Abstract
Background
Osteoprotegerin (OPG) is involved in the regulation of bone turnover through binding to the receptor activator of nuclear factor κB ligand (RANKL), and has also been reported to be a potential survival factor for several different cell types. The survival effects are mediated through inhibition of the activity of tumour necrosis factor related apoptosis inducing ligand (TRAIL). Both breast and prostate cancer cells produce sufficient amounts of OPG to be protected against the effects of TRAIL in vitro.
Aims
To investigate the spatial expression of OPG, RANKL, and TRAIL in non‐neoplastic breast tissue and breast cancer, and its relation with oestrogen receptor (ER) expression.
Methods
Forty breast cancers (20 ER+, 20 ER−) and five non‐neoplastic breast tissue samples were stained with antibodies against OPG, RANKL, and TRAIL.
Results
OPG was not expressed in non‐neoplastic breast tissue except when colocalised with altered columnar epithelium. RANKL was expressed at the apical surface of luminal epithelial cells and TRAIL was expressed in myoepithelial cells. All three proteins were expressed in some breast cancers but showed no significant association with tumour type. OPG expression showed a significant positive correlation with ER expression (p = 0.011).
Conclusions
This is the first published study of the spatial expression of OPG, RANKL, and TRAIL in breast tissue and breast cancer. The localisation of each protein was specific and they were not colocalised. This specificity may provide a useful marker of functional differentiation in breast cancer; for example, TRAIL expression as a marker of myoepithelial differentiation.
Keywords: osteoprotegerin, receptor activator of nuclear factor κB ligand, tumour necrosis factor related apoptosis inducing ligand, breast tumours, oestrogen receptor
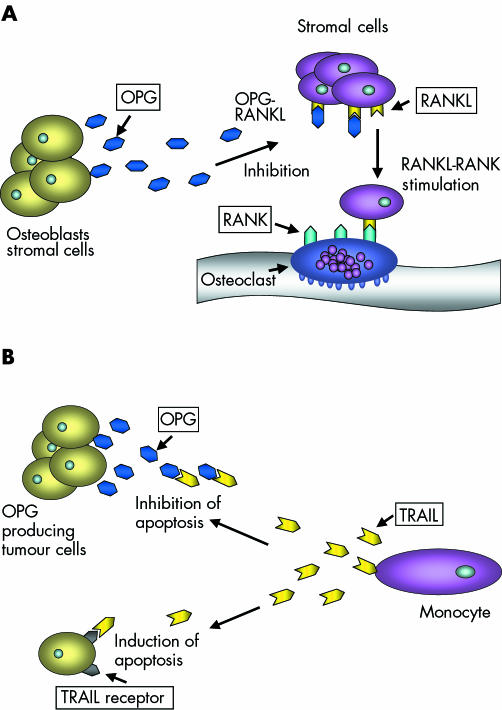
The development and spread of tumours is associated with the ability of malignant cells to avoid detection and subsequent elimination by the immune system, to grow in non‐native sites, to induce blood vessel formation, and to avoid programmed or induced cell death. Among the mechanisms tumour cells have developed to gain these advantages are the expression of molecules that (1) interfere with the induction of apoptotic cell death and (2) eliminate key elements of the apoptotic pathway.1 Induction of apoptosis involves both intracellular and extracellular pathways, some of which are regulated by members of the tumour necrosis factor (TNF) family.2 A key member of this group of molecules is TNF related apoptosis inducing ligand (TRAIL),3 which binds to several receptors on the cell surface that express intracellular death domains, including DR4 and DR5.4,5,6 Binding of TRAIL to these receptors results in activation of a series of different caspases, ultimately causing the initiation of apoptosis. TRAIL is produced in tumours by invading monocytes as a response to interferon γ or interferon α, and is the principal mediator of tumour cell death caused by these immune cells. Altered expression and function of TRAIL has been reported in association with several different tumour types, including human breast tumours.7 A proposed mechanism whereby tumour cells may be protected against the effects of TRAIL is through the production of soluble decoy receptors, such as osteoprotegerin (OPG).4,8 Originally identified as a modulator of bone resorption,9 the wide tissue distribution of OPG suggests that this molecule has additional functions (fig 1). One example is reported in a study by Yun et al, using opg−/− mice, where B cell development and function was found to be altered compared with mice expressing OPG.10 In vitro, OPG acts as a survival factor for human breast and prostate cancer cells, in addition to myeloma cells, through inhibition of TRAIL induced apoptosis.11,12,13 Whether this potential OPG linked survival system operates in vivo remains to be established, but there are reports that high expression of OPG in tumours may be associated with poor prognosis in gastric carcinoma.14 In contrast, using reverse transcription polymerase chain reaction, Reinholz et al found no link between increased expression of OPG mRNA and increased invasiveness in a study of human breast cancer.15
Figure 1 The suggested roles of osteoprotegerin (OPG) in (A) bone remodelling and (B) cell survival. RANKL, receptor activator of nuclear factor κB ligand; TRAIL, tumour necrosis factor related apoptosis inducing ligand.
“A proposed mechanism whereby tumour cells may be protected against the effects of TNF related apoptosis inducing ligand is through the production of soluble decoy receptors, such as osteoprotegerin”
Although OPG mRNA is expressed in normal breast tissue, it does not seem to play an essential role in breast development, because OPG knockout mice show no abnormalities in breast development or function.16 In contrast, the high affinity OPG binding molecule RANKL is essential for the development of lactating breast lobules in the mouse. Fata et al reported that RANKL knockout mice could not successfully nurse their young because of the lack of formation of functional breast lobules.17 RANKL is therefore established as a regulator of both bone homeostasis and functional breast development, showing that a single molecule can have different key functions in unrelated organs.18 RANKL mRNA is reported to be expressed in human breast tumours, but it is not known whether this molecule is associated with the development of breast cancer.15 In vitro studies have shown that a variety of human tumour cells (including breast) do not express RANKL when grown in culture unless co‐cultivated with other cell types.18
In summary, OPG and two of its established ligands, TRAIL and RANKL, are reported to be expressed at different levels both in normal breast tissue and in breast tumours. It is unclear to what extent these molecules are involved in the development of breast cancer, and the relation between these molecules within human cancer tissue samples has not been defined. Because complex interactions between TNF family members are involved in the regulation of cell survival, a key process in tumour development, the expression of these molecules in tumours may be relevant. Importantly, OPG has a higher affinity for RANKL than for TRAIL,19 and the potential effects of OPG within tumours probably depends on the local concentrations of OPG/RANKL/TRAIL and the ratio between the molecules.20
To characterise the relations between members of the OPG–RANKL system and TRAIL in breast tumours, we sought to define the expression patterns of the OPG, TRAIL, and RANKL proteins within serial sections from human primary breast cancer tissue, in addition to samples of non‐malignant breast. Because we have found differences in OPG expression between hormone dependent and independent breast cancer cells in vitro, we also investigated whether OPG expression correlated with oestrogen receptor (ER) or progesterone receptor (PR) status in this tumour sample set. To explore further the correlations between cell culture model systems and clinically obtained cancer tissue, we also analysed OPG expression by immunohistochemistry (IHC) in MCF‐7 (ER positive) and MDA‐MB‐436 (ER negative) cell lines.
Materials and methods
Patient samples
This retrospective, correlative study received approval by the Memorial Sloan‐Kettering Cancer Center institutional review board. Paraffin wax embedded primary breast cancer tissues, removed for clinical indications from women treated at the Memorial Sloan‐Kettering Cancer Center, were identified. To test the preclinical finding that hormone sensitivity affected OPG expression, ER and PR positive tumours (ER/PR+; n = 20) and ER and PR negative tumours (ER/PR−; n = 20) were chosen. Selected specimens were either positive, or negative, for both hormone receptors; hormone receptor status was assayed by IHC and reported in the medical record for clinical indications. There has been no reported study of IHC for OPG in primary human breast tissue; therefore, non‐malignant breast tissue was also analysed for comparison of intensity of staining between various breast tissue types and to aid in the development of a scoring system. Non‐malignant breast tissue served as a control (n = 5). A database was created from the pertinent clinical information abstracted from the medical records.
Immunohistochemistry
IHC studies were performed on paraffin wax blocks from tissue fixed in 10% neutral buffered formalin. Tissue sections (4 μm thick) were prepared and mounted on polylysine coated slides. Sections were dewaxed and hydrated to 70% ethanol and incubated in 3% hydrogen peroxide in methanol for 15 minutes, followed by three washes in phosphate buffered saline. The sections were then microwaved for 10 minutes in 0.1M Tris/HCl at full power, washed in phosphate buffered saline and blocked with casein (Vector Laboratories Ltd, Peterborough, UK) for 30 minutes. The slides were incubated with either mouse monoclonal anti‐OPG (MAB 8051; R&D Systems, Abingdon, Oxford, UK; 1/250 dilution), mouse monoclonal anti‐TRAIL (MAB 687; R&D Systems; 1/100 dilution), or goat polyclonal anti‐RANKL (SC‐7627; Santa Cruz Technology, Santa Cruz, California, USA; 1/100 dilution) for one hour, washed, and incubated for 30 minutes with antimouse/goat biotin conjugated secondary antibody (30 μl/2 ml of casein solution diluted 1/50; Vector Laboratories Ltd). Slides were washed and developed using the ABC Elite kit (Vector Laboratories Ltd), followed by diaminobenzidine and a 20 second counterstain with haematoxylin. Negative controls (no primary antibody) were included in all runs.
The immunohistochemical staining was analysed by an experienced breast pathologist (SSC). The staining in the breast cancers was intratumorally heterogeneous but relatively dichotomous—tumours that were classified as negative showed no staining at all. If more than 10% of the tumour showed strong staining it was classified as positive for the molecule in question.
Cell lines and tissue culture
The human breast cancer cell lines MCF‐7 (ER+) and MDA‐MB‐436 (ER−) were obtained from the American Type Culture Collection, Mannassas, Virginia, USA. The cell lines were routinely maintained in RPMI 1640 supplemented with fetal bovine serum (10%) and L‐glutamine (2mM). For the generation of conditioned medium all cell lines were seeded into 75 cm2 flasks and grown for five days. Conditioned medium was collected over these cultures from days 1–5 and accumulated OPG concentrations were measured. All medium/reagents were from Life Technologies, Paisley, Scotland, UK. Plastics were purchased from Costar Ltd, Buckinghamshire, UK.
Enzyme linked immunosorbent assay
The concentration of OPG in the culture medium was determined using an enzyme linked immunosorbent assay method. Briefly, 96 well plates were coated with 2 μg/ml mouse monoclonal antihuman OPG (R&D Systems). An OPG standard curve was generated using recombinant human OPG (R&D Systems) at concentrations from 31.24 to 2000 pg/ml. The secondary antibody was biotinylated antihuman OPG (R&D Systems) at 200 ng/ml, and detection was done using streptavidin–horseradish peroxidase (R&D Systems) in combination with TMB substrate (Sigma RBI, Poole, Dorset, UK). The reaction was stopped after five to 20 minutes of incubation in the dark by the addition of 50 μl 2M H2SO4. The absorbance was read at 450 nm on a Dynatech plate reader and analysed using Revelation software.
Biostatistics
The primary aim of our study was to define OPG expression in ER/PR+ and ER/PR− primary breast cancer tissue specimens, to confirm preclinical findings and lend support to the continuing study with this preclinical model. The study was designed to detect a 40% difference in the frequency of OPG positivity in these two groups with 80% power (5% significance), which required 20 specimens for each group. OPG expression was assessed in five non‐malignant breast tissue specimens as a control.
Results
Non‐malignant tissue: expression of OPG, TRAIL, and RANKL
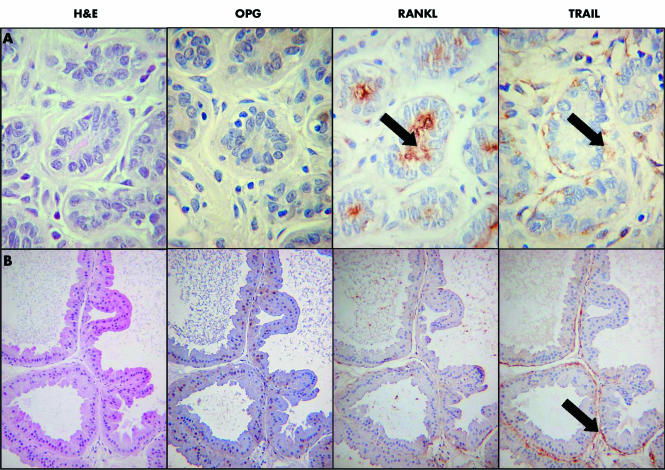
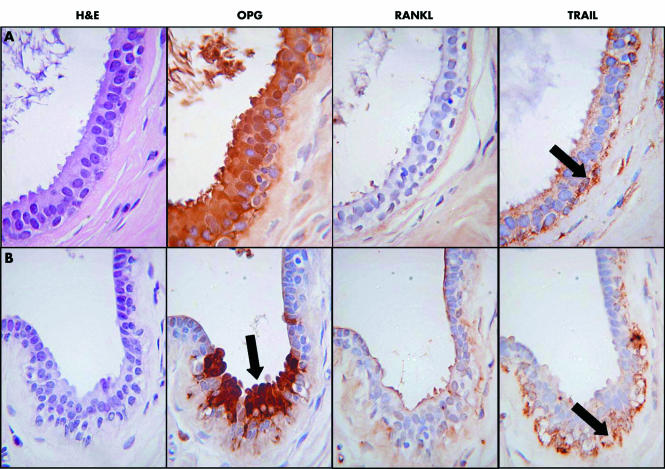
There was a highly consistent pattern of expression of all three proteins in non‐malignant breast tissue. RANKL was present in the apical membrane of luminal epithelial cells in non‐malignant lobules and ducts (fig 2), whereas it was not detected in the myoepithelial cells of these ducts, or in epithelia showing columnar alteration. TRAIL was localised to the myoepithelial cells in non‐malignant lobules and ducts, but was absent from epithelial cells (fig 2). TRAIL was in some cases also expressed in macrophages, fibroblasts, and endothelial cells (data not shown). OPG was not present in non‐malignant lobules and ducts but was strongly expressed in any epithelium showing columnar alteration (fig 3). OPG was expressed in endothelial cells, but was absent from myoepithelial cells.
Figure 2 (A) Normal breast lobule. None of the epithelial cells is positive for OPG (osteoprotegerin). There is RANKL (receptor activator of nuclear factor κB) staining at the surface of the luminal epithelial cells (arrowed). TRAIL (tumour necrosis factor related apoptosis inducing ligand) staining is found in the basal myoepithelial cells (arrowed). (B) An area of apocrine metaplasia. There is no staining for OPG or RANKL, TRAIL is expressed in the myoepithelial cells (arrowed) but not in the epithelial cells, which show apocrine metaplasia.
Figure 3 (A) An area of columnar cell metaplasia in a small duct. There is strong cytoplasmic and nuclear staining for OPG (osteoprotegerin), but the luminal epithelial cells are negative for OPG and TRAIL (tumour necrosis factor related apoptosis inducing ligand), although the myoepithelial cells are still positive for TRAIL (arrowed). (B) Focal area of columnar cell metaplasia in a duct. There is strong cytoplasmic and nuclear staining for OPG which exactly colocalises with the columnar cell change (arrowed). The myoepithelial cells are still positive for TRAIL (arrowed). H&E, haematoxylin and eosin.
Breast cancer tissue: expression of OPG, TRAIL, and RANKL
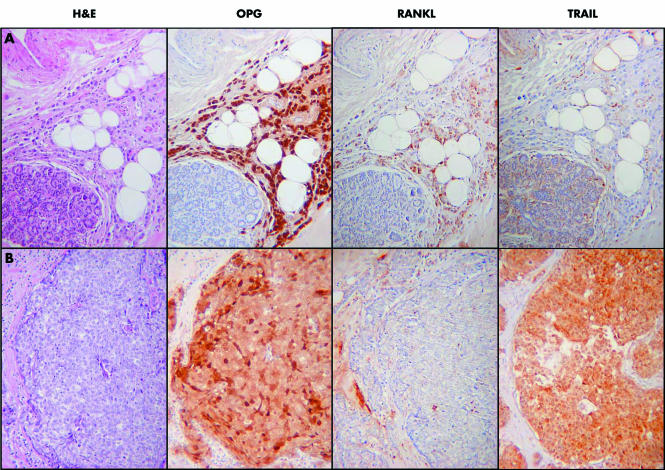
The expression of all three proteins was clearly demonstrated, but was heterogeneous in the cancer tissues. There were areas of strong positivity for OPG in 55% of the tumours, for RANKL in 60%, and for TRAIL in 58% (fig 4). However, there was no consistent pattern of expression in particular tumour types (such as lobular cancer), and no apparent association between the expression of the three proteins. Of note, in the 11 breast cancer specimens that included an in situ component, there was complete concordance between expression of the three proteins in the in situ and invasive components of the tumours (data not shown). Table 1 summarises the staining patterns for OPG, RANKL, and TRAIL in invasive breast cancer, ductal carcinoma in situ, and lobular carcinoma in situ.
Figure 4 (A) A grade 2 lobular breast cancer. There is strong nuclear and cytoplasmic staining for OPG (osteoprotegerin), which contrasts with the negative normal lobule in the bottom left of the image. There is weaker focal staining for RANKL (receptor activator of nuclear factor κB ligand) and the tumour is negative for TRAIL (tumour necrosis factor related apoptosis inducing ligand). (B) A grade 3 ductal breast cancer of no specific type. There is strong nuclear and cytoplasmic staining for OPG, strong cytoplasmic staining for TRAIL, but the tumour is negative for RANKL. H&E, haematoxylin and eosin.
Table 1 Staining patterns for OPG, RANKL, and TRAIL in breast tumours.
| Invasive cancer | DCIS | LCIS | |
|---|---|---|---|
| OPG | 22/40 | 5/11 | 1/1 |
| RANKL | 24/40 | 2/11 | 1/1 |
| TRAIL | 23/40 | 5/11 | 0/1 |
Total number of specimens containing invasive mammary carcinoma, 40; DCIS is present in 11 specimens, LCIS is present in 1 specimen.
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand; TRAIL, tumour necrosis factor related apoptosis inducing ligand.
Correlation between OPG and ER/PR status in vitro and in vivo
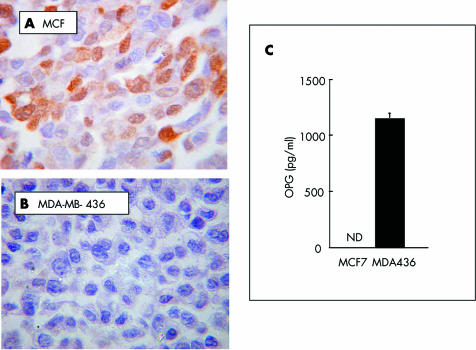
As shown in fig 5, there is a clear difference in the ability of the two breast cancer cell lines to express and release OPG in vitro. The hormone dependent cell line MCF7 showed strong intracellular staining for OPG, but did not release detectable amounts of OPG into the medium (fig 5A, C). In contrast, the hormone independent cell line, MDA‐MB‐436, was negative for intracellular OPG, but did release substantial amounts into the medium (fig 5B, C). The pronounced difference between the ER status and OPG expression in vitro prompted us to investigate the correlation between OPG expression and ER/PR status in vivo. As shown in table 2, there was a significant correlation between ER/PR positivity and OPG expression in these specimens (χ2 test; p = 0.011). To evaluate the potential for bias as a result of the small sample size, we also analysed the OPG and hormone receptor status data with Yate's continuity correction to remove such bias. The Yate's correction yielded a value of p = 0.026.
Figure 5 Expression of OPG (osteoprotegerin) by breast cancer cell lines in vitro. Hormone dependent (A; MCF7) and independent (B; MDA‐MB‐436) cells were grown under standard conditions for 72 hours, and OPG expression was determined in the cell pellets by immunohistochemistry and in conditioned medium by enzyme linked immunosorbent assay (C). ND, not detectable.
Table 2 Correlation between OPG expression and ER/PR positivity of breast tumours.
| OPG negative (%) | OPG positive (%) | p Value | |
|---|---|---|---|
| ER/PR− (n = 20) | 13 (65%) | 7 (35%) | |
| ER/PR+ (n = 20) | 5 (25%) | 15 (75%) | 0.011 |
| Total (n = 40) | 18 | 22 |
Analysis with the χ2 test yields p = 0.011. To evaluate the potential for bias as a result of the small sample size, we also analysed the OPG and hormone receptor status data with Yate's continuity correction to remove such bias. The Yate's correction yields a value of p = 0.026.
ER, oestrogen receptor; OPG, osteoprotegerin; PR, progesterone receptor.
Discussion
The local cellular microenvironment probably plays an important role in the development of cancer, and in the establishment and growth of metastases. Our current study, by examining tissues en block, rather than using microdissection and laser capture techniques, provides insight into the expression patterns of OPG, TRAIL, and RANKL in several cell types in serial sections from human primary breast cancer tissue, and in non‐malignant tissue. The functions and site of origin of these molecules in the tumour specimens are currently unknown, but complex interactions between members of the TNF superfamily have been shown to regulate cell survival in vitro, and similar systems are expected to operate in vivo. The data presented here demonstrate the presence of OPG, TRAIL and RANKL in clinically relevant primary breast cancer specimens. These results support the preclinical data and confirm the correlation between ER/PR status and OPG expression. However, the function of OPG, TRAIL, and RANKL in these human breast cancer tissues is yet to be determined, and studies to explore correlations with clinical outcome and to utilise preclinical models are ongoing.
Published studies have demonstrated the expression of these molecules at the mRNA level; however, data have often been obtained using mixed cell populations and the cellular localisation of the different molecules has not been adequately described. Thomas et al reported the expression of OPG mRNA in 12 different samples of human primary breast tumours (infiltrating ductal carcinoma), but did not detect RANKL expression in the samples.21 In contrast, Reinholz et al reported the presence of RANKL mRNA transcripts in a series of human breast tumours.15 TRAIL has been reported to be expressed both at the mRNA and the protein level in human ductal invasive mammary carcinomas, but in the same report the authors could not detect TRAIL protein in normal breast tissue.1 We have shown that OPG, RANKL, and TRAIL are expressed in primary breast specimens and generated data regarding the localisation of these proteins within clinically relevant human specimens.
We found that there was a highly consistent pattern of expression of OPG, RANKL, and TRAIL in non‐neoplastic breast tissue, and each protein was localised to a different cell type or cellular subcompartment. Although IHC is only a surrogate indicator of the site of protein production, the patterns were very clear and consistent, and suggest that there may not be close functional relations between these three proteins in breast tissue. However, soluble factors like OPG have the potential to elicit an effect on surrounding cell types that express the appropriate receptors/ligands. Therefore, it is possible that our observation of the highly localised expression of OPG by epithelial cells with columnar change, combined with TRAIL expressed by the adjacent myoepithelial cells, allows for OPG–TRAIL interactions between these closely associated cell types. Columnar alteration is a relatively recently recognised change that has gained prominence because of its association with microcalcification, and thus detection through mammographic screening.22 Columnar epithelial change is probably of little clinical relevance23; it is mainly important because it is associated with microcalcification and is therefore present in lesions removed for this screen detected abnormality.
Over half of the invasive cancers were strongly positive for each of the proteins of interest. Our study is the first of its type, and is pilot in design; therefore, it did not have the power to make a definitive assessment of expression patterns in relation to histological type, or to detect an association between the expression of the three proteins. Any protein that is expressed in a subset of an otherwise relatively homogeneous population of tumours (for example, invasive breast cancer of the ductal type) could potentially be related to prognosis, and this should be explored in a much larger population of breast cancers. The data generated in our study will aid in designing future investigations. Because myoepithelial cells in the background breast tissue were positive for TRAIL, this molecule may be a marker of myoepitheliomatous differentiation, which might be expected to have a different biological behaviour.24 A consistent finding was that the pattern of protein expression was identical in invasive and in situ components in those cases where both were present. This could be related to the genetic changes, such as chromosomal loss, which have been identified in in situ carcinoma and even earlier cancer precursors.25 This result warrants further exploration, and we are currently investigating this in a larger set of cases.
“Increased knowledge of the different biological functions of osteoprotegerin and related molecules is crucial, because these potent regulators of biological pathways are currently being investigated as possible therapeutic agents”
Our present study is the first that relates OPG expression to ER/PR status in human primary breast cancer, and we found that 75% of ER/PR+ tumours expressed OPG, which supports and gives relevance to the preclinical modelling. The importance of the correlation between ER/PR+ and ER/PR− breast tumour expression of OPG is yet to be defined, and studies investigating a clinical correlation are ongoing. Of note, OPG expression in osteoblasts is influenced by oestrogen, and Rumpler et al showed that ERα binds to an oestrogen response element and regulates the transcription of OPG in these cells.26 It has been proposed that oestrogen exerts its anti‐resorptive effects partly through stimulation of OPG expression in osteoblasts,27 and that the RANKL–OPG system may also be affected by phyto‐oestrogens,28 but the clinical relevance of OPG and ER signalling in human breast cancers is unknown.
In addition to the possible role of OPG as a survival factor for breast cancer cells, OPG may be linked to tumour cells gaining a growth advantage in certain microenvironments. OPG signalling is important for osteoclast activation and differentiation, and within the bone microenvironment may influence the activity of micrometastatic breast cancer. When breast cancer metastasises, it frequently spreads to the bone, and approximately 70% of all patients with metastatic breast cancer have osseous involvement.29 Whether or not tumour expression of OPG, TRAIL, and/or RANKL alters the bone microenvironment to favour tumour growth requires further exploration. Clinical investigations of OPG and/or RANKL have been performed. Studies include the report by Brown et al, demonstrating RANKL and OPG expression in prostate cancer specimens30; that of Huang et al reporting expression in osseous metastases31; and that of Good et al reporting expression in benign bone tumours, primary malignant tumours, and bone metastases.32 Grimaud et al reported that the ratio of RANKL to OPG is related to the extent of osteolysis occurring in osseous metastases in several tumour types.20 Of note, these authors report that OPG and RANKL colocalised in a variety of tumour types, but our study shows the lack of colocalisation between OPG, TRAIL, and RANKL in primary breast cancers. These differences may be the result of variations in the tissue of origin or assay techniques. The clinical usefulness of serum concentrations of OPG and/or RANKL are being explored.33,34 However, because it is impossible to determine the cellular origin(s) of molecules present in serum, the potential contribution of human tumours to the concentration of serum OPG/RANKL remains unknown.
Increased knowledge of the different biological functions of OPG and related molecules is crucial, because these potent regulators of biological pathways are currently being investigated as possible therapeutic agents. The OPG signalling system is being explored as a means of altering the course of bone metastases, in addition to osteoporosis, and new therapeutic agents targeting this pathway are presently undergoing clinical trials. The potential clinical application of interfering with the OPG pathway is in treating osteoporosis, cancer related osteolytic bone lesions, and hypercalcaemia. OPG, soluble RANK, or RANKL/RANK blocking antibodies may be of therapeutic value for patients with multiple myeloma and metastatic osseous metastases of solid tumours. These molecules, along with therapeutic agents that effect TRAIL signalling, may ultimately have a role in preventing bone metastasis or enhancing the effects of chemotherapy.35
Take home messages
OPG (osteoprotegerin), RANKL (receptor activator of nuclear factor κB ligand), and TRAIL (tumour necrosis factor related apoptosis inducing ligand) expression is limited to specific cell types in non‐malignant breast tissue, with OPG being strongly associated with epithelial cells displaying columnar change
We found a correlation between OPG expression and oestrogen/progesterone receptor positivity in breast tumours, consistent with the data from breast cancer cell lines grown in vitro
OPG, RANKL, and TRAIL were expressed in more than half of the breast tumour tissues examined, their localisation was specific, and they were not colocalised
This specificity may provide a useful marker of functional differentiation in breast cancer; for example, TRAIL expression as a marker of myoepithelial differentiation
In conclusion, we have found that in non‐malignant breast tissue, the expression of OPG, RANKL, and TRAIL is limited to specific cell types, with OPG being strongly associated with epithelial cells displaying columnar change. Our current study was powered to determine the potential correlation between OPG expression and ER/PR expression status of breast tumours, and we established a correlation (p = 0.011). This is consistent with the data obtained when studying breast cancer cell lines grown in vitro—MDA‐MB‐436/231 (hormone independent) and MCF7 (hormone dependent) cell lines. The ER–PR–OPG correlation and the frequency of OPG, TRAIL, and RANKL staining have been taken into account during the statistical planning of further studies designed to explore our interesting findings. In the 40 breast tumours we examined, all three molecules were expressed by more than half of the tumours, but there was no correlation between OPG, RANKL, and TRAIL expression. The potential role of OPG in modifying tumour cell survival in vivo is an innovative area of research, and has implications for our understanding of the development of breast cancer and the establishment of metastatic disease.
Acknowledgements
This study was supported by Weston Park Cancer Appeal, Sheffield, UK and the Research Directors of the University of Sheffield, Sheffield, UK. We are grateful for the expert technical assistance provided by Mrs J Lippitt and Mrs A Evans.
Abbreviations
ER - oestrogen receptor
IHC - immunohistochemistry
OPG - osteoprotegerin
PR - progesterone receptor
RANK(L) - receptor activator of nuclear factor κB (ligand)
TNF - tumour necrosis factor
TRAIL - tumour necrosis factor related apoptosis inducing ligand
References
- 1.Hu W, Kavanagh J J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 200312721–729. [DOI] [PubMed] [Google Scholar]
- 2.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signalling, biology and potential for cancer therapy. Cytokine Growth Factor Rev 200314337–348. [DOI] [PubMed] [Google Scholar]
- 3.Pitti R M, Marsters S A, Ruppert S.et al Induction of apoptosis by Apo‐2 ligand, a new member of the tumour necrosis factor cytokine family. J Biol Chem 199627112687–12690. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan P, Marsters S A, Pitti R M.et al Control of TRAIL induced apoptosis by a family of signalling and decoy receptors. Science 1997277818–821. [DOI] [PubMed] [Google Scholar]
- 5.Pan G, O'Rourke K, Chinnaiyan A M.et al The receptor for the cytotoxic ligand TRAIL. Science 1997276111–113. [DOI] [PubMed] [Google Scholar]
- 6.MacFarlane M, Ahmad M, Srinivasula S M.et al Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem 199727225417–25420. [DOI] [PubMed] [Google Scholar]
- 7.Herrnring C, Reimer T, Jeschke U.et al Expression of the apoptosis inducing ligands FasL and TRAIL in malignant and benign human breast tumours. Histochem Cell Biol 2000113189–194. [DOI] [PubMed] [Google Scholar]
- 8.Emery J G, McDonnell P, Burke M B.et al Osteoprotegerin is a receptor for cytotoxic ligand TRAIL. J Biol Chem 1998273143636–143637. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda E, Goto M, Mochizuki S.et al Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 1997234137–142. [DOI] [PubMed] [Google Scholar]
- 10.Yun T J, Tallquist M D, Aicher A.et al Osteoprotegerin, a crucial regulator of bone metabolism also regulates B cell development and function. J Immunol 20011661482–1491. [DOI] [PubMed] [Google Scholar]
- 11.Holen I, Croucher P I, Hamdy F C.et al Osteoprotegerin is a survival factor for human prostate cancer cells. Cancer Res 2002621619–1623. [PubMed] [Google Scholar]
- 12.Neville‐Webbe H L, Cross N A, Eaton C L.et al Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL‐induced apoptosis. Breast Cancer Res Treat 200486269–279. [DOI] [PubMed] [Google Scholar]
- 13.Shipman C M, Croucher P I. Osteoprotegerin is a soluble decoy receptor for tumour necrosis factor‐related apoptosis‐inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res 200363912–916. [PubMed] [Google Scholar]
- 14.Ito R, Nakayama H, Yoshida K.et al Expression of osteoprotegerin correlates with aggressiveness and poor prognosis of gastric carcinoma. Virchows Arch 2003443146–151. [DOI] [PubMed] [Google Scholar]
- 15.Reinholz M M, Iturria S J, Roche P C. Differential gene expression of TGF‐beta family members and osteopontin in breast tumour tissue: analysis by real‐time quantitative PCR. Breast Cancer Res Treat 200274255–269. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno A, Amizuka N, Irie K.et al Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 1998247610–615. [DOI] [PubMed] [Google Scholar]
- 17.Fata J E, Kong Y ‐ Y, Li J.et al The osteoclast differentiation factor osteoprotegerin‐ligand is essential for mammary gland development. Cell 200010341–50. [DOI] [PubMed] [Google Scholar]
- 18.Martin T J, Gillespie M T. Receptor activator of nuclear factor kappa B ligand (RANKL): another link between breast and bone. Trends Endocrinol Metab 2001122–4. [DOI] [PubMed] [Google Scholar]
- 19.Truneh A, Sharma S, Silverman C.et al Temperature‐sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem 200027523319–23325. [DOI] [PubMed] [Google Scholar]
- 20.Grimaud E, Soubigou L, Couillaud S.et al Receptor activator of nuclear factor {kappa}B ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol 20031632021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas R J, Guise T A, Yin J J.et al Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 19991404451–4458. [DOI] [PubMed] [Google Scholar]
- 22.Fraser J L, Raza S, Chorny K.et al Columnar alteration with prominent apical snouts and secretions: a spectrum of changes frequently present in breast biopsies performed for microcalcifications. Am J Surg Pathol 1998221521–1527. [DOI] [PubMed] [Google Scholar]
- 23.Schnitt S J, Vincent‐Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol 200310113–124. [DOI] [PubMed] [Google Scholar]
- 24.Schnitt S J. Flat epithelial atypia—classification, pathologic features and clinical significance. Breast Cancer Res 20035263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eusebi V, Feudale E, Foschini M P.et al Long term follow‐up of in situ carcinoma of the breast. Semin Diagn Pathol 199411223–235. [PubMed] [Google Scholar]
- 26.Rumpler M, Vagra F, Nemeth P.et al Identification of an estrogen response element in the osteoprotegerin promoter. Bone 200333S17 (abstract 28) [Google Scholar]
- 27.Bord S, Ireland D C, Beavan S R.et al The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 200332136–141. [DOI] [PubMed] [Google Scholar]
- 28.Crisafulli A, Altavilla D, Squadrito G.et al Effects of the phytoestrogen genistein on the circulating soluble receptor activator of nuclear factor kappaB ligand–osteoprotegerin system in early postmenopausal women. J Clin Endocrinol Metab 200489188–192. [DOI] [PubMed] [Google Scholar]
- 29.Coleman R E, Rubens R D. The clinical course of bone metastases from breast cancer. Br J Cancer 19875561–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown J M, Corey E, Lee Z D.et al Osteoprotegerin and RANK ligand expression in prostate cancer. Urology 200157611–616. [DOI] [PubMed] [Google Scholar]
- 31.Huang L, Cheng Y Y, Chow L T.et al Tumour cells produce receptor activator of NF‐κB ligand (RANKL) in skeletal metastases. J Clin Pathol 200255877–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Good C R, O'Keefe R J, Puzas J E.et al Immunohistochemical study of receptor activator of nuclear factor kappa‐B ligand (RANK‐L) in human osteolytic bone tumours. J Surg Oncol 200279174–179. [DOI] [PubMed] [Google Scholar]
- 33.Lipton A, Ali S M, Leitzel K.et al Serum osteoprotegerin levels in healthy controls and cancer patients. Clin Cancer Res 200272306–2310. [PubMed] [Google Scholar]
- 34.Terpos E, Szydlo R, Apperley J F.et al Soluble receptor activator of nuclear factor κB ligand–osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 20031021064–1069. [DOI] [PubMed] [Google Scholar]
- 35.Mundy G R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 20028584–593. [DOI] [PubMed] [Google Scholar]