Abstract
Objective
To report the case of five month old female baby with a history of episodes of gastro‐oesophageal reflux and pneumonia. Her sudden death offered a unique insight into the possible role of delayed neuronal maturation and hypoplasia of the hypoglossal nucleus in representing a likely morphological substrate of sudden death.
Methods
Morphometric analysis was carried out with an Image‐Pro Plus Image analyser (Media Cybernetics) on both sides of the brain stem.
Results
Hypoplasia and neuronal immaturity of the hypoglossal nucleus were demonstrated, accompanied by hypoplasia of the arcuate nucleus.
Conclusions
Much attention should be paid to the possible role of the hypoglossal nucleus in determining a lethal outcome in infancy through impairment of deglutition and subsequent recurrent episodes of pneumonia, and as a necropsy finding.
Keywords: hypoglossal nucleus hypoplasia, hypoglossal nucleus neuronal immaturity, aspiration pneumonia, passive smoking, borderline SIDS
The sudden infant death syndrome (SIDS), or cot death, is the commonest form of death in infancy and is defined as the sudden death of an infant under one year of age which remains unexplained after a thorough case investigation, including performance of a complete necropsy examination, examination of the death scene, and a review of the clinical history.1
Congenital abnormalities of the cardiac conduction system2 and of the medullary arcuate nucleus3,4 have been described as possible morphological substrates for sudden reflexogenic infant death.4,5 Hypoplasia of the arcuate nucleus, an important cardiorespiratory centre on the ventral medullary surface of the brain stem, is characterised by a great morphological variability3 and has been detected in over 50% of SIDS cases3,4 and also in sudden perinatal deaths.4,6 This can be associated with alterations in other brain stem structures, such as hypoplasia of the parabrachial/Kölliker‐fuse complex and the respiratory reticular formation.4,7,8
However, while the arcuate nucleus is of great interest, particularly in view of the frequency and pathogenic implications of alterations of this structure,4,5,7 the hypoglossal nucleus is rarely considered in the aetiopathogenesis of SIDS. Few, if any, cases of sudden infant death have been reported where hypoplasia and neuronal immaturity of this nucleus has been found.
Our purpose is to report a case of five month old female baby with a history of episodes of gastro‐oesophageal reflux and pneumonia, who died suddenly. This offered a unique insight into the possible role of delayed neuronal maturation and hypoplasia of the hypoglossal nucleus as a likely morphological substrate of sudden death.
Case report
A five month old girl was found unresponsive in her cot. Emergency help was summoned. The baby was taken to an emergency department where all possible resuscitation efforts were attempted without success, and she was pronounced dead on the day after arrival.
The baby was the firstborn child, born at term after an uncomplicated pregnancy, with a birth weight of 2980 g. She seemed to be normal at birth and continued in apparent good health until she was admitted to hospital at the age of one month because of an episode of apnoea. The baby was taken into an emergency department where resuscitative manoeuvres were successfully undertaken. Chest x ray showed extensive parenchymal thickening involving the upper median field of the right lung and lesser parenchymal thickening in the homolateral basal site. The other investigations carried out (cerebral ultrasound, echocardiography, blood culture, urine culture, faeces culture, pharyngeal swab) were within normal limits. During her hospital stay, the baby was given wide spectrum antibiotic therapy with ampicillin and gentamicin, and then with cephtriaxone, as well as gastric protection with ranitidine and intravenous rehydration. She did not have any evident symptoms, had no fever, and fed regularly; her bowel function gradually normalised. She was discharged six days later in good general condition after a further chest x ray had confirmed a normal picture. After hospital discharge, the baby continued well except for sporadic episodes of “gastro‐oesophageal reflux”, as reported by her parents. The infant was breast fed and the mother was a cigarette smoker (“less than one pack/day” reported by the mother upon inquiry).
A necropsy examination was requested because of the clinical suspicion of SIDS. The case was referred to our Institute of Pathology, University of Milan, for specialised studies.
Methods
A complete necropsy was carried out, including close examination of the central and peripheral autonomic nervous structures involved in cardiorespiratory reflexogenesis and of the cardiac conduction system, according to our guidelines.4,6,8,9
The brain stem was divided into four blocks. The first, or cranial, block extended from the border between the medulla oblongata and pons up to the upper pole of the olivary nucleus. The second, or intermediate, block corresponding to the submedian area of the inferior olivary nucleus, took the obex as the reference point and extended 2 to 3 mm above and below the obex itself. The third, or caudal, block included the lower pole of the inferior olivary nucleus and the lower adjacent area of the medulla oblongata. A fourth block of the brain stem included the ponto‐mesencephalic portion.4,5,7,8,9
Transverse serial sections were made through each block at intervals of 30 μm (levels). For each level, 12 sections 5 μm thick were obtained, three of which were routinely stained for histological examination using alternately haematoxylin‐eosin, Bielschowsky, and Klüver‐Barrera stains; the other nine were saved and stained as deemed necessary for further investigations.4,5,7,8,9 The average number of sections was 1100, corresponding to about 90 levels. The pertinent nuclei were outlined, namely the arcuate nucleus, the hypoglossal nucleus, the dorsal vagus motor nucleus, the tractus solitarii nucleus, the ambiguus nucleus, the trigeminal tract and nucleus, and the ventrolateral reticular formation in the medulla; and the locus coeruleus and the parabrachial/Kölliker‐Fuse complex in the pons.4,9
Morphometric analysis was carried out with an Image‐Pro Plus Image analyser (Media Cybernetics, Silver Spring, Maryland, USA) on both sides of the brain stem. The analysis was done at the same defined histological level of the medulla for comparison between this case and an age matched control who died of hypertrophic cardiomyopathy. In the hypoglossal and arcuate nuclei the following parameters were evaluated on the right and left side as mean values: nuclear area (expressed as mm2); neuronal density (expressed as the number of neurones per mm2); the profile of the neurones (neuronal nuclear and cytoplasmic area, expressed as μm2); the nucleus to cytoplasm ratio (N/C); and the form factor of the neurones (the FF index), according to the formula: 4πarea/perimeter2 (the FF index value of a perfect circle is 1.0). The outer boundary of the hypoglossal and arcuate nuclei was delineated on both sides of the medulla in the same specimen.
The cardiac conduction system was removed in two blocks: the first included the sinoatrial node and the crista terminalis, the second contained the atrioventricular node, the His bundle down to the bifurcation, and the bundle branches. These two blocks were cut serially at intervals of 40 μm (levels) and alternately stained with haematoxylin‐eosin and Azan, as previously described.2,4,6,9
Immunohistochemistry was carried out on lung sections by the ABC (avidin‐biotin complex) method, using antibodies against CD 68 antigen. A lung section was stained with Sudan III to visualise areas of fat deposition.
Pathological findings
At necropsy, the external examination was normal for age and sex. The white female infant had a body weight of 6810 g and body length of 66.7 cm. Macroscopic examination did not reveal malformations or organ malposition, nor marks of violence.
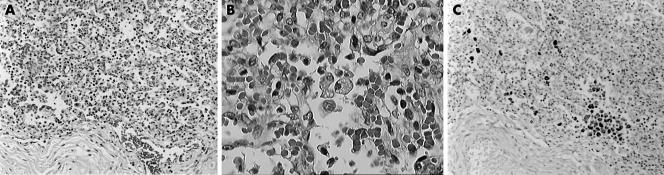
Histopathological examination of the lungs showed bilateral diffuse pneumonia with marked inflammatory infiltrates both in the bronchial branches and in the alveolar cavities, associated with reactive lymph node hyperplasia in the hilar and mediastinic lymph nodes. The pulmonary phlogistic infiltrate consisted of numerous granulocytes, including eosinophils, lymphocytes, monocytes, and CD68+ macrophages and multinuclear macrophages (giant cells), with intracellular deposits of lipidic material consistent with milk ingestion (fig 1, panels A to C). The affected alveoli were in part stuffed with macrophages and foreign body giant cells with foamy cytoplasm which contained large amounts of fat, much of it sudanophil. The neutrophilic exudate was accompanied by dark blue bacterial colonies. The localised foreign body giant cell response to the aspirated material was accompanied by sequels of previous similar aspiration episodes represented by chronic abscessing inflammation, with large areas of pink necrotic tissue bordered by granulation tissue and endoalveolar fibrosis.
Figure 1 (A) Histopathological appearance of diffuse pneumonia. Haematoxylin‐eosin, original magnification ×200. (B) Detail of (A) at higher magnification. White cell infiltrate with granulocytes, lymphocytes, monocytes, macrophages and giant cells with intracellular deposits of lipid material consistent with milk ingestion. Haematoxylin‐eosin, ×400. (C) The same field as (A): macrophages marked by immunohistochemical staining for CD68, ×200.
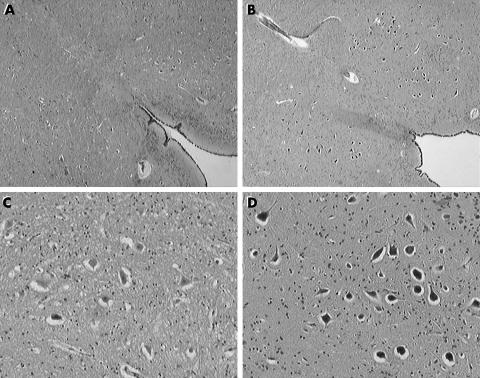
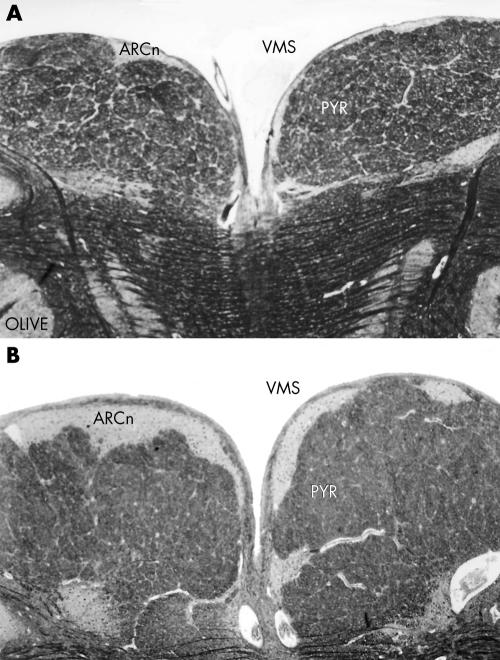
Examination of the brain stem on serial sections revealed hypoplasia and neuronal immaturity of the hypoglossal nucleus (fig 2) and bilateral hypoplasia of the arcuate nucleus (fig 3), as confirmed by two dimensional morphometric reconstruction and comparison with an age matched control case. The morphometric results are shown in table 1.
Figure 2 Hypoplasia and neuronal immaturity of the hypoglossal nucleus (A, C), compared with an age matched control case (B, D). (A, B) Haematoxylin‐eosin, original magnification ×25; (C, D) haematoxylin‐eosin, original magnification ×100.
Figure 3 Bilateral hypoplasia of the arcuate nucleus (A), compared with an age matched control case (B). ARCn, arcuate nucleus; OLIVE, principal inferior olivary nucleus; PYR, pyramid; VMS, ventral medullary surface. Klüver‐Barrera stain, original magnification ×25.
Table 1 Morphometric variables of hypoglossal and arcuate nuclei in this case of borderline SIDS and an age matched control.
| Morphometric variables | Borderline SIDS | Control case | ||
|---|---|---|---|---|
| Hypoglossal N | Arcuate N | Hypoglossal N | Arcuate N | |
| Nuclear area (mm2) | 0.62 | 0.15 | 0.74 | 0.64 |
| Neuronal density (No of neurones/mm2) | 62 | 189.5 | 66 | 246 |
| Neuronal nuclear area (μm2) | 60.40 | 53.95 | 113.35 | 88.8 |
| Neuronal cytoplasmic area (μm2) | 384.85 | 132.15 | 523.30 | 205.5 |
| Nuclear/cytoplasmic ratio (N/C) | 0.18 | 0.44 | 0.24 | 0.45 |
| Neuronal form factor (FF index) | 1.7 | 1.3 | 1.9 | 1.35 |
N, nucleus; SIDS, sudden infant death syndrome.
No abnormalities of the other cardiorespiratory medullary nuclei—that is, the dorsal vagus motor, the tractus solitarii, the ambiguus, the trigeminal nuclei, the ventrolateral reticular formation, and the parabrachial/Kölliker‐Fuse complex—were observed.
Histological examination of the cardiac conduction system showed the sino‐atrial node and its adjacent ganglia to be normal. Islands of conduction tissue, known as persistent fetal dispersion, were observed in the central fibrous body, as well as areas of resorptive degeneration2 in the atrioventricular node.
Discussion
In sudden unexpected infant and perinatal death, frequent developmental abnormalities in the brain stem, particularly in the arcuate nucleus, have been identified.3,4,5,6,7 Sometimes the arcuate nucleus hypoplasia is associated with alterations in other brain stem structures, such as the respiratory reticular formation, and with pulmonary hypoplasia in near term fetuses.4 Recently, in cases of sudden unexpected perinatal and infant death, we observed an intense somatostatin positivity in the hypopglossus nucleus, as well as in other brain stem nuclei involved in respiratory activity.10
The most important pathogenic hypotheses of SIDS are a respiratory (apnoea), cardiac (arrhythmogenic), or visceral dyskinesia (glottal spasm and/or oesophagogastric reflux).2,11,12 It has been stressed that the autonomic nervous system (respiratory, cardiovascular, and upper digestive) interact in all such hypotheses, even in that of a cardiac arrhythmogenic dyskinesia, as the conduction system is subject to strict autonomic control.4,5,6,7
The borderline SIDS or “grey zone” cases have been described as cases in which anatomo‐pathological findings alone might not have accounted for the sudden deaths, if it had not been for the location or concomitant presence of brain stem abnormalities, which could have played a triggering role in causing the sudden death.13 SIDS grey zone or borderline cases have been described as consistent with the triple risk model of SIDS, a hypothesis postulating an underlying biological vulnerability to exogenous stressors or triggering factors at a critical developmental period.13,14 Previously we reported cases of SIDS grey zone in which only our further investigations on serial sections successfully identified anatomo‐pathological findings that probably represented the morphological substrates for a sudden reflexogenic death.13 Cases were classified as borderline in relation to the location of inflammatory infiltrates,15 or even a haemangioendothelioma,16 in centres controlling the cardiorespiratory and arousal activities. Such lesions alone might or might not have accounted for sudden death if it had not been for the location which could have played a triggering role.13,15,16 Krous et al,17 in the new definitional and diagnostic approach of SIDS, while not mentioning the SIDS grey zone cases, described as “unclassified sudden infant deaths” those cases that do not meet the criteria for a diagnosis of SIDS and for which alternative diagnoses of natural or unnatural conditions were equivocal. However, they anticipated that such new definitions of SIDS would have been modified to accommodate new understandings of SIDS and sudden infant death.17
Take home messages
Hypoplasia and neuronal immaturity of the hypoglossal nucleus were found in a child dying suddenly at 5 months of age.
Attention should be paid to the possible role of the hypoglossal nucleus in the pathogenesis of SIDS, by causing impairment of deglutition and recurrent episodes of pneumonia.
The anomalous morphological brain stem (figs 2 and 3) and cardiac conduction findings detected in the present case represent a plausible basis for the diagnosis of SIDS.2,3,4,5 Our pulmonary anatomo‐pathological examination showed bilateral aspiration pneumonia with confluent foci, accompanied by areas of endoalveolar fibrosis which are consistent with sequels of previous episodes of aspiration pneumonia. On the basis of the clinical history, it would seem that in this case the aspiration of milk and subsequent pneumonia had manifested in recurrent episodes. Therefore at the time of death the baby had suffered a new acute episode of lipidic pneumonia caused by aspiration, probably similar to that which led to the hospital admission at the age of one month. The hypoplasia and neuronal immaturity of the hypoglossal nucleus seem to have been responsible for the baby's symptoms of deglutition impairment with subsequent episodes of recurrent pneumonia, one of which required a hospital admission. Milk aspiration can be attributed to functional swallowing disturbances caused by congenital alteration of the hypoglossal nucleus—that is, hypoplasia and neuronal immaturity. In our case this congenital hypoglossal abnormality was associated with arcuate nucleus hypoplasia, a frequent pathological substrate of sudden infant and perinatal death. Thus the case could be diagnosed as SIDS borderline, and the progressively worsening pulmonary symptoms should be considered secondary to the congenital anomalies of the brain stem nuclei rather than being a localised illness.
Maternal smoking has been reported to be significantly associated with SIDS18 and may have played a role in the congenital abnormalities of the hypoglossal and arcuate nuclei in this case. We have recently demonstrated a significantly increased incidence of cytoarchitectural alterations of the brain stem nuclei in stillborn infants and SIDS victims with smoking mothers compared with victims with non‐smoking mothers.19 In this case, the maternal cigarette smoke could have played a triggering role in determining death in a vulnerable infant and as well, and could have contributed to the abnormalities of both the hypoglossal (fig 2) and the arcuate nucleus (fig 3).
We are convinced that much attention should be paid to the possible role of the hypoglossal nucleus in determining a lethal outcome in infancy through deglutition impairment and subsequent recurrent episodes of aspiration pneumonia—in this case at the age of one month and as a necropsy finding at five months.
Acknowledgements
This study was supported by the “Lino Rossi” Research Centre for the study and prevention of unexpected perinatal death and sudden infant death syndrome (SIDS) funding (Rectorial Decree No 225678 of 23/04/04), and by the Ministry of Foreign Affairs (joint project of particular relevance No 269/P/0085087 “Anatomopathologic and genetic study of unexplained perinatal death and SIDS”), Milan, Italy. We wish to express our gratitude to Dr Graziella Alfonsi and Dr Lorella Terni for their skilful technical assistance.
Abbreviations
SIDS - sudden infant death syndrome
Footnotes
URL: http://users.unimi.it/∼pathol/pathol_e.html
References
- 1.Willinger M, James L S, Catz C. Defining the Sudden Infant Death Syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health Development. Pediatric Pathol 199111677–684. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Matturri L, Rossi L.et al Crib death: further support for the concept of fatal cardiac electrical instability as the final common pathway. Int J Cardiol 20039217–26. [DOI] [PubMed] [Google Scholar]
- 3.Matturri L, Ottaviani G, Alfonsi G.et al Study of the brainstem, particularly the arcuate nucleus, in sudden infant death syndrome (SIDS) and sudden intrauterine unexplained death (SIUD). Am J Forensic Med Pathol 20042544–48. [DOI] [PubMed] [Google Scholar]
- 4.Matturri L, Ottaviani G, Lavezzi A M. Techniques and criteria in pathologic and forensic‐medical diagnostics of sudden unexpected infant and perinatal death. Am J Clin Pathol 2005124259–268. [DOI] [PubMed] [Google Scholar]
- 5.Matturri L, Ottaviani G, Lavezzi A M. Sudden infant death triggered by dive reflex. J Clin Pathol 20055877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ottaviani G, Lavezzi A M, Rossi L.et al Sudden unexpected death of a term fetus in a anticardiolipin positive mother. Am J Perinatal 20042179–83. [DOI] [PubMed] [Google Scholar]
- 7.Matturri L, Ottaviani G, Benedetti G.et al Unexpected perinatal death and sudden infant death syndrome (SIDS): anatomo‐pathological and legal aspects. Am J Forensic Med Pathol 200526155–160. [PubMed] [Google Scholar]
- 8.Lavezzi A M, Ottaviani G, Rossi L.et al Hypoplasia of the parabrachial/Kölliker‐fuse complex in perinatal death. Biol Neonate 20048692–97. [DOI] [PubMed] [Google Scholar]
- 9.Matturri L, Ottaviani G, Alfonsi G.et al Guidelines in pathological and forensic‐medical diagnostics of sudden unexpected infant (SIDS) and fetal death. Lombardy Region Project for reduction of the risk for SIDS and/or Sudden Fetal Death. Available at: http://users.unimi.it/ ˜pathol/sids/linee_guida_e.html Accessed: 1 June, 2005
- 10.Lavezzi A M, Ottaviani G, Matturri L. Role of somatostatin and apoptosis in breathing control in sudden perinatal and infant unexplained death. Clin Neuropathol 200423304–310. [PubMed] [Google Scholar]
- 11.Guntheroth W G, Spiers P S. Prolongation of the QT interval and the sudden infant death syndrome. Pediatrics 199910813–814. [DOI] [PubMed] [Google Scholar]
- 12.Guntheroth W G, Spiers P S. Are bedding and rebreathing suffocation a cause of SIDS? Pediatr Pulmonol 199622335–341. [DOI] [PubMed] [Google Scholar]
- 13.Ottaviani G, Matturri L, Bruni B.et al Sudden infant death syndrome “gray zone” disclosed only by a study of the brain stem on serial sections. J Perinat Med 200533165–169. [DOI] [PubMed] [Google Scholar]
- 14.Filiano J J, Kinney H C. A perspective on neuropathologic findings in victims of sudden infant death syndrome: the triple risk model. Biol Neonate 199465194–197. [DOI] [PubMed] [Google Scholar]
- 15.Matturri L, Ottaviani G, Ramos S G.et al Discrete T‐lymphocytic leptomeningitis of the ventral medullary surface in a case of sudden unexpected infant death. Adv Clin Pathol 19982313–316. [PubMed] [Google Scholar]
- 16.Matturri L, Ottaviani G, Rossi L. Sudden and unexpected infant death due to an hemangioendotelioma located in the medulla oblongata. Adv Clin Pathol 1999329–33. [PubMed] [Google Scholar]
- 17.Krous H F, Beckwith J B, Byard R W.et al Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 2004114234–238. [DOI] [PubMed] [Google Scholar]
- 18.Tong E K, England L, Glantz S A. Changing conclusions on secondhand smoke in a sudden infant death syndrome review funded by the tobacco industry. Pediatrics 2005115e356–e366. [DOI] [PubMed] [Google Scholar]
- 19.Lavezzi A M, Ottaviani G, Matturri L. Adverse effects of prenatal tobacco smoke exposure on biological parameters of the developing brainstem. Neurobiol Dis 200520601–607. [DOI] [PubMed] [Google Scholar]