Abstract
Background/Aims
Idiopathic interstitial pneumonias (IIPs) are a diverse grouping of chronic pulmonary diseases characterised by varying degrees of pulmonary fibrosis. The triggers of the fibroproliferative process in IIP remain enigmatic but recent attention has been directed towards chemokine involvement in this process.
Methods
The expression of two chemokine receptors, CCR7 and CXCR4, and their respective ligands, CCL19, CCL21, and CXCL12, were examined in surgical lung biopsies (SLBs) from patients with IIP. Transcript and protein expression of these receptors and their ligands was compared with that detected in histologically normal margin SLBs.
Results
CCR7 and CXCR4 were detected by gene array and real time polymerase chain reaction analysis and CCR7, but not CXCR4, expression was significantly raised in usual interstitial pneumonia (UIP) relative to biopsies from patients diagnosed with non‐specific interstitial pneumonia (NSIP) or respiratory bronchiolitis/interstitial lung disease (RBILD). CCR7 protein was expressed in interstitial areas of all upper and lower lobe UIP SLBs analysed. CCR7 expression was present in 50% of NSIP SLBs, and CCR7 was restricted to blood vessels and mononuclear cells in 75% of RBILD SLBs. Immune cell specific CXCR4 expression was seen in IIP and normal margin biopsies. CCR7 positive areas in UIP biopsies were concomitantly positive for CD45 (the leucocyte common antigen) but CCR7 positive areas in all IIP SLBs lacked the haemopoietic stem cell antigen CD34, collagen 1, and α smooth muscle actin.
Conclusion
This molecular and immunohistochemical analysis showed that IIPs are associated with abnormal CCR7 transcript and protein expression.
Keywords: chemokine, chemokine receptor, idiopathic interstitial pneumonia, fibrocyte
The major factors driving the dominant pulmonary fibrotic response associated with various histologically distinct forms of idiopathic interstitial pneumonia (IIP) remain poorly defined, thereby contributing to the lack of effective clinical treatments for these diseases.1 Although many of these diseases exhibit a fibroproliferative response in the alveolar microenvironment leading to respiratory impairment, the degree of fibrotic change varies considerably among them.2,3 Equally perplexing is the clear demonstration that anti‐inflammatory agents provide therapeutic benefit in less severe forms of IIP, such as non‐specific interstitial pneumonia (NSIP) and respiratory bronchiolitis/interstitial lung disease (RBILD), whereas they often fail to prevent respiratory failure in patients with the most severe and deadly form of IIP—usual interstitial pneumonia (UIP).4,5,6 The existence of distinct foci of fibroblasts, or fibroblastic foci, is an important pathological feature associated with a poor prognosis and a fatal outcome.7 Newer treatments may follow after the elucidation of the shared and divergent events that contribute to pulmonary fibrotic changes during the initiation and maintenance of IIP.8
“The existence of distinct foci of fibroblasts, or fibroblastic foci, is an important pathological feature associated with a poor prognosis and a fatal outcome in idiopathic interstitial pneumonia”
CC chemokine receptor 7 (CCR7; which binds MIP‐3β/CCL19 and 6‐Ckine/CCL219) and CX chemokine receptor 4 (CXCR4; which binds SDF‐1/CXCL1210) are chemokine receptors that were widely recognised as being limited in their expression to cells of haemopoietic origin.11,12 During immune responses, CCR7 expression facilitates the movement of mature dendritic cells13 to lymphoid tissues, regulates the localisation of T helper type 1 and 2 cells within the spleen,14 and directs T helper type 2 cells to the allergic lung.15 CXCR4 expression and effects were largely limited to bone marrow derived immune cells.16,17 Recent evidence showing that the expression of CCR7 and CXCR4 by breast tumour cells allows these cells to metastasise to the lung has modified this immunocentric paradigm.18 Breast cells do not express these chemokine receptors or respond to the ligands that bind them.18 This novel observation has been extended to several metastasising tumour types, including melanoma19 and various forms of leukaemia.20,21
Through extension to IIP, particularly UIP, the profibrotic mediator milieu present during chronic pulmonary fibrosis may promote CCR7 and/or CXCR4 expression by tissue resident fibroblasts or myofibroblasts. Subsequently, chemokine receptor expression by these cells may permit these resident fibroblasts to become inappropriately activated and/or promote their migration towards areas of focal injury, thereby forming fibroblastic foci. Conversely, recruited collagen producing bone marrow cells or fibrocytes22 may be the key instigators of the fibrotic response. In recent studies examining the involvement of fibrocytes in experimental fibrosis, it has been shown that fibrocytes express functional CCR7 and CXCR4 and that ligands for these receptors regulate their movement into the lung.23,24,25 Thus, the formation of fibroblastic foci and the amplification of the fibrotic response may depend partly on the pathological response of tissue resident fibroblasts to chemokine ligands that they typically have no ability to respond to, or the recruitment of fibrocytes.
In our present study, we first hypothesised that CCR7 and/or CXCR4 expression would be high in IIP surgical lung biopsies (SLBs) and not normal margin SLBs. We tested this hypothesis through a detailed gene transcript and protein analysis of SLBs from IIP and normal margin tumour patient groups. Chemokine and chemokine receptor transcript analysis revealed that CCR7, but not CXCR4, was highly expressed in focal interstitial areas within IIP, particularly all UIP biopsies analysed, but not in normal margin biopsies. We next hypothesised that CCR7 positive areas in IIP biopsies contained fibrocytes. However, given that the cells present in CCR7 positive areas did not coexpress fibrocyte markers, such as CD34 and collagen 1, we cannot conclude that the CCR7 positive cells are bone marrow derived cells. Our final hypothesis was that the CCR7 positive cells in IIP biopsies were activated resident tissue fibroblasts. In addition, our immunohistochemical analysis of SLBs failed to support the hypothesis that the CCR7 positive cells were myofibroblasts, based on the absence of the myofibroblast marker α smooth muscle actin (αSMA). Thus, our study demonstrated increased expression of CCR7 in severe IIP, and this expression was localised to focal fibrotic areas devoid of fibrocytes and myofibroblasts.
Methods
Surgical lung biopsies
The institutional review board at the University of Michigan Medical School approved our study and informed consent was obtained from each patient before inclusion. Members of the Michigan endstage lung disease network referred patients upon suspicion of IIP, as determined from a compilation of clinical,4 physiological,26 radiographical,27 and pathological28 findings. None of the patients enrolled in the present study had undergone previous biopsy surgery or received treatment for IIP. SLBs were performed as part of the evaluation for the University of Michigan Specialized Center of Research in the pathobiology of fibrotic lung disease between May 2000 and August 2004. Histologically normal lung biopsies with no pathological evidence of disease were obtained from the distant margins of resected specimens in patients undergoing thoracic resection for lung cancer. Each SLB was processed separately using sterile technique in a laminar flow hood and processed for molecular, protein, and immunohistochemical analysis (see below).
Preparation of RNA and cDNA from SLBs
The portion of each SLB used for molecular analysis was snap frozen in liquid nitrogen and stored at −80°C. For RNA isolation, these samples were thawed on ice, mechanically homogenised in TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, California, USA), and total RNA was then prepared according to the manufacturer's instructions. Purified RNA from SLBs was subsequently reverse transcribed into cDNA using a BRL reverse transcription kit and oligo (dT) 12–18 primers. The amplification buffer contained 50mM KCl, 10mM Tris/HCl (pH 8.3), and 2.5mM MgCl2.
Gene array analysis
Non‐Rad GEArray gene array membranes from SuperArray Inc (Bethesda, Maryland, USA) were used to analyse changes in gene profiles for human chemokines and chemokine receptors, as described previously.29 Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as the internal positive control because it was consistently expressed in all SuperArrays analysed in our present study.
Real time Taqman PCR analysis
Human CCR7, CXCR4, CCL19, CCL21, and CXCL12 gene expression in IIP and normal margin SLBs was analysed by a real time quantitative reverse transcription polymerase chain reaction (PCR) procedure using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, California, USA), as described previously.30 The cDNAs from upper and lower lobe SLBs were analysed for chemokine receptor, chemokine, and GAPDH (an internal control) expression. All primers and probes used were purchased from Applied Biosystems. Chemokine gene expression was normalised to GAPDH before the fold change in chemokine expression was calculated. The fold increases in cytokine gene expression were calculated by comparing gene expression in all patients with that detected in the upper or lower lobes of the normal margin tumour patient group.
ELISA analysis
Human CCL19, CCL21, and CXCL12 concentrations were measured in 50 µl cell free supernatant samples from homogenised IIP and normal margin SLBs using a standardised sandwich enzyme linked immunosorbent assay (ELISA) technique (R&D Systems, Minneapolis, Minnesota, USA). Recombinant human cytokines were used to generate standard curves from which the concentrations present in the samples were derived. The limit of detection for CCL19, CCL21, and CXCL12 in these ELISAs was consistently above 50 pg/ml. The cytokine values in each sample were normalised to total protein concentrations in each sample using the Bradford assay.
Immunohistochemistry
Routine immunochemistry techniques were used to detect CCR7, CXCR4, CD45, CD34, and αSMA) in SLBs, as described previously.31 The following target antibodies and their appropriate isotype controls were used: mouse antihuman CCR7 antibody and mouse IgMκ purchased from BD Biosciences PharMingen (San Diego, California, USA); mouse antihuman CXCR4 and mouse IgG2B purchased from R&D Systems; mouse antihuman CD45, CD34, and collagen 1 antibodies purchased from Abcam (Cambridge, Massachusetts, USA) and mouse IgG1 purchased from R&D Systems. Upper and lower lobe SLBs from 10 patients with UIP, six with NSIP, and five with RBILD were analysed using these immunohistochemical techniques. The normal margins from resected tumours were analysed in five patients. Serial sections (5 μm thick) were analysed from each SLB and colocalisation of CCR7 with other antigens was performed on immediately adjacent serial sections.
Statistical analysis
All results are expressed as mean (SEM). ANOVA analysis and the Kruskal‐Wallis or the Tukey‐Kramer multiple comparisons tests were used to reveal significant differences among and between the IIP and non‐IIP patient groups. The data were presumed to be non‐parametric because of the inclusion of gene (by Taqman) and protein (by ELISA) values that were below the limit of detection and thus assigned a value of zero. Significance was set at p < 0.05.
Results
SuperArray analysis of CCR7, CXCR4, CCL19, CCL21, and CXCL12 transcripts in IIP and normal margin SLBs
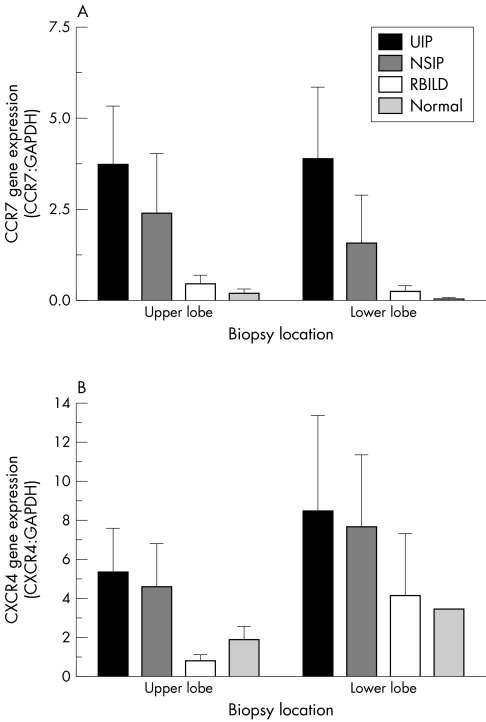
The presence of CCR7 and CXCR4 in IIP and normal margin SLBs was analysed using a specific SuperArray GEArray. Although this is a qualitative and not a quantitative technique, the expression of CCR7 (fig 1A) and CXCR4 (fig 1B) was normalised to that of GAPDH, and the values are shown for both receptors. The highest relative expression of CCR7 and CXCR4 was seen in upper and lower lobe biopsies from the UIP patient group (n = 7). Lower and upper lobe biopsies from the NSIP (n = 6), RBILD (n = 6), and normal margin (n = 5) tumour patient groups were similarly analysed. Although significant differences were not detected, overall, the relative expression of both chemokine receptors appeared to follow a disease severity pattern as follows: UIP > NSIP > RBILD > normal margin.
Figure 1 SuperArray gene analysis of (A) CCR7 and (B) CXCR4 gene expression in upper and lower lobe surgical lung biopsies from UIP (n = 7), NSIP (n = 6), RBILD (n = 6), and normal margin (n = 5) tumour patient groups. The data shown are mean (SEM). No significant differences were detected. CCR7, CC chemokine receptor 7; CXCR4, CX chemokine receptor 4; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; UIP, usual interstitial pneumonia.
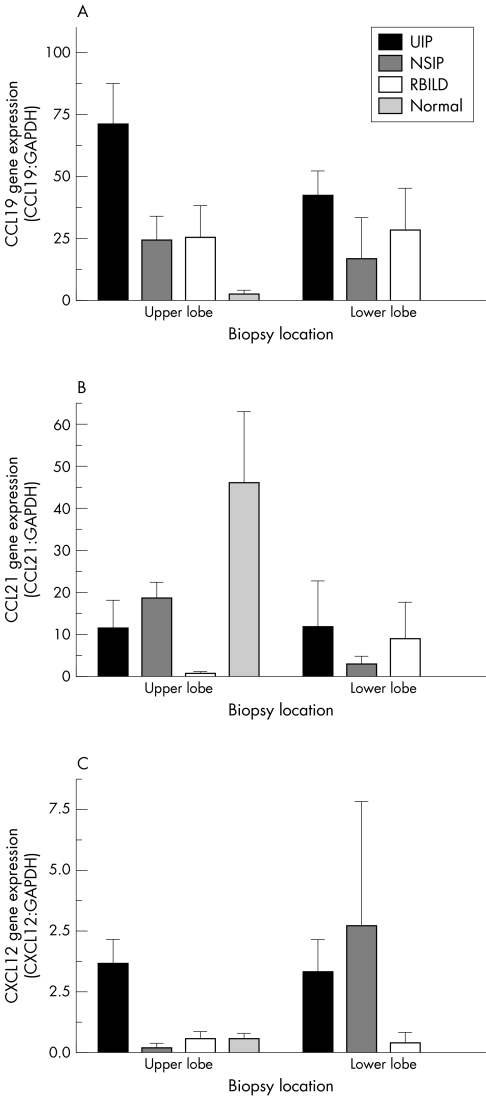
The two CCR7 ligands identified to date are CCL1932 and CCL21.33 CCL19 (or ELC, exodus‐3, and CKβ‐1134) is constitutively expressed in afferent lymph endothelium and the T cell area of lymph nodes, thereby accounting for its major role in the movement of dendritic cells and T cells to these immune sites.13 Concurrent studies showed that CCL21 (or SLC, exodus‐2, TCA‐4, and CKβ‐99) had similar functions and a similar pattern of expression.33 However, both chemokines have been detected in the lung35 and kidney,36 suggesting that they may have roles outside immune surveillance. Our analysis of the relative transcript expression of CCL19 revealed higher amounts of this CCR7 ligand in UIP upper and lower lobe biopsies (fig 2A). Similar amounts of CCL19 transcript were detected in NSIP and RBILD biopsies, and the lowest amounts were seen in normal margin biopsies. However, no clear patterns of CCL21 (fig 2B) and CXCL12 (fig 2C) transcript expression were seen among the IIP and normal margin biopsies.
Figure 2 SuperArray gene analysis of (A) CCL19, (B) CCL21, and (C) CXCL12 gene expression in upper and lower lobe surgical lung biopsies from UIP (n = 7), NSIP (n = 6), RBILD (n = 6), and normal margin (n = 5) tumour patient groups. The data shown are mean (SEM). No significant differences were detected. CCL, CC chemokine receptor ligand; CXCL, CX chemokine receptor ligand; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; UIP, usual interstitial pneumonia.
Quantitative real time PCR analysis of CCR7, CXCR4, CCL19, CCL21, and CXCL12 transcript expression in IIP and normal margin SLBs
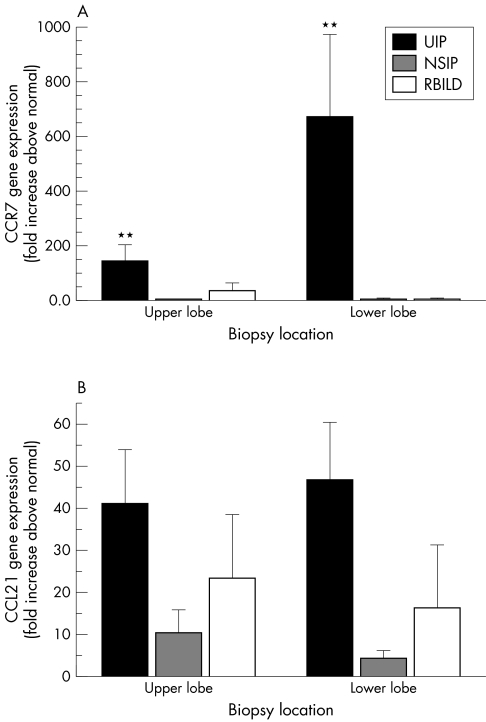
Taqman real time PCR analysis of upper and lower lobe biopsies from the four patient groups revealed a significant increase in CCR7 transcript expression in both upper and lower SLBs from patients with UIP (n = 18) compared with the appropriate biopsy from the normal margin tumour patient group (n = 5) (fig 3A). Mean CCR7 transcript values were approximately 146 (SEM, 58) and 675 (SEM, 300) fold higher in upper and lower lobe UIP SLBs, respectively, compared with the appropriate lobe from the normal margin patient group. Approximately fivefold greater CCR7 transcript expression was detected in the lower lobe UIP biopsies compared with upper lobe UIP biopsies. Mean CCR7 transcript values were increased fourfold (SEM, 1) in both upper and lower lobe NSIP SLBs compared with normal margin SLBs. Mean CCR7 transcript values were also increased in RBILD SLBs relative to normal margin SLBs: 36 (SEM, 28) fold in upper SLBs and 4.4 (SEM, 3.2) fold in lower lobe biopsies. Increases in CCR7 transcript expression in NSIP and RBILD SLBs relative to normal margin SLBs were not significant.
Figure 3 Quantitative Taqman polymerase chain reaction analysis of CCR7 and CCL21 gene expression in upper and lower lobe surgical lung biopsies from patients with UIP (n = 18), NSIP (n = 8), RBILD (n = 6), and normal margins (n = 6). The data shown are mean (SEM). ** p ⩽ 0.01 compared with the appropriate lobe from the normal margin tumour patient group. CCR7, CC chemokine receptor 7; CCL21, CC chemokine receptor 4 ligand; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; UIP, usual interstitial pneumonia.
These findings were unique to CCR7 because Taqman PCR analysis of CXCR4 found no significant increase in CXCR4 transcript expression in IIP SLBs relative to normal margin SLBs (data not shown). Finally, no clear patterns or significant differences in CCL19 (data not shown), CCL21 (fig 3B), and CXCL12 (data not shown) transcript expression were apparent or detected among the IIP and normal margin tumour patient groups examined. However, the greatest increase in CCL21 transcript expression relative to the normal margin tumour patient group was seen in the upper and lower lobe biopsies from the UIP patient group (more than 40 times higher than normal margin SLB values). Greater increases in CCL21 transcript expression were seen in upper and lower RBILD SLBs compared with NSIP SLBs. Together, these data suggested that CCR7 transcript expression was very much increased in UIP, and that increases in CCR7 transcript expression were also present in less severe forms of IIP.
ELISA analysis of CCL19, CCL21, and CXCL12 protein in IIP and normal margin SLBs
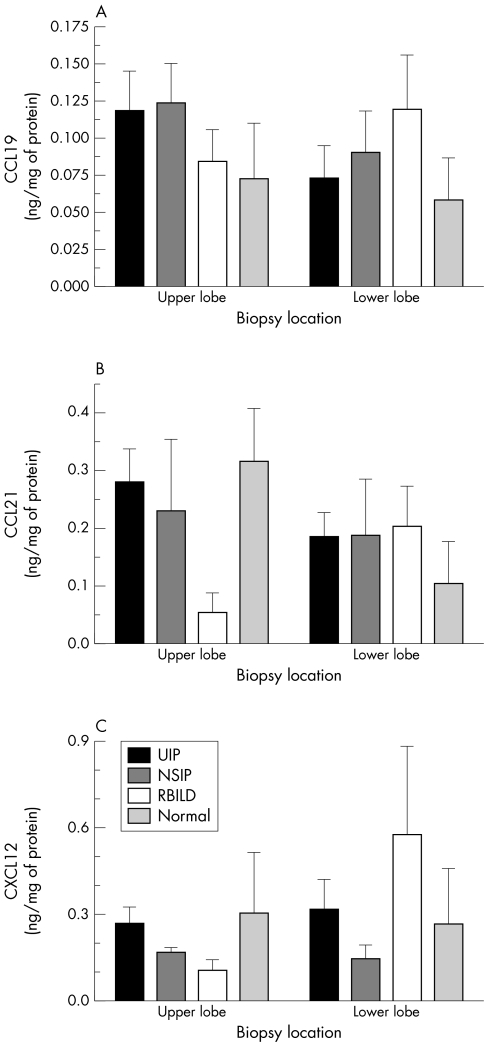
Figure 4 shows the concentrations of CCL19 (fig 4A), CCL21 (fig 4B), and CXCL12 (fig 4C) proteins in cell free supernatants from homogenised SLBs. No clear patterns of CCL expression were noted, and no significant differences were detected among the patient groups analysed. Thus, these data suggested that the fibrotic events in IIP were not associated with the increased generation of CCR7 and CXCR4 ligands within the lung.
Figure 4 Enzyme linked immunosorbent assay analysis of CCL19, CCL21, and CXCL12 in upper and lower lobe surgical lung biopsies from patients with UIP (n = 18), NSIP (n = 6), RBILD (n = 6), and normal margins (n = 5). The data shown are mean (SEM). CCL, CC chemokine receptor ligand; CXCL, CX chemokine receptor ligand; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; UIP, usual interstitial pneumonia.
Focal interstitial CCR7 protein expression in IIP SLBs
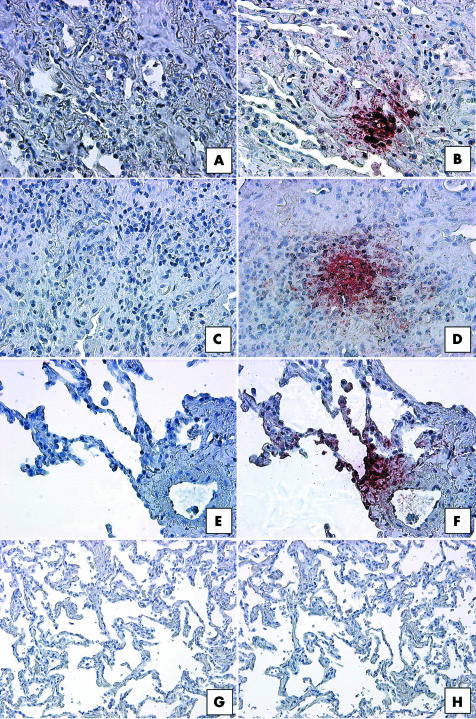
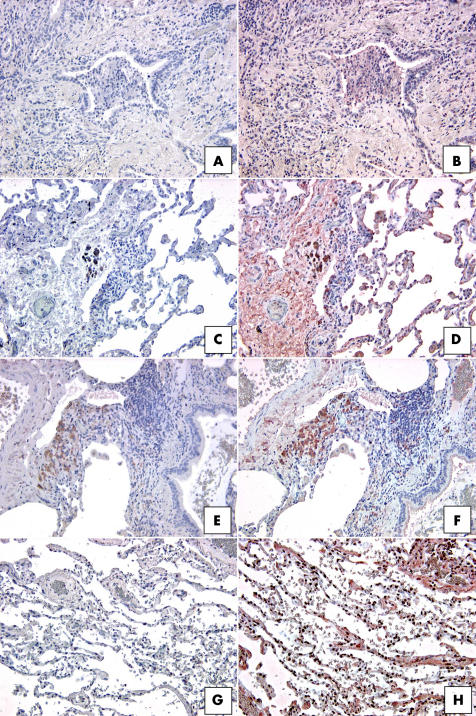
The SuperArray and real time PCR transcript analysis suggested that CCR7 expression was much higher in IIP SLBs than in normal margin SLBs; therefore, we undertook immunohistochemical analysis of whole lung sections from IIP and normal margin SLBs to confirm these observations. As shown in fig 5, focal interstitial CCR7 expression was seen in SLBs from the UIP, NSIP, and RBILD patient groups. Although we found CCR7 expression in macrophages in the patients with RBILD (fig 5F), the focal interstitial pattern of CCR7 expression was not exclusively associated with immune cells in UIP (fig 5B) and NSIP biopsies (fig 5D). Multiple areas of focal CCR7 protein expression were present in interstitial areas of all upper and lower lobe SLBs from the UIP patient group (n = 10). Of note, we found that the foci of CCR7 expression did not exclusively comprise fibroblasts—other cells, including mononuclear and epithelial cells, also appeared to express this chemokine receptor. Both NSIP and RBILD subtypes expressed less CCR7 than the more severe subtype UIP. Although 50% of these biopsies showed CCR7 staining, far fewer focal areas of CCR7 expression were seen in upper and lower lobe SLBs in the NSIP patient group (n = 6). CCR7 was frequently restricted to blood vessels and mononuclear cells in three quarters of the RBILD (n = 5) SLBs, but CCR7 staining was infrequently detected in interstitial areas of the biopsy samples from this IIP group. In contrast to the prominent staining seen in UIP SLBs, no CCR7 expression was detected by immunohistochemical analysis in upper and lower SLBs from patients with normal margins (n = 5) (fig 5H).
Figure 5 Representative immunohistochemical analysis of CCR7 in SLBs from (A, B) UIP, (C, D) NSIP, (E, F) RBILD, and (G, H) normal margin tumour patient groups. Panels (A), (C), (E), and (G) show control staining. CCR7 immunoreactivity (red staining) was seen in focal areas in SLBs from (B) UIP, (D) NSIP, and (F) RBILD, but not (H) normal margins. Original magnification, ×200. CCR7, CC chemokine receptor 7; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
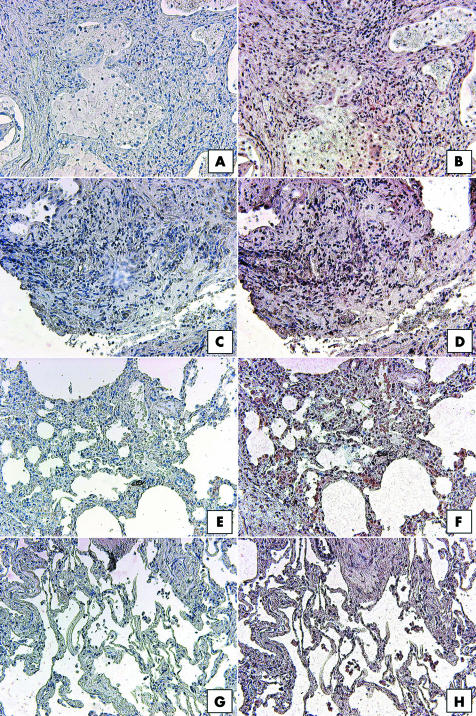
In the same IIP and normal margin SLBs analysed for CCR7, CXCR4 staining appeared to be limited to immune cells, and more importantly, the expression of this chemokine receptor was not focal and did not differ among the four patient groups analysed. All IIP and normal margin biopsies analysed showed strong mononuclear cell associated CXCR4 expression. This is highlighted in fig 6, which shows representative CXCR4 staining in UIP (fig 6B), NSIP (fig 6D), RBILD, (fig 6F), and normal margin (fig 5D) SLBs. Thus, an immunohistochemical survey revealed that CCR7, unlike CXCR4, was prominently expressed in IIP SLBs but not in normal margin SLBs, and that CCR7 expression appeared to be localised to focal interstitial areas in IIP.
Figure 6 Representative immunohistochemical analysis of CXCR4 in SLBs from (A, B) UIP, (C, D) NSIP, (E, F) RBILD, and (G,H) normal margin tumour patient groups. Panels (A), (C), (E), and (G) show control staining. CXCR4 immunoreactivity (red staining) was seen in mononuclear cells present in IIP and normal margin SLBs. Original magnification, ×200. CXCR4, CX chemokine receptor 4; IIP, idiopathic interstitial pneumonia; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
CCR7 positive areas in IIP SLBs are CD45 positive but CD34 and collagen 1 negative
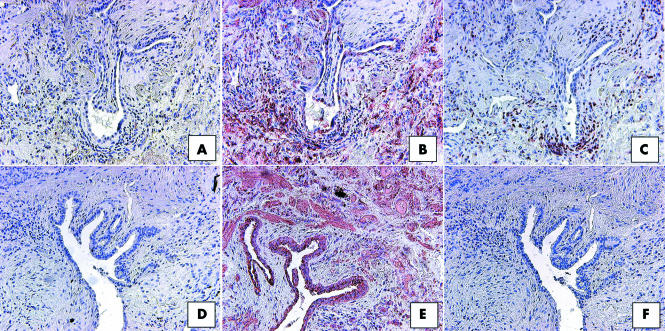
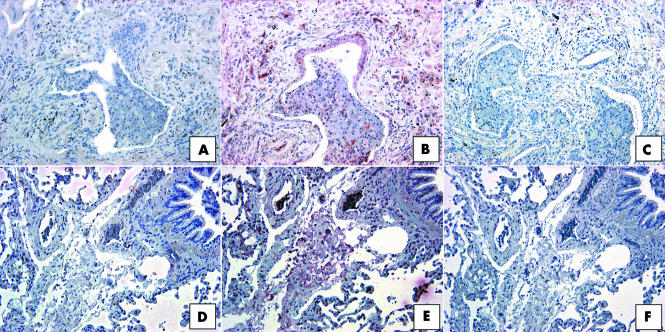
To elucidate the identity of the cells making up the CCR7 positive areas in IIP SLBs, we performed additional staining on serial sections for the expression of CD45 and CCR7 (fig 7). Upon examination of UIP SLBs, the immunohistochemical expression of CCR7 (fig 7B) was found to overlap partly with that of CD45 (fig 7C). Cells expressing both CCR7 and CD45 appeared to be mononuclear cells. In contrast, although CCR7 was highly expressed in NSIP biopsies (fig 7E), the immunolocalisation of this chemokine receptor did not correspond with that of CD45. Similar immunohistochemical studies in RBILD SLBs revealed the same lack of colocalisation of CCR7 and CD45 (not shown). Therefore, dual CD45 and CCR7 expression on mononuclear cells was present in UIP but not in other forms of IIP.
Figure 7 Representative immunohistochemical analysis of (B, E) CCR7 and (C, F) CD45 in serial histological sections from (A–C) UIP and (D–F) NSIP patient groups. Panel (A) and (D) show control staining. In UIP SLBs, CCR7 immunoreactivity (red staining in B) partially overlapped with CD45 immunoreactivity (C), with most of the dual staining associated with mononuclear cells (C). (A, F) In serial histological sections, CCR7 and CD45 did not appear to colocalise in NSIP SLBs. Original magnification, ×200. CCR7, CC chemokine receptor 7; NSIP, non‐specific interstitial pneumonia; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
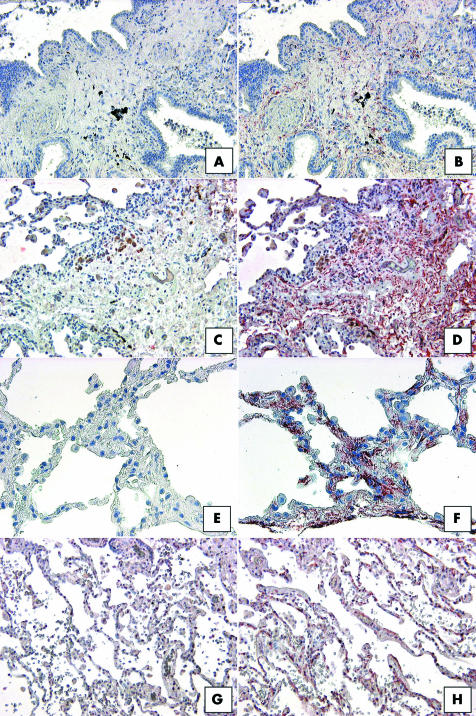
As described above, CD34 is a haemopoietic stem cell antigen that has been identified on peripheral blood fibrocytes.23 We next examined whether this marker could be identified in histological sections from IIP and normal margin biopsies (fig 8). Interestingly, very little CD34 expression was seen in UIP biopsies (fig 8B), and when it was detected this antigen was predominately localised to the interstitial areas of the biopsy. The highest expression of CD34 was seen in NSIP biopsies, where this marker was highly expressed in interstitial areas and on mononuclear cells (fig 8D). Strong CD34 expression was also detected in interstitial areas in RBILD (fig 8F) and normal margin (fig 8H) SLBs. Further examination of the colocalisation of CD34 and CCR7 did not reveal areas in IIP or normal margin SLBs that showed dual expression of these proteins (not shown). Thus, CD34 was prominently expressed in less severe forms of IIP and in normal margin SLBs.
Figure 8 Representative immunohistochemical analysis of CD34 in SLBs from (A, B) UIP, (C, D) NSIP, (E, F) RBILD, and (G, H) normal margin tumour patient groups. Panels (A), (C), (E), and (G) show control staining. CD34 immunoreactivity (red staining) was seen in interstitial areas in all patient SLBs. (D) The highest expression of CD34 was seen in SLBs from the NSIP patient group. Original magnification, ×200. NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
Collagen 1 is prominently expressed by fibrocytes and these cells generate collagen upon recruitment to sites of tissue injury.23 Figure 9 summarises the immunohistochemical analysis of collagen 1 protein expression in IIP and normal margin SLBs. Collagen 1 was immunolocalised to interstitial areas in UIP (fig 9B), NSIP (fig 9D), RBILD (fig 9F), and normal margin (fig 9H) tumour patient biopsies. The highest expression of collagen 1 was seen in UIP SLBs, but high collagen 1 protein expression was also noted in normal margin SLBs. To determine whether collagen 1 and CCR7 colocalised in IIP biopsies, staining was also carried out on serial sections to determine the expression of both proteins (fig 10). Although collagen 1 was highly expressed in all UIP SLBs (fig 10B), CCR7 was not expressed in areas with high collagen 1 expression (fig 10C). A similar pattern was seen in NSIP (not shown) and RBILD (fig 10E,F) SLBs, in which collagen 1 (fig 10E) did not appear to colocalise with CCR7 expression (fig 10F). Thus, CCR7 positive areas in IIP SLBs were not also positive for collagen 1.
Figure 9 Representative immunohistochemical analysis of collagen 1 in SLBs from (A, B) UIP, (C, D) NSIP, (E, F) RBILD, and (G, H) normal margin tumour patient groups. Panels (A), (C), (E), and (G) show control staining. Collagen immunoreactivity (red staining) was diffusely present throughout SLBs from (B) UIP, (D) NSIP, (F) RBILD, and (H) normal margin tumour patient groups. Original magnification, ×200. NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
Figure 10 Representative immunohistochemical analysis of (B, E) collagen 1 and (C, F) CCR7 in SLBs from (A–C) UIP and (D–F) RBILD patient groups. Panels (A) and (D) show control staining. Collagen 1 immunoreactivity (red staining) was present in (B) UIP and (E) RBILD patient groups. However, in serial histological sections, areas that were immunoreactive for collagen 1 lacked CCR7 expression (C, UIP; F, RBILD). Original magnification, ×200. CCR7, CC chemokine receptor 7; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; UIP, usual interstitial pneumonia.
CCR7 positive areas in IIP SLBs lack αSMA expression
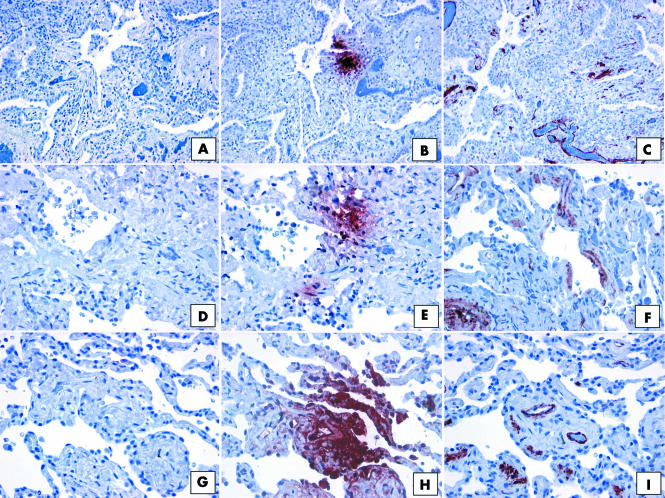
Figure 11 shows representative immunohistochemical staining for CCR7 and αSMA in histological lung sections from patients diagnosed with UIP (fig 11A–C), NSIP (fig 11D–F), and RBILD (fig 11G–I). In all three patient groups, foci of CCR7 (fig 11B,E,H) expression were seen in interstitial areas of the lung, as described above. However, areas that were positive for CCR7 did not overlap with those that were positive for αSMA in serial sections (fig 11C,F,I).
Figure 11 Representative immunohistochemical analysis of α smooth muscle actin (αSMA) in SLBs from (A–C) UIP, (D–F) NSIP, and (G–I) RBILD patient groups. Panels (A), (D), and (G) show control staining. CCR7 immunoreactivity (red staining) was seen in focal areas of SLBs from patients with (B) UIP, (E) NSIP, and (H) RBILD. In serial histological tissue sections, αSMA expression (red staining) was also seen in (C) UIP, (F) NSIP, and (I) RBILD patient groups. However, no overlap between CCR7 and αSMA expression was seen. Original magnification, 85259 ×200. CCR7, CC chemokine receptor 7; NSIP, non‐specific interstitial pneumonia; RBILD, respiratory bronchiolitis/interstitial lung disease; SLB, surgical lung biopsy; αSMA, α smooth muscle actin; UIP, usual interstitial pneumonia.
Discussion
The overall aim of our study was to examine the pattern of CCR7, CXCR4, CCL19, CCL21, and CXCL12 expression in lung biopsy samples from IIP and normal margin tumour patient groups. Quantitative transcript analysis and qualitative protein analysis revealed that CCR7, but not CXCR4, was dramatically increased in UIP relative to normal margin SLBs. UIP SLBs, and to a lesser extent NSIP SLBs, exhibited focal interstitial areas of intense CCR7 protein expression that were not concomitantly positive for CD34, collagen 1, or αSMA. Focal expression of CCR7 protein was seen in all UIP SLBs and smaller proportions of the NSIP and RBILD biopsies. In UIP SLBs, the focal interstitial expression of CCR7 protein appeared to include some CD45 positive cells,37 but most CCR7 postive cells lacked CD45 expression and there was no overlap between CCR7 and CD45 expression in biopsies from patients with NSIP and RBILD. Although it is possible that CCR7 expression in interstitial areas of UIP and NSIP biopsies may represent the recruitment of bone marrow derived fibrocytes,22 we saw no evidence that CCR7 positive areas in these biopsies were positive for fibrocyte markers, such as CD34 and collagen 1. In addition, these CCR7 positive cells lacked the myofibroblast marker αSMA. These data lead us to speculate that focal CCR7 protein expression may indicate sites of idiopathic injury, and the inappropriate activation of resident fibroblasts, rather than the recruitment of fibrocytes expressing CCR7.
Although their role in human disease has yet to be established, and no fibrocyte specific cell surface marker has been identified, several experimental fibrosis models point to a role for circulating cells of bone marrow origin in fibrotic events in the skin38 and lung.24,25,39,40 Most of these experimental studies use bone marrow chimaera models constructed by the adoptive transfer of murine bone marrow transgenically expressing chimaeric green fluorescent protein into irradiated mice. Green fluorescent protein positive bone marrow derived cells, which assumed macrophage‐like39 and/or fibroblast‐like24,25 morphology, were shown to be major contributors to tissue fibrosis. In addition, these bone marrow derived cells were manganese superoxide dismutase sensitive,39 and expressed collagen 1,24,25,40 CD34,40 CD45,25 telomerase reverse transcriptase,24 CCR7,24 and CXCR4.24,25 Nevertheless, controversy persists as to whether fibrocytes ultimately differentiate into myofibroblasts once they localise in the lung. It is presently assumed that fibrocytes recruited into the bronchial tissue after allergen exposure40 or into damaged cutaneous sites23 differentiate into myofibroblasts, whereas fibrocytes attracted to the lung during interstitial fibrosis caused by bleomycin do not express αSMA and cannot be induced to express this myofibroblast marker.24 Added controversy regarding these cells is the observation that not all bone marrow derived cells appear to be deleterious during tissue repair. Accordingly, Ortiz and colleagues41 have shown that murine mesenchymal stem cells migrate to the injured lung, assume an epithelium‐like phenotype, and reduce inflammation and collagen deposition in bleomycin challenged mice. Clearly, further investigation is required to delineate the role of bone marrow derived cells in the clinical fibrotic response.
“Focal CCR7 protein expression may indicate sites of idiopathic injury, and the inappropriate activation of resident fibroblasts, rather than the recruitment of fibrocytes expressing CCR7”
Impetus to examine CCR7 and CXCR4 in SLBs from patients with IIP stemmed from previous studies showing that CCL21, a ligand of the CCR7 chemokine receptor, was a potent stimulus for fibrocyte chemotaxis in vitro and in vivo,23 and that CXCR4 positive bone marrow derived cells contribute to experimentally induced fibrosis.24,25 It is postulated that fibrocytes can rapidly enter sites of tissue injury through CCR7, CXCR4, or possibly other chemokine receptor dependent chemotactic events and contribute to the pathogenesis of pulmonary fibrosis.25 In our present study, focal areas of CCR7 expression were examined for the presence of putative fibrocyte markers, including CD45, CD34, and collagen 1.23,42 CD45 was the only marker that was present in the focal areas of CCR7 expression, and colocalisation of CD45 and CCR7 was seen only in mononuclear cells present within UIP, and not other IIP biopsies. In addition, all of these markers were detected in normal margin SLBs although our immunohistochemical techniques failed to detect CCR7 in these biopsies. Although our findings do not rule out the possibility that the CCR7 positive cells seen in patient biopsies are fibrocytes, it is possible that the expression of these markers is quickly downregulated upon the migration of fibrocytes from the circulation into the lung. The observation that CXCR4 was not present in focal areas of IIP SLBs also does not rule out the importance of this chemokine receptor in the recruitment of fibrocytes and/or collagen producing bone marrow derived cells to the lung. A diffuse pattern of CXCR4 expression was seen in all SLBs analysed and most of the cells expressing this chemokine receptor appeared to be mononuclear cells.
Given the abnormal synthetic and proliferative characteristics of UIP fibroblasts (highlighted by histologically distinct fibroblastic foci7), we hypothesised that these cells may also be inappropriately responding to chemokine ligands that usually regulate the function of immune cells. Tissue resident fibroblasts comprise a major component of the lung architecture, and many investigators have proposed that the inappropriate activation of these cells underlies the pathological fibrotic response seen in UIP and possibly other forms of IIP.43,44,45,46 The factors that contribute to this inappropriate activation have yet to be elucidated, but data from several laboratories indicate that chemokines may play a role in this process.8,47 For example, it is now widely recognised that UIP fibroblasts from fibrotic lungs migrate faster than those from control lungs,43 and there is some suggestion from mouse studies that chemokines have a major role in this respect.48 Further impetus to examine the possibility that CCR7 and CXCR4 may play a role in the aberrant migratory activity of cells in IIP was derived from recent discoveries in the field of cancer biology, which have documented the expression of a unique repertoire of chemokine receptors on breast cancer cells that explain, in part, the metastatic properties of breast tumours.18
Thus, our present study demonstrated the focal interstitial expression of CCR7 in IIP. The identity of the cells expressing this receptor has yet to be fully elucidated, but areas containing focal CCR7 expression were not positive for CD34 and collagen 1. CCR7 expressing cells in the interstitial areas of IIP biopsies also did not express αSMA, thereby ruling out the possibility that these cells were myofibroblasts. Future studies with primary fibroblast and myofibroblast cell lines grown from the same SLBs analysed in our study are under way, and preliminary data suggest that the synthetic and proliferative properties of IIP fibroblasts, but not normal margin fibroblasts, are differentially influenced by exogenous CCL19 and CCL21. We recognise that other chemokines and their receptors have been described in human pulmonary fibrosis,49 and it is possible that CCR7 and its ligands are working in concert with other chemotactic cytokines to bring about the lethal fibroproliferative response often seen in IIP. Nonetheless, these studies are of potential therapeutic importance, given that it may be possible to inhibit the migration of fibrocytes and/or the activation of resident pulmonary fibroblasts in response to CCR7 specific chemokines, thereby preventing the excessive production of extracellular matrix in favour of appropriate lung repair.
Take home messages
Focal interstitial expression of the CCR7 chemokine was seen in idiopathic interstitial pneumonia (IIP)
CCR7 expression was significantly raised in the most severe form of IIP—usual interstitial pneumonia—compared with non‐specific interstitial pneumonia and respiratory bronchiolitis/interstitial lung disease
Although the identity of the cells expressing this receptor is not clear, our results suggest that they are activated resident pulmonary fibroblasts rather than infiltrating fibrocytes or myofibroblasts
Acknowledgements
The authors express their gratitude to R Kunkel for her artistic assistance during the preparation of this manuscript. This study was supported by a grant from the National Heart Lung and Blood Institute P50 HL56402 (CMH, SLK, KRF, FJM, and GBT). The tissue procurement core of the University of Michigan Comprehensive Cancer Center also supported these investigations, in part, through grant CA46952.
Abbreviations
CCR/L - CC chemokine receptor/ligand
CXCR/L - CX chemokine receptor/ligand
ELISA - enzyme linked immunosorbent assay
GAPDH - glyceraldehyde 3‐phosphate dehydrogenase
IIP - idiopathic interstitial pneumonia
NSIP - non‐specific interstitial pneumonia
PCR - polymerase chain reaction
RBILD - respiratory bronchiolitis/interstitial lung disease
SLB - surgical lung biopsy
αSMA - α smooth muscle actin
UIP - usual interstitial pneumonia
References
- 1.Green F H. Overview of pulmonary fibrosis. Chest 2002122(suppl 6)334S–9S. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson A G, Colby T V, du Bois R M.et al The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 20001622213–2217. [DOI] [PubMed] [Google Scholar]
- 3.Chapman H A. Disorders of lung matrix remodeling. J Clin Invest 2004113148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty K R, Toews G B, Lynch J P., 3rdet al Steroids in idiopathic pulmonary fibrosis: a prospective assessment of adverse reactions, response to therapy, and survival. Am J Med 2001110278–282. [DOI] [PubMed] [Google Scholar]
- 5.Lynch J P, 3rd, White E, Flaherty K. Corticosteroids in idiopathic pulmonary fibrosis. Curr Opin Pulm Med 20017298–308. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty K R, Thwaite E L, Kazerooni E A.et al Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 200358143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King T E, Jr, Schwarz M I, Brown K.et al Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 20011641025–1032. [DOI] [PubMed] [Google Scholar]
- 8.van den Blink B, Jansen H M, Peppelenbosch M P. Idiopathic pulmonary fibrosis: molecular mechanisms and possible therapeutic strategies. Arch Immunol Ther Exp (Warsz) 200048539–545. [PubMed] [Google Scholar]
- 9.Nagira M, Imai T, Hieshima K.et al Molecular cloning of a novel human CC chemokine secondary lymphoid‐tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem 199727219518–19524. [DOI] [PubMed] [Google Scholar]
- 10.Forster R, Kremmer E, Schubel A.et al Intracellular and surface expression of the HIV‐1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol 19981601522–1531. [PubMed] [Google Scholar]
- 11.Kim C H, Broxmeyer H E. SLC/exodus2/6Ckine/TCA4 induces chemotaxis of hematopoietic progenitor cells: differential activity of ligands of CCR7, CXCR3, or CXCR4 in chemotaxis vs. suppression of progenitor proliferation. J Leukoc Biol 199966455–461. [DOI] [PubMed] [Google Scholar]
- 12.Kim C H, Rott L, Kunkel E J.et al Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest 20011081331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Palermo B, Lenig D.et al Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 1999291617–1625. [DOI] [PubMed] [Google Scholar]
- 14.Randolph D A, Huang G, Carruthers C J.et al The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science 19992862159–2162. [DOI] [PubMed] [Google Scholar]
- 15.Hammad H, Lambrecht B N, Pochard P.et al Monocyte‐derived dendritic cells induce a house dust mite‐specific Th2 allergic inflammation in the lung of humanized SCID mice: involvement of CCR7. J Immunol 20021691524–1534. [DOI] [PubMed] [Google Scholar]
- 16.Ward S G, Westwick J. Chemokines: understanding their role in T‐lymphocyte biology. Biochem J 1998333457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalo J A, Lloyd C M, Peled A.et al Critical involvement of the chemotactic axis CXCR4/stromal cell‐derived factor‐1 alpha in the inflammatory component of allergic airway disease. J Immunol 2000165499–508. [DOI] [PubMed] [Google Scholar]
- 18.Muller A, Homey B, Soto H.et al Involvement of chemokine receptors in breast cancer metastasis. Nature 200141050–56. [DOI] [PubMed] [Google Scholar]
- 19.Murakami T, Cardones A R, Hwang S T. Chemokine receptors and melanoma metastasis. J Dermatol Sci 20043671–78. [DOI] [PubMed] [Google Scholar]
- 20.Tavor S, Petit I, Porozov S.et al CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res 2004642817–2824. [DOI] [PubMed] [Google Scholar]
- 21.Ghobrial I M, Bone N D, Stenson M J.et al Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B‐cell chronic lymphocytic leukemia/small lymphocytic lymphoma. Mayo Clin Proc 200479318–325. [DOI] [PubMed] [Google Scholar]
- 22.Bucala R, Spiegel L A, Chesney J.et al Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994171–81. [PMC free article] [PubMed] [Google Scholar]
- 23.Abe R, Donnelly S C, Peng T.et al Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 20011667556–7562. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto N, Jin H, Liu T.et al Bone marrow‐derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004113243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips R J, Burdick M D, Hong K.et al Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004114438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty K R, Martinez F J. The role of pulmonary function testing in pulmonary fibrosis. Curr Opin Pulm Med 20006404–410. [DOI] [PubMed] [Google Scholar]
- 27.Kazerooni E A, Martinez F J, Flint A.et al Thin‐section CT obtained at 10‐mm increments versus limited three‐level thin‐section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997169977–983. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society, European Respiratory Society (ATS/ERS) international multidisciplinary consensus classification of idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002165277–304. [DOI] [PubMed] [Google Scholar]
- 29.Choi E S, Jakubzick C, Carpenter K J.et al Enhanced monocyte chemoattractant protein‐3/CC chemokine ligand‐7 in usual interstitial pneumonia. Am J Respir Crit Care Med 2004170508–515. [DOI] [PubMed] [Google Scholar]
- 30.Jakubzick C, Choi E S, Kunkel S L.et al Impact of interleukin‐13 responsiveness on the synthetic and proliferative properties of Th1‐ and th2‐type pulmonary granuloma fibroblasts. Am J Pathol 20031621475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubzick C, Choi E S, Kunkel S L.et al Augmented pulmonary IL‐4 and IL‐13 receptor subunit expression in idiopathic interstitial pneumonia. J Clin Pathol 200457477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida R, Imai T, Hieshima K.et al Molecular cloning of a novel human CC chemokine EBI1‐ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem 199727213803–13809. [DOI] [PubMed] [Google Scholar]
- 33.Campbell J J, Bowman E P, Murphy K.et al 6‐C‐kine (SLC), a lymphocyte adhesion‐triggering chemokine expressed by high endothelium, is an agonist for the MIP‐3beta receptor CCR7. J Cell Biol 19981411053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte‐specific CC chemokines and their receptors. J Leukoc Biol 199762634–644. [DOI] [PubMed] [Google Scholar]
- 35.Gunn M D, Tangemann K, Tam C.et al A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 199895258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banas B, Wornle M, Berger T.et al Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol 20021684301–4307. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Schaerli P, Loetscher P.et al Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 1998282760–2769. [DOI] [PubMed] [Google Scholar]
- 38.Fathke C, Wilson L, Hutter J.et al Contribution of bone marrow‐derived cells to skin: collagen deposition and wound repair. Stem Cells 200422812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epperly M W, Guo H, Gretton J E.et al Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 200329213–224. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt M, Sun G, Stacey M A.et al Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003171380–389. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz L A, Gambelli F, McBride C.et al Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 20031008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartlapp I, Abe R, Saeed R W.et al Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 2001152215–2224. [DOI] [PubMed] [Google Scholar]
- 43.Suganuma H, Sato A, Tamura R.et al Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 199550984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogaboam C M, Smith R E, Kunkel S L. Dynamic interactions between lung fibroblasts and leukocytes: implications for fibrotic lung disease. Proc Assoc Am Physicians 1998110313–320. [PubMed] [Google Scholar]
- 45.Ramos C, Montano M, Garcia‐Alvarez J.et al Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 200124591–598. [DOI] [PubMed] [Google Scholar]
- 46.Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross‐talk disorder. Respir Res 200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selman M. Plunging into the chaos of the cytokine/chemokine cocktail in pulmonary fibrosis: how many and how important are they? Am J Respir Crit Care Med 2003168730–731. [DOI] [PubMed] [Google Scholar]
- 48.Moore B B, Paine R, 3rd, Christensen P J.et al Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 20011674368–4377. [DOI] [PubMed] [Google Scholar]
- 49.Kunkel S L, Lukacs N W, Strieter R M.et al The role of chemokines in the immunopathology of pulmonary disease. Forum (Genova) 19999339–355. [PubMed] [Google Scholar]