymixin B (fig 3A) did not abolish the MCP-1 stimulating activity of decorin. Interestingly, the expression of other inflammatory cytokines, such as IL1β and TNFα, was not altered by decorin (data not shown).
Figure 3 Impact of decorin on monocyte chemoattracting protein 1 (MCP-1) expression in mononuclear cells (MNC). MNC were isolated from blood by gradient centrifugation (A) or from stroma embedded pancreatic inflammatory infiltrates by laser capture microdissection (B). (A) In vitro, MNC isolated from donors were exposed to decorin (10 μg/ml) in the presence (or not) of 150μM Polymixin B. Chemokine expression was analysed by quantitative reverse transcription polymerase chain reaction (QRT-PCR) using mRNA prepared from MNC after three hours of stimulation. To compare samples, MCP-1 expression was normalised using the housekeeping genes hypoxanthine phosphoribosyl transferase and cyclophillin B and presented as a ratio of transcripts detected in stimulated versus non-stimulated cultures (fold increase over medium). A summary of three independent experiments using different donors is presented. (B) In situ, circulating (decorin negative environment) and resident (decorin positive environment) MNC were compared for their ability to express MCP-1, tumour necrosis factor α (TNFα) and interleukin 1β (IL1β). Blood from donors (n = 6) and patients with CP (n = 7) and CP tissue (n = 4) were used for MNC isolation, mRNA preparation, and QRT-PCR analysis. Normalisation of expression was performed as described above.
To assess the in vivo relevance of this finding, we compared in situ MCP-1 expression in PBMC versus MNC from laser capture microdissected pancreatic inflammatory infiltrates (fig 3B). To avoid contamination, pure stroma without cellular infiltrates was also laser capture microdissected and analysed for MCP-1 expression. Surprisingly, the constitutive expression of MCP-1 in circulating MNC did not differ between donors and patients with CP, whereas IL1β and TNFα were upregulated in the patient's PBMC. However, the number of MCP-1 transcripts expressed in CP MNC of pancreatic tissue was 300-fold higher than that in circulating PBMC (compared with < 10 copies/μl cDNA in pure stroma). In contrast to MCP-1, expression of IL1β and TNFα was lower in tissue than in CP PBMC. Although MCP-1 expression was lower than that of IL1β and TNFα in the blood of both donors and patients with CP, the pattern was reversed in CP MNC infiltrates, where IL1β and TNFα expression was much lower than that of MCP-1, resembling the profile of decorin induced cytokine production in vitro.
Discussion
Our study shows for the first time that decorin is overexpressed in the pancreas of patients suffering from CP. Decorin expression was high in the ECM of all patients, independent of the underlying aetiology of CP. Decorin is known to exert both profibrotic and antifibrotic functions, depending on the experimental system.11,12,19,20,21 For example, decorin was used as an antifibrotic agent to reduce muscle fibrosis after injury.22 However, in several syndromes characterised by progressive fibrosis (cirrhosis of the liver, renal glomerulosclerosis, pulmonary granuloma), overexpression of decorin was associated with the severity of the fibrotic reaction.23,24,25,26,27,28,29 The antifibrotic activity of decorin was initially linked to its ability to bind and retain TGFβ1, preventing its downstream signalling.13 However, other data showed that raised amounts of decorin in TGFβ1 transgenic mice did not inhibit the action of TGFβ1 in the liver.30 In our opinion, decorin contributes to the progressive fibrotic reaction in CP and, as in cirrhosis of the liver, is a product of activated fibroblasts; namely, the pancreatic stellate cells. Until recently, ECM proteoglycans were considered to be harmless constituents of newly formed fibrotic tissue. However, increasing evidence indicates the crucial impact of these proteins on the outcome of inflammatory diseases and cancer.9,31,32
CP is a progressive inflammatory disorder, characterised by an irreversible loss of pancreatic parenchyma, with a consequent decline in exocrine and endocrine functions.1,2 The histological hallmarks of CP are extreme desmoplasia, proliferation of nerves and blood vessels, and massive penetration of the pancreas by inflammatory cells. Despite evidence that cytokines, chemokines, and infiltrating MNC are involved in the pathogenesis of CP, the major question of a molecular factor governing the switch from acute to chronic inflammation, and the mechanism responsible for continuous inflammation in the absence of primary stimulus remains unsolved. This question is of particular importance, because it would help to explain the observation that surgical resection of the inflammatory mass in the pancreatic head of patients with advanced CP can influence the natural course of the disease.33,34 According to our data, the main factors leading to the chronicity of the inflammatory process are probably in the fibrotic tissue.
“In our opinion, decorin contributes to the progressive fibrotic reaction in chronic pancreatitis and, as in cirrhosis of the liver, is a product of activated fibroblasts; namely, the pancreatic stellate cells”
The resolution of an inflammatory process is dependent on the proper localisation and activation of lymphocytes and macrophages. Therefore, after having shown that decorin was highly upregulated and abundantly expressed in the pancreatic ECM of patients with CP, we hypothesised that decorin, previously shown to modulate some macrophage functions, may provide a maintaining structural stimulus for the ongoing inflammatory process in CP.14,15,17 Because the chemokine MCP-1 has been implicated in the pathogenesis of various disorders characterised by chronic inflammation—including psoriasis, rheumatoid arthritis, and CP—we investigated whether there was a link between decorin and MCP-1.8,35,36,37 We found that decorin induced the expression of MCP-1 in mononuclear cells in vitro. This effect was specific, because other inflammatory cytokines, such as IL1 and TNFα, were not decorin specific targets. In addition, a comparison of cytokine expression by circulating MNC and pancreas bound resident MNC revealed a similar pattern (strong upregulation of MCP-1, but not IL1β and TNFα genes). Such a biased induction may have clear clinical consequences. Signalling through CCR2 (and possibly, other G protein coupled receptors), MCP-1 is able to attract monocytes, dendritic cells, and T cells to the sites of inflammation.38 Although some reports endowed MCP-1 with direct macrophage activating activity,39 MCP-1 was found to induce a mechanism that prevents the effective resolution of inflammation: through the reduction of IL12 production by macrophages and the enhancement of IL4 production by activated T cells, MCP-1 promotes T helper type 2 (Th2) effector cell development.40 Although the physiological importance of this pathway was supported by reduced Th2 responses and an overt reaction to infection in MCP-1 depleted mice, the puzzling shift from Th1 to Th2 responses was seen in CCR2 deficient mice.41 In addition to the proposed existence of additional receptors for MCP-1, the ability of CCR2 to bind and deplete its ligands was anticipated as a possible explanation. For example, CCL7 binds CCR2 and CCR3. In the absence of CCR2, CCL7 may cause increased CCR3 triggering, favouring Th2 responses over Th1 responses.40 Similarly, one can speculate that the high concentrations of MCP-1 in CP may saturate CCR2 and thus redirect CCL7 to CCR3. The result of such a scenario in CP would be insufficient production of TNFα, IL1β, IL12, and interferon γ, which are necessary for the appropriate activation and execution of the antigen presenting, phagocytic, and cytotoxic functions of macrophages. Together with the previously described ability of decorin to increase the survival and the scavenger receptor mediated retention of macrophages,15,17 this suggests that decorin may be a crucial factor for the continuous MCP-1 mediated recruitment of non-optimally activated macrophages and lymphocytes in foci that are remote from areas containing damaged acini. Certainly, we cannot exclude the possibility that other surrounding factors may alter the responses of MNC to decorin in diseased pancreatic tissue.
Take home messages
Decorin was overexpressed in chronic pancreatitis (CP) and is a molecular marker of desmoplasia in CP
Excessive decorin expression may contribute to the imbalance of stromal–neuro–immune interactions in pancreatic tissue, resulting in neurotrophy and continuous monocyte chemoattracting protein 1 mediated recruitment and deregulation of mononuclear cells
The hypertrophic stroma may be the driving force behind the chronic inflammation and continuous tissue destruction seen in patients with CP
These data provide a molecular basis for surgical resection of diseased tissue as a treatment option in CP
Our finding that pancreatic nerves are stained with decorin specific antibodies requires special attention. Although it is not clear yet whether neural structures produce decorin themselves or just bind pancreatic stellate cell released proteoglycan, this localisation of decorin is intriguing in light of the recently described ability of decorin to promote axon growth and the frequent localisation of MNC infiltrates in close proximity to nerves in CP. Injury of these hypertrophic nerves by MNC may be an additional factor responsible for the severe pain attacks experienced by patients with CP.42
In conclusion, being a structural element of ECM in CP, accumulated decorin may contribute to the imbalance of stromal–neuro–immune interactions in pancreatic tissue, resulting in neurotrophy and continuous MCP-1 mediated recruitment and deregulation of MNC. Decorin might perpetuate inflammation in CP in the absence of primary stimuli. This finding is of particular importance because it suggests that the hypertrophic stroma may be the driving force behind the chronic inflammation and continuous tissue destruction seen in patients with CP, and supports the role of pancreatic head resection as a treatment in patients with advanced disease.33,34
Abbreviations
CP - chronic pancreatitis
CPB - cyclophillin B
ECM - extracellular matrix
IL - interleukin
HPRT - hypoxanthine phosphoribosyl transferase
MCP-1 - monocyte chemoattracting protein 1
MNC - mononuclear cells
PBMC - peripheral blood mononuclear cells
TBS - Tris buffered saline
TGFβ1 - transforming growth factor β1
Th1/2 - T helper type 1/2
TNFα - tumour necrosis factor α
QRT-PCR - quantitative reverse transcription polymerase chain reaction
References
- 1.Shrikhande S V, Martignoni M E, Shrikhande M.et al Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg 2003901565–1572. [DOI] [PubMed] [Google Scholar]
- 2.Bockman D E, Muller M, Buchler M.et al Pathological changes in pancreatic ducts from patients with chronic pancreatitis. Int J Pancreatol 199721119–126. [DOI] [PubMed] [Google Scholar]
- 3.Emmrich J, Weber I, Nausch M.et al Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion 199859192–198. [DOI] [PubMed] [Google Scholar]
- 4.Bateman A C, Turner S M, Thomas K S.et al Apoptosis and proliferation of acinar and islet cells in chronic pancreatitis: evidence for differential cell loss mediating preservation of islet function. Gut 200250542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goecke H, Forssmann U, Uguccioni M.et al Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery 2000128806–814. [DOI] [PubMed] [Google Scholar]
- 6.Hunger R E, Mueller C, Z'Graggen K.et al Cytotoxic cells are activated in cellular infiltrates of alcoholic chronic pancreatitis. Gastroenterology 19971121656–1663. [DOI] [PubMed] [Google Scholar]
- 7.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998338436–445. [DOI] [PubMed] [Google Scholar]
- 8.Saurer L, Reber P, Schaffner T.et al Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology 2000118356–367. [DOI] [PubMed] [Google Scholar]
- 9.Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 2001189266–274. [DOI] [PubMed] [Google Scholar]
- 10.Okamura Y, Watari M, Jerud E S.et al The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 200127610229–10233. [DOI] [PubMed] [Google Scholar]
- 11.Ameye L, Young M F. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 200212107R–16R. [DOI] [PubMed] [Google Scholar]
- 12.Danielson K G, Baribault H, Holmes D F.et al Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997136729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Mann D M, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990346281–284. [DOI] [PubMed] [Google Scholar]
- 14.Comalada M, Cardo M, Xaus J.et al Decorin reverses the repressive effect of autocrine-produced TGF-beta on mouse macrophage activation. J Immunol 20031704450–4456. [DOI] [PubMed] [Google Scholar]
- 15.Xaus J, Comalada M, Cardo M.et al Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27(Kip1) and p21(Waf1). Blood 2001982124–2133. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt G, Robenek H, Harrach B.et al Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol 19871041683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago-Garcia J, Kodama T, Pitas R E. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem 20032786942–6946. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Kleeff J, Li J.et al Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene 20042371–81. [DOI] [PubMed] [Google Scholar]
- 19.Fukui N, Fukuda A, Kojima K.et al Suppression of fibrous adhesion by proteoglycan decorin. J Orthop Res 200119456–462. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura N, Hart D A, Boorman R S.et al Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res 200018517–523. [DOI] [PubMed] [Google Scholar]
- 21.Giri S N, Hyde D M, Braun R K.et al Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol 1997541205–1216. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Li Y, Foster W.et al Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 200328365–372. [DOI] [PubMed] [Google Scholar]
- 23.Dudas J, Kovalszky I, Gallai M.et al Expression of decorin, transforming growth factor-beta 1, tissue inhibitor metalloproteinase 1 and 2, and type IV collagenases in chronic hepatitis. Am J Clin Pathol 2001115725–735. [DOI] [PubMed] [Google Scholar]
- 24.Kovalszky I I, Nagy J O, Gallai M.et al Altered proteoglycan gene expression in human biliary cirrhosis. Pathol Oncol Res 1997351–58. [DOI] [PubMed] [Google Scholar]
- 25.Hogemann B, Edel G, Schwarz K.et al Expression of biglycan, decorin and proteoglycan-100/CSF-1 in normal and fibrotic human liver. Pathol Res Pract 1997193747–751. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer L, Raslik I, Grone H J.et al Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J 200115559–561. [DOI] [PubMed] [Google Scholar]
- 27.Cs-Szabo G, Ragasa-San Juan D, Turumella V.et al Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine 2002272212–2219. [DOI] [PubMed] [Google Scholar]
- 28.Malmstrom J, Larsen K, Hansson L.et al Proteoglycan and proteome profiling of central human pulmonary fibrotic tissue utilizing miniaturized sample preparation: a feasibility study. Proteomics 20022394–404. [DOI] [PubMed] [Google Scholar]
- 29.Bensadoun E S, Burke A K, Hogg J C.et al Proteoglycans in granulomatous lung diseases. Eur Respir J 1997102731–2737. [DOI] [PubMed] [Google Scholar]
- 30.Kovalszky I, Nagy P, Szende B.et al Experimental and human liver fibrogenesis. Scand J Gastroenterol Suppl 199822851–55. [DOI] [PubMed] [Google Scholar]
- 31.Bissell M J, Radisky D. Putting tumours in context. Nat Rev Cancer 2001146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbett S A, Schwarzbauer J E. Fibronectin–fibrin cross-linking; a regulator of cell behavior. Trends Cardiovasc Med 19988357–362. [DOI] [PubMed] [Google Scholar]
- 33.Beger H G, Schlosser W, Friess H M.et al Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg 1999230512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartel M, Tempia-Caliera A A, Wente M N.et al Evidence-based surgery in chronic pancreatitis. Langenbecks Arch Surg 2003388132–139. [DOI] [PubMed] [Google Scholar]
- 35.Daly C, Rollins B J. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation 200310247–257. [DOI] [PubMed] [Google Scholar]
- 36.Ross E L, D'Cruz D, Morrow W J. Localized monocyte chemotactic protein-1 production correlates with T cell infiltration of synovium in patients with psoriatic arthritis. J Rheumatol 2000272432–2443. [PubMed] [Google Scholar]
- 37.Ellingsen T, Buus A, Stengaard-Pedersen K. Plasma monocyte chemoattractant protein 1 is a marker for joint inflammation in rheumatoid arthritis. J Rheumatol 20012841–46. [PubMed] [Google Scholar]
- 38.Charo I F, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T cell polarization. Microcirculation 200310259–264. [DOI] [PubMed] [Google Scholar]
- 39.Biswas S K, Sodhi A. In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J Interferon Cytokine Res 200222527–538. [DOI] [PubMed] [Google Scholar]
- 40.Luther S A, Cyster J G. Chemokines as regulators of T cell differentiation. Nat Immunol 20012102–107. [DOI] [PubMed] [Google Scholar]
- 41.Traynor T R, Herring A C, Dorf M E.et al Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol 20021684659–4666. [DOI] [PubMed] [Google Scholar]
- 42.Davies J E, Tang X, Denning J W.et al Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci 2004191226–1242. [DOI] [PubMed] [Google Scholar]