Abstract
Background
An association between cow's milk hypersensitivity (CMH) and gastro–oesophageal reflux disease (GERD) in childhood has been reported in the past decade.
Aim
To assess whether biopsies from the upper gastrointestinal tract of children with cow's milk sensitive GERD have a specific allergic inflammatory pattern, and to compare two different techniques for measuring inflammatory cells in gastrointestinal biopsies.
Methods
GERD was diagnosed by means of endoscopy and oesophageal pH monitoring. Hypersensitivity to cow's milk was determined by an elimination diet and cow's milk challenge. Allergic inflammatory cells in upper gastrointestinal biopsies were identified by immunohistochemistry and their numbers were assessed by two different methods—counting the number of cells/high power field and using the computerised Cast‐Grid system.
Results
Cow's milk sensitive GERD was identified in 10 of 17 children with severe GERD (median age, 7.8 years). Biopsies from children with endoscopic oesophagitis had significantly increased numbers of mast cells and T cells. No differences in the number of eosinophils, mast cells, or T cells were found between children with CMH and those with primary GERD. Several differences were found between the two different histological quantification methods.
Conclusions
CMH was found not only in infants but also in school age children with GERD. Histology did not identify the cow's milk sensitive GERD subgroup. The computerised histological method provides a more complete evaluation based upon total biopsy area, and helped to limit the bias of uneven biopsy size.
Keywords: food allergy, gastro–oesophageal reflux disease, eosinophils, quantification, children
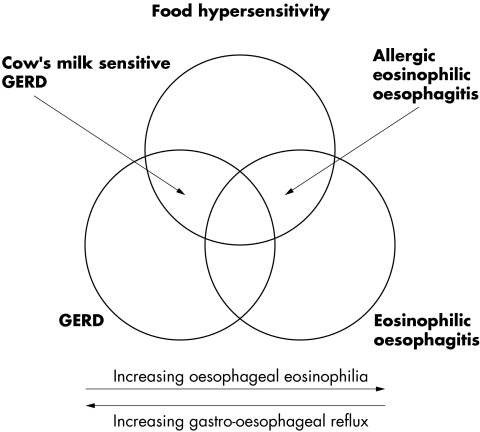
An association between gastro–oesophageal reflux disease (GERD) and hypersensitivity to cow's milk has been described in several publications.1,2,3 A phasic pH pattern in the oesophagus and increased concentrations of IgG anti‐β lactoglobulin antibody in serum have been suggested as characteristics of this subgroup of patients with cow's milk sensitive GERD.4 It is currently unknown whether a specific allergic inflammatory pattern is characteristic for these patients. Increased basal zone, papillary length, and infiltration by eosinophils are the classic histological hallmarks of GERD.5 The presence of oesophageal eosinophils was first described as a diagnostic feature of paediatric GERD by Winter et al.6 However, there are several causes of oesophageal eosinophilia other than acid reflux. A distinct disease process with intense eosinophilic infiltration in the oesophageal mucosa has been described as eosinophilic oesophagitis. Eosinophilic oesophagitis is clinically characterised by reflux‐like symptoms, dysphagia, and frequent food impactions,7 and endoscopically by rings, webs, longitudinal tears, and a fragile mucosa.8 Histological findings include oesophageal eosinophilia of more than 10–20 eosinophils/high power field (HPF). Normal or only slightly increased acid reflux is found in this group of patients.9 The efficacy of an elimination diet on clinical symptoms and eosinophilic infiltration has been documented in children10 and adolescents/adults with eosinophilic oesophagitis.9 However, no consensus has been reached with regard to the number of oesophageal eosinophils needed to qualify for the diagnosis of eosinophilic oesophagitis. As few as five eosinophils/HPF have been reported as characteristic of eosinophilic oesophagitis.7 At present, our understanding of the interaction of GERD, food hypersensitivity, and oesophageal eosinophilia may be visualised by a Venn diagram (fig 1).
Figure 1 A model showing the interactions between gastro–oesophageal reflux, food hypersensitivity, and eosinophilic oesophagitis. Each entity may exist alone, although overlapping states exist as cow's milk sensitive gastro–oesophageal reflux disease (GERD) and allergic eosinophilic oesophagitis. Pure GERD is characterised by increased oesophageal acid exposure and minimal eosinophilic infiltration in oesophageal biopsies, whereas the opposite is seen in eosinophilic oesophagitis
“There are several causes of oesophageal eosinophilia other than acid reflux”
The aim of our study was to evaluate infiltration by eosinophils, mast cells, and T cells in a group of infants and children with cow's milk sensitive GERD identified by strict criteria for both GERD and cow's milk hypersensitivity.11 This patient group was compared with infants/children with severe GERD and negative milk challenge results and a control group. A further aim was to compare two methods of enumerating eosinophils: (1) counting the number of cells/HPF and (2) assessing with the number of cells in the total biopsy area, using the Olympus Cast‐Grid system, and expressing the results as the median number of cells/106 μm2 epithelium.
Material and methods
Patients and algorithm
During a two year period, 51 infants and children were referred for evaluation of GERD to a tertiary centre of paediatric gastroenterology at the department of paediatrics, Odense University Hospital, Denmark. In total, 42 children completed evaluation for GERD and cow's milk hypersensitivity. Initially, pH monitoring was performed for 48 hours. Eighteen infants and children were identified with severe GERD and completed a four to six week cow's milk elimination diet before a challenge procedure was performed according to current EAACI guidelines.12 Infants/children with a positive reaction continued on the elimination diet, whereas negative responders abandoned the diet and started proton pump inhibitor treatment until follow up endoscopy and pH monitoring. Detailed information on the clinical, endoscopic, and allergic characteristics of the cow's milk sensitive GERD group has been published.11
GERD diagnosis
Upper endoscopy was performed under general anaesthesia using an Olympus Gif N230 videoscope. pH monitoring was conducted with either the Digitrapper MkIII (n = 9) or the Digitrapper pH (n = 33) monitor for 48 hours. Project criteria for severe GERD were endoscopic oesophagitis according to the LA criteria13 and/or a reflux index (fraction of recording time with oesophageal pH < 4) >10% at least on one of the two recording days.
Allergy investigations
The challenge was performed as a double blind, placebo controlled procedure in children greater than 3 years of age and as an open procedure in infants and children less than 3 years of age.
Histology
Two biopsy specimens were obtained from the oesophagus, approximately 3 and 5 cm above the Z‐line; in addition, one biopsy was taken from the antrum of the stomach and one from the first part of the duodenum. Biopsies were placed on Millipore filter paper with the mucosa upwards and fixed in 10% neutral buffered formaldehyde. Oesophageal biopsy specimens were analysed for thickness of basal zone and elongation of the papillae after staining with haematoxylin and eosin. Data from postmortem studies were used as reference material for these parameters: upper limit (mean, + 3 SD) for basal zone, 24%; upper limit for papillary length, 53% of total epithelium.14
Immunohistochemistry protocol
Mast cells were identified with a monoclonal anti‐tryptase antibody (AA1; DakoCytomation, Glostrup, Denmark), eosinophils with a monoclonal antibody to major basic protein (BMK‐13; Monosan, Uden, the Netherlands), and T cells with a polyclonal anti‐CD3 antibody.
We evaluated the optimal antigen retrieval method for each antibody. For CD3 staining microwave heating in 10mM Tris with 0.5mM EGTA at pH 9.0 for 26 minutes proved optimal. For eosinophil major basic protein antibody BMK‐13 and mast cell tryptase antibody AA1 an enzymatic retrieval protocol was superior to the heat induced epitope retrieval method. BMK‐13 retrieval was done using protease (1/200) for 15 minutes at 37°C, and treatment with pepsin (1/20) for 15 minutes at 37°C was used for AA1.
For the final analysis, 4 µm thick sections were cut from neutral buffered formaldehyde fixed, paraffin wax embedded tissue blocks. Sections were mounted on ChemMate™ capillary gap slides (DakoCytomation), dried at 60°C, dewaxed, and hydrated. Before antigen retrieval, endogenous peroxidase was blocked by incubation in 1.5% hydrogen peroxide in Tris buffered saline (pH 7.4) for 10 minutes. The antibodies were incubated for 60 minutes at room temperature. Immunostaining was automated using the EnVision+™ HRP detection systems K4001 (AA1 and BMK‐13) and K4003 (CD3) (DakoCytomation) on the TechMate™ 500 instrument (DakoCytomation). DAB+ K3468 was used as the chromogen (DakoCytomation). Immunostaining was followed by brief nuclear counterstaining in Mayer's haematoxylin. Finally, coverslips were mounted with AquaTex (Merck, Darmstadt, Germany).
Measurement of inflammatory cells
Two different methods were used to measure the numbers of immunostained cells. Initially, the biopsy area was scanned at a low power magnification and the area where inflammation was most dense was identified. The numbers of cells/HPF were then counted. In addition, a second method using a computerised principle to assess the total biopsy area was applied. The biopsy area was measured by the Olympus Cast‐Grid computer system (Olympus, Albertslund, Denmark) by delineating the borders of the biopsy at ×100 magnification. The area was expressed as μm2 epithelium. The area was then scanned at ×200 magnification and immunopositive cells were marked and automatically counted. T cells were too numerous to count in the total biopsy area, so that the Cast‐Grid Meander sampling function was used instead. A fraction of the total biopsy area, in this case 25%, was randomly projected on to the computer screen and the cells were counted. The number of cells was expressed as median number of cells/106 μm2 epithelium. Only intact cells with a distinct nucleus were counted. T cells were not counted by the HPF method in the antrum and duodenum because they were so abundant.
Only immunopositive cells in the squamous epithelium were counted in the oesophageal biopsy specimens, because the lamina propria was not constantly present. Counting was carried out in a blinded manner using coded slides.
Statistics
Groups were compared by the Mann‐Whitney U test, Kruskal‐Wallis test, and Fisher's exact test when appropriate. Paired data were analysed by the Wilcoxon signed rank test. Significance was set at p < 0.05.
Ethics
The protocol was approved by the regional committee for ethics in medical research for the Vejle and Funen counties, Denmark. Informed consent was obtained from the parents or legal guardians of the children.
Results
Patients
Based upon the diagnostic criteria 10 infants and children (median age, 7.8 years; range, 2–178 months) with severe GERD and CMH were identified. This group was compared with a group of infants/children with severe primary GERD (n = 7) and a control group without GERD (n = 24). No differences were seen between the groups with regard to age or sex.
Biopsy characteristics
To assess the number of inflammatory cells a total of 272 oesophageal, 148 antral, and 159 duodenal biopsy specimens were immunostained and the biopsy area and number of cells measured. The area of the oesophageal biopsies (median, 838 000 × 106/μm2 epithelium) was significantly smaller (p < 0.001) than that of the antral (median, 1216 000 × 106/μm2) and duodenal biopsies (median, 1294 000 × 106/μm2).
Epithelial changes induced by increased cell turnover
A significant difference (p = 0.0001) was found in the thickness of the basal zone between endoscopically normal patients (median, 10%; range, 5–30%) and those with endoscopic oesophagitis (median, 40%; range 30–70%). Similar findings were noted for papillary length; patients with a normal endoscopy had a median papillary length of 50% (range, 20–80%) and those with endoscopic oesophagitis had a median value of 85% (range, 60–95%; p = 0.001). No differences were seen between the two GERD groups.
Cellular infiltration in the oesophagus according to endoscopic findings
Significantly higher numbers of mast cells, eosinophils, and T cells were found in the oesophageal biopsies from infants/children with endoscopic oesophagitis. However, a lack of consistency was seen between the HPF and the Cast‐Grid methods (table 1).
Table 1 Numbers of mast cells, eosinophils, and T cells in oesophageal biopsies from infants and children with and without endoscopic oesophagitis (LA grade 1 or above).
| Normal endoscopy (n = 33) | Endoscopic oesophagitis (n = 7) | p Value | ||||
|---|---|---|---|---|---|---|
| Cells/HPF | Cast‐Grid | Cells/HPF | Cast‐Grid | Cells/HPF | Cast‐Grid | |
| Mast cells | 3 (0–17) | 13.5 (0–26) | 6 (1–43) | 32.5(7–158) | 0.22 | 0.03 |
| Eosinophils | 0 (0–6) | 0 (0–13) | 2 (0–36) | 3 (0–130) | 0.01 | 0.14 |
| T cells | ND | 109 (26–547) | ND | 321 (94–909) | ND | 0.03 |
Significant differences were noted for all parameters but with a lack of consistency between the two different methods.
Data are presented as median (range).
HPF, high power field; ND, not done.
Cellular infiltration according to clinical group
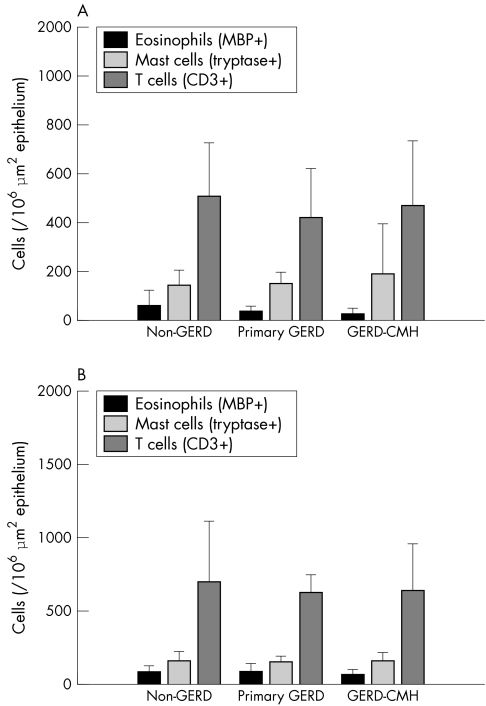
No significant differences were found between the clinical groups for mast cell, eosinophil, and T cell numbers in all biopsies using the two methods (table 2; fig 2).
Table 2 Cell numbers in oesophageal biopsies from the different patient groups.
| Primary GERD | GERD‐CMH | Controls | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Cells/HPF | Cast‐Grid | Cells/HPF | Cast‐Grid | Cells/HPF | Cast‐Grid | Cells/HPF | Cast‐Grid | |
| Mast cells | 3 (1–8) | 17 (7–91) | 3 (1–43) | 15 (0–158) | 3 (1–17) | 15 (9–26) | 0.95 | 0.91 |
| Eosinophils | 0 (0–3) | 0 (0–8) | 0 (0–36) | 1 (0–130) | 0 (0–6) | 0 (0–13) | 0.63 | 0.65 |
| T cells | ND | 140 (104–787) | ND | 321 (64–908) | ND | 108 (25–298) | ND | 0.10 |
Data are presented as median (range).
Because of the minimal infiltration by eosinophils in most of the oesophageal biopsies the median values are zero. No significant difference was noted between the groups.
CMH, cow's milk hypersensitivity; GERD, gastro–oesophageal reflux disease; HPF, high power field; ND, not done.
Figure 2 Numbers of inflammatory cells in biopsies from the antrum and the duodenum. No differences were seen between the clinical groups. Based upon Cast‐Grid data.
Oesophageal eosinophils in different patient groups
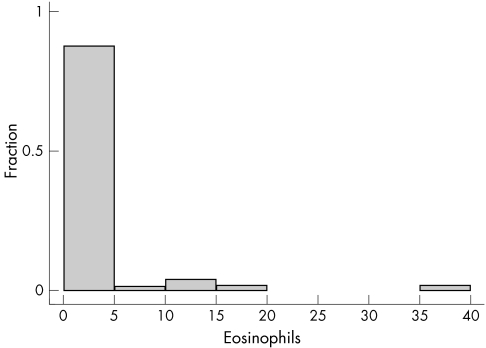
In general, few eosinophils were found in the oesophageal biopsies. In total, biopsies from 13 of 41 patients contained oesophageal eosinophils (range, 1–41/HFP). Counts of more than 10/HPF were only noted in four patients (fig 3). Table 3 categorises patients into those with and without eosinophils in the oesophagus. Again, significantly more endoscopy positive infants/children had eosinophils, but no significant differences were seen between the groups.
Figure 3 Eosinophils in each high power field in oesophageal biopsies. Eosinophils were found in the oesophageal biopsies of only 13 of the 41 patients. One to 10 eosinophils were found in nine patients, 10–20 in three patients, and 20–40 in one patient.
Table 3 Categorisation of infants/children according to the presence or absence of oesophageal eosinophils.
| No eosinophils | Eosinophils present (>1) | |
|---|---|---|
| Endoscopic findings* | ||
| Normal endoscopy (n = 33) | 25 | 8 |
| Oesophagitis (n = 7) | 2 | 5 |
| Clinical group† | ||
| Controls (n = 24) | 18 | 6 |
| Primary GERD (n = 7) | 4 | 3 |
| GERD–CMH (n = 10) | 6 | 4 |
*Fishers exact test, p = 0.03; Pearson χ2 test, p = 0.54. Significant differences were noted for endoscopic status only and not GERD group.
CMH, cow's milk hypersensitivity; GERD, gastro–oesophageal reflux disease.
Changes in inflammatory cells after treatment
Based on the Cast‐Grid method, the follow up biopsies in the cow's milk sensitive GERD group showed a significant increase in numbers of eosinophils in the biopsies from the antrum and the duodenum after the elimination diet. Antrum numbers before the diet were 22 cells/106 μm2 epithelium but after the diet they were 77 cells/106 μm2 (p = 0.05). In the duodenum these figures were 58 cells/106 μm2 and 115 cells/106 μm2, respectively (p = 0.01). In the oesophageal biopsies reduced numbers of eosinophils and T cells were found after the diet period, but this finding did not reach significance (p = 0.06 and 0.09, respectively). Follow up biopsy after proton pump inhibitor treatment in the primary GERD group showed a trend towards reduced numbers of oesophageal mast cells (p = 0.06 for the HPF method and p = 0.08 for the Cast‐Grid method) and no changes in eosinophils.
Discussion
With the use of strict criteria for both GERD and cow's milk hypersensitivity we identified a group of infants and children suffering from cow's milk sensitive GERD. This group was characterised by significantly higher oesophageal acid exposure compared with a group of infants/children with primary GERD and a significant reduction in oesophageal acid exposure after the elimination diet.11 We compared the numbers of inflammatory cells in upper gastrointestinal tract biopsies from these patient groups.
Few studies have assessed the physiological numbers of eosinophils in paediatric gastrointestinal tract biopsies. Lowichik and Weinberg counted gastrointestinal tract eosinophils in a postmortem study of 44 infants/children who died suddenly and unexpectedly.15 A gradient of increasing numbers of eosinophils/HFP was seen from the fundus of the stomach towards the terminal ileum. Similar studies by Shub and colleagues16 and Black and colleagues14 reported that the paediatric oesophagus is normally devoid of eosinophils, making the presence of even a few eosinophils/HFP clinically important.
In our present study, we hypothesised that increased numbers of mast cells and eosinophils might be characteristic of patients with cow's milk sensitive GERD compared with primary GERD. This hypothesis could not be confirmed. Both the HPF and the Cast‐Grid methods revealed non‐significant differences in the number of inflammatory cells compared with primary GERD and controls. These data are in contrast to those of Justinich et al,17 who reported significantly higher numbers of mast cell and eosinophils in oesophageal biopsies from infants/children with assumed allergic oesophagitis. In our study, both the HPF and the Cast‐Grid methods revealed a reduction in oesophageal eosinophils, which was almost significant (p = 0.06), and simultaneously increased numbers of eosinophils in the antrum and the duodenum (p = 0.05 and p = 0.01, respectively) after the elimination diet in the cow's milk sensitive group only. This finding poses an important question, namely: is it possible that the gastrointestinal tract eosinophils were degranulated at the time of the initial biopsy and “stable and stainable” after the elimination diet? We are not able to answer this question directly because of the descriptive nature of the immunohistochemical investigations. Electron microscopy assessment of eosinophils in biopsy specimens of oesophagitis have indicated signs of activation, including the lucent nature of the granule core proteins and inverted core to matrix densities.18 In animal models, a specific time course association has been noted in the accumulation and activation of eosinophils in the gastrointestinal tract mucosa during and after challenge. In a rodent model of food allergy, mast cells showed a characteristic biphasic pattern, with peaks at 30 minutes and again at 72 hours. The numbers of eosinophils decreased immediately after antigen challenge, but later increased and reached a peak at 48 hours.19
Experimental studies have shown that eosinophils accumulate in the gastrointestinal tract under the influence of eotaxin‐1.20,21 The role of eotaxin in clinical cow's milk sensitive GERD has been illustrated in a recent study including nine patients with cow's milk sensitive GERD.22 Upregulated eotaxin‐1 expression, assessed by immunohistochemistry and a semiquantative grading system, was found in the basal and papillary epithelium of the oesophagus. However, the upregulation of this potent eosinophilic chemokine was only associated with oesophageal eosinophilia in one of nine patients. This observation may be explained by the pH dependence of eotaxin‐1 and CCR3 receptor binding and signalling.23 Thus, the cow's milk sensitive infants/children in the study by Butt et al were characterised by higher oesophageal eotaxin concentrations but, as in our study, not by increased numbers of oesophageal eosinophils.22 In our present study, we evaluated a monoclonal eotaxin antibody as a potential immunohistochemical marker. Using tissue blocks containing multiple organs we could not demonstrate sufficient specificity in the immunohistochemical staining patterns. We cannot exclude the possibility that this lack of specificity resulted from the use of paraffin wax embedded tissue blocks rather than cryopreparations.
The potential effects of degranulation products on upper gut motility have been illustrated in a murine model of eosinophilic gastrointestinal tract inflammation.24 Upon food challenge, the mice developed delayed gastric emptying and gastromegaly, both of which predispose to transient lower oesophageal sphincter relaxation—the most important pathophysiological mechanism for paediatric GERD.25 In addition, Hogan et al detected degenerative changes of enteric nerves in close proximity to eosinophils, indicating that eosinophilic granules have a direct effect on the enteric nervous system.24
The possible role of mast cells has been illustrated in a rodent model of IgE mediated food allergy.26 Food challenge induced mast cell degranulation, significantly delayed gastric emptying, and increased gastric acid secretion compared with controls.
Take home messages
Biopsies from children with endoscopic oesophagitis had significantly increased numbers of mast cells and T cells
There were no differences in the number of eosinophils, mast cells, or T cells between patients with cow's milk hypersensitivity and those with primary gastro–intestinal reflux disease
Eosinophils were reduced in the oesophagus, although this was not quite significant, and increased in the antrum and duodenum after the elimination diet in the cow's milk sensitive group
The computerised histological method provided a more complete evaluation based upon total biopsy area and helped to limit the bias of uneven biopsy size
In our present study there were no differences in the numbers of CD3 positive T cells in the antrum and duodenum of the different patient groups. The population of duodenal T cells may display different functional and cytokine profiles, as recently documented by Beyer et al.27 When milk specific duodenal T cells were isolated from children who were allergic to cow's milk, a high proliferative response and a distinct T helper type 2 profile were seen after restimulation with cow's milk.
“Despite sharing an association with food allergy, cow's milk sensitive gastro–oesophageal reflux disease and eosinophilic oesophagitis appear to be two distinct entities”
We used two different methods for enumerating inflammatory cells. The HPF method relies on counting the numbers of cells in each HFP (×400 magnification) in the most densely inflamed area. This method is used in most publications investigating eosinophilic oesophagitis. For the experienced pathologist the HFP method is simple to use and not time consuming. The disadvantages of the method are that only the most densely infiltrated area is evaluated. Secondly, the HFP method is not adjustable for variations in biopsy size. As an example, the biopsy areas in our study varied by a factor of 20. We also used a computer assisted semiautomatic system (Cast‐Grid, Olympus). This system is based upon the principles of stereology,28 and has to our knowledge not previously been used in the enumeration of gastrointestinal tract inflammatory cells. Using the Cast‐Grid system, the total area of the single biopsy is determined and cellular infiltration expressed as an average of the cellular content of the biopsy. This method is time consuming but limits the possible bias of an uneven distribution of cells and an uneven sample size. Discrepancies were noted between the two methods—the significant increase in eosinophils after the elimination diet was seen only with the Cast‐Grid method. Furthermore, the Cast‐Grid method revealed significantly higher numbers of oesophageal mast cells in infants/children with endoscopic oesophagitis, whereas the HPF method showed significantly higher number of eosinophils. The Cast‐Grid system may be regarded as a more objective method and might particularly be important in studies with a limited number of patients.
In conclusion, histological characterisation was performed using a new method in a group of infants and children with cow's milk sensitive GERD. Infants and children with endoscopic oesophagitis had higher numbers of oesophageal mast cells, eosinophils, and T cells, and had elongated papillae and an increased basal zone. No significant differences were seen in the numbers of eosinophils, mast cells, or T cells in upper gastrointestinal tract biopsies from infants and children with cow's milk hypersensitivity and severe GERD compared with those with primary GERD and controls. Despite sharing an association with food allergy, cow's milk sensitive GERD and eosinophilic oesophagitis appear to be two distinct entities.
Acknowledgements
Mr O Nielsen is acknowledged for skilled technical assistance during the process of immunohistochemistry.
Abbreviations
GERD - gastro–oesophageal reflux disease
HPF - high power field
References
- 1.Iacono G, Carroccio A, Cavataio F.et al Gastroesophageal reflux and cow's milk allergy in infants: a prospective study. J Allergy Clin Immunol 199697822–827. [DOI] [PubMed] [Google Scholar]
- 2.Milocco C, Torre G, Ventura A. Gastro–oesophageal reflux and cows' milk protein allergy. Arch Dis Child 199777183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamer B, Chilarski A, Lange A.et al Gastroesophageal reflux in infants with food allergy. Med Sci Monit 20006348–352. [PubMed] [Google Scholar]
- 4.Cavataio F, Iacono G, Montalto G.et al Gastroesophageal reflux associated with cow's milk allergy in infants: which diagnostic examinations are useful? Am J Gastroenterol 1996911215–1220. [PubMed] [Google Scholar]
- 5.Ismail‐Beigi F, Horton P F, Pope C E. Histological consequences of gastroesophageal reflux in man. Gastroenterology 197058163–174. [PubMed] [Google Scholar]
- 6.Winter H S, Madara J L, Stafford R J.et al Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology 198283818–823. [PubMed] [Google Scholar]
- 7.Orenstein S R, Shalaby T M, Di Lorenzo C.et al The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol 2000951422–1430. [DOI] [PubMed] [Google Scholar]
- 8.Straumann A, Rossi L, Simon H U.et al Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc 200357407–412. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz J E, Spergel J M, Ruchelli E.et al Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 200398777–782. [DOI] [PubMed] [Google Scholar]
- 10.Kelly K J, Lazenby A J, Rowe P C.et al Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid‐based formula. Gastroenterology 19951091503–1512. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen R G, Bindslev‐Jensen C, Kruse‐Andersen S.et al Severe gastroesophageal reflux disease and cow's milk hypersensitivity in infants and children: disease association and evaluation of a new challenge procedure. J Pediatr Gastroenterol Nutr 200439383–391. [DOI] [PubMed] [Google Scholar]
- 12.Bindslev‐Jensen C. Standardization of double‐blind, placebo‐controlled food challenges. Allergy 200156(suppl 67)75–77. [DOI] [PubMed] [Google Scholar]
- 13.Lundell L R, Dent J, Bennett J R.et al Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 199945172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black D D, Haggitt R C, Orenstein S R.et al Esophagitis in infants. Morphometric histological diagnosis and correlation with measures of gastroesophageal reflux. Gastroenterology 1990981408–1414. [PubMed] [Google Scholar]
- 15.Lowichik A, Weinberg A G. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol 19969110–114. [PubMed] [Google Scholar]
- 16.Shub M D, Ulshen M H, Hargrove C B.et al Esophagitis: a frequent consequence of gastroesophageal reflux in infancy. Pediatrics 1985107881–884. [DOI] [PubMed] [Google Scholar]
- 17.Justinich C J, Kalafus D, Esposito P.et al Mucosal mast cells distinguish allergic from gastroesophageal‐reflux induced esophagitis [abstract]. J Pediatr Gastroenterol Nutr 199623342 [Google Scholar]
- 18.Justinich C J, Ricci A, Jr, Kalafus D.et al Activated eosinophils in esophagitis in children: a transmission electron microscopic study. J Pediatr Gastroenterol Nutr 199725194–198. [DOI] [PubMed] [Google Scholar]
- 19.Yang P ‐ C, Berin M, Yu L.et al Mucosal pathophysiology and inflammatory changes in the late phase of the intestinal allergic reaction in the rat. Am J Pathol 2001158681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews A N, Friend D S, Zimmermann N.et al Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A 1998956273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan S P, Mishra A, Brandt E B.et al A critical role for eotaxin in experimental oral antigen‐induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci U S A 2000976681–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt A M, Murch S H, Ng C L.et al Upregulated eotaxin expression and T cell infiltration in the basal and papillary epithelium in cows' milk associated reflux oesophagitis. Arch Dis Child 200287124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dairaghi D J, Oldham E R, Bacon K B. Chemokine receptor CCR3 function is highly dependent on local pH and ionic strength. J Biol Chem 199727228206–28209. [DOI] [PubMed] [Google Scholar]
- 24.Hogan S P, Mishra A, Brandt E B.et al A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol 20012353–360. [DOI] [PubMed] [Google Scholar]
- 25.Omari T I, Barnett C P, Benninga M A.et al Mechanisms of gastro–oesophageal reflux in preterm and term infants with reflux disease. Gut 200251475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catto‐Smith A G, Patrick M K, Scott R B.et al Gastric response to mucosal IgE‐mediated reactions. Am J Physiol 1989257(5 Pt 1)G704–G708. [DOI] [PubMed] [Google Scholar]
- 27.Beyer K, Castro R, Birnbaum A.et al Human milk‐specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol 2002109707–713. [DOI] [PubMed] [Google Scholar]
- 28.Gundersen H J. Stereology—or how figures for spatial shape and content are obtained by observation of structures in sections. Microsc Acta 198083409–426. [PubMed] [Google Scholar]