Abstract
Eosinophilic abscess inciting a granulomatous response has rarely been reported and appears not to have been described in the setting of a neoplasm. In this report, a case is described where a granulomatous response occurred around eosinophilic abscesses in a patient with Langerhans cell histiocytosis, an association which has not previously been documented. On histology, the excised lymph node showed the presence of eosinophilic abscess and necrosis surrounded by granulomas, which in turn were surrounded by Langerhans cells, a feature confirmed on immunohistochemistry. Although rare, this case highlights the importance of careful examination of eosinophilic abscess with granulomatous inflammation in order to exclude an underlying neoplasm.
Keywords: granulomatous inflammation, eosinophilic abscess, Langerhans cell histiocytosis
The association of granulomatous inflammation in response to eosinophilic abscess in the setting of a bona fide tumour has not been reported. In this paper, we document the presence of granulomatous inflammation in conjunction with eosinophilic abscess in the setting of Langerhans cell histiocytosis.
Case report
A 30 year old Chinese man who worked as a car polisher presented with left neck swelling of two months' duration. On clinical examination, there was left cervical lymphadenopathy but otherwise physical examination was unremarkable. Full blood count showed the presence of an increased total white count (15.39×109/l; normal range 4.0–10.6×109/l) associated with hypereosinophilia of 20.5% (normal range 0–6%). All other blood investigations were within normal limits. Following fine needle aspiration, the lymph node was excised. Subsequently bone marrow trephine aspirate and biopsy were carried out, revealing the presence of erythroid hyperplasia and increased eosinophils only. He underwent chemotherapy and was free of disease for the last two years since the lymph node excision.
Pathological findings
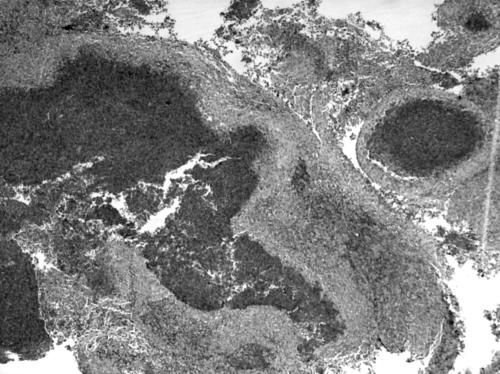
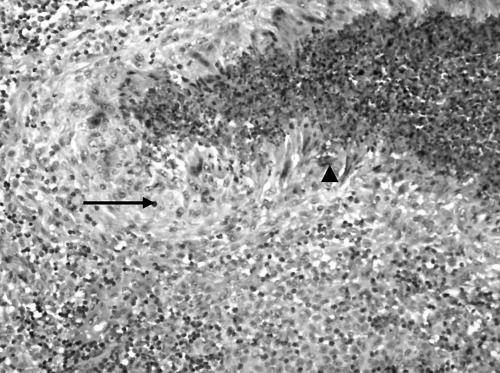
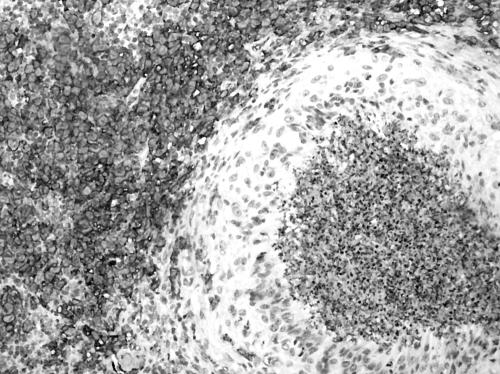
The excised lymph node was received in three pieces which aggregated 1.5×1×1 cm and was associated with yellowish speckles. On light microscopy, there were small to large serpinginous areas of necrosis consisting of eosinophilic material associated with basophilic nuclear material (fig 1). A rim of pale cells could be identified around the necrosis which on high power examination revealed epithelioid histiocytes and occasional multinucleated giant cells. Closer examination of the areas of necrosis revealed the presence of eosinophils and Charcot Leyden crystals (fig 2). On closer scrutiny, the epithelioid histiocytes were rimmed by another population of histiocyte‐like cells with grooved ovoid nuclei and moderately abundant cytoplasm. These histiocyte‐like cells were accompanied by eosinophils and were interpreted as Langerhans cells. Some discernible native lymph node tissue with germinal centres was noted. No evidence of vasculitis was present. No abnormal lymphoid proliferation was otherwise identified. Ziehl‐Neelsen and methenamine silver stains did not reveal any acid‐fast bacilli or fungal organisms, respectively. On immunohistochemistry, the Langerhans cells stained for S‐100 (Dako Z0311) and CD1a (Venta 250–2729) (fig 3). The stains for CD15 (Venta 760–2504) and CD30 (Dako M0751) were negative in the areas with Langerhans cells. CD68 (Dako M0814) highlighted the presence of epithelioid histiocytes and also stained some of the Langerhans cells. The light microscopy findings in conjunction with the immunostains corroborated a diagnosis of Langerhans cell histiocytosis.
Figure 1 Low power view of a fragmented piece of tissue with eosinophilic abscess containing necrotic debris. (Haematoxylin and eosin stain, original magnification ×40.)
Figure 2 On medium power, an eosinophilic abscess was rimmed by epithelioid histiocytes (arrow) which were in turn rimmed by Langerhans cells with grooved nuclei. Note the presence of Charcot‐Leyden crystals (arrowhead). (Haematoxylin and eosin, original magnification ×200.)
Figure 3 Antibodies against CD1a decorating the Langerhans cells but sparing the epithelioid histiocytes as well as area of eosinophilic abscess and necrosis. Similar staining pattern seen with S‐100. (Avidin biotin complex method, original magnification ×200.)
Discussion
The presence of granulomatous inflammation in association with eosinophilic abscess warrants consideration of several differential diagnoses. An important possibility to be considered is the limited form of Churg‐Strauss syndrome resulting in a lymphadenitis, where a granulomatous response around an eosinophilic abscess containing Charcot Leyden crystals is seen.1 However, in this forme fruste of Churg‐Strauss syndrome, there is vasculitis of the arterioles and venules associated with activated lymphoid cells, a feature not seen in our case. Another important possibility to be considered is Kimura's disease, which is associated with peripheral eosinophilia on blood counts, as in our case, and can rarely manifest an eosinophilic granulomatous reaction.2 However, in Kimura's disease, there is a proliferation of capillaries within the stroma around the germinal centres which was not seen in our case.3 The presence of Langerhans cells also militates against a diagnosis of Kimura's disease. Hodgkin's lymphoma with large Reed‐Sternberg cells within the milieu of inflammatory cells, including significant numbers of eosinophils at times and concomitant presence of granulomas, enters the differential diagnosis.1 However, the Reed‐Sternberg cells lack the longitudinal nuclear grooves seen in Langerhans cells and instead possess prominent inclusion‐like nucleoli. In addition, the characteristic staining of Reed‐Sternberg cells for CD15 and CD30 is absent in Langerhans cell histiocytosis.
Other important causes of granulomatous inflammation such as mycobacteria and fungal infections have to be excluded, and the performance of histochemistry stains such as Ziehl‐Neelsen and methenamine silver would be appropriate, even in our case. Parasitic infections4,5 such as Dirofilaria can manifest with a rich infiltrate of eosinophils and granulomatous inflammation; however, in such situations, a careful search for oocysts and parasites within the necrotic areas or within the lumina of the blood vessels will be necessary.
Langerhans cell histiocytosis is a disease where there is a proliferation of neoplastic Langerhans cells which typically express CD1a and S‐100 on immunohistochemistry and show the presence of Birbeck granules on ultrastructural examination.6,7,8 It affects a wide age range and may involve a single organ or multiple organs, the prognosis being influenced by the number of organs involved. Langerhans cell histiocytosis can affect the lymph node as a localised condition or as part of a systemic disease.7,8 Langerhans cell histiocytosis involving the lymph node typically shows a preferential proliferation of Langerhans cells within the sinuses with secondary infiltration of the paracortical regions associated with eosinophils.6 The formation of eosinophilic abscesses with necrosis can occasionally be seen and in such instances Charcot Leyden crystals may be identified. However, the formation of granulomas around necrotic eosinophilic abscesses has not been described previously. Thus Langerhans cell histiocytosis with eosinophilic abscess and necrosis in the correct light microscopic findings should be considered as a possible association with, or causation of, granuloma formation. We postulate that the formation of granulomas in our case may be the result of phagocytosis of apoptotic eosinophils by macrophages as well as host defence to the granules of the eosinophils and Charcot Leyden crystals, a situation analogous to that of a case of Kimura's disease.2
In summary, a hitherto undescribed phenomenon of granulomatous inflammation around eosinophilic abscess in the setting of Langerhans cell histiocytosis is presented. While the granulomatous inflammation is likely to be related to the eosinophils rather than as a direct consequence of Langerhans cells, Langerhans cell histiocytosis should be carefully excluded in cases where granulomas and eosinophilic abscess are intimately related. In such instances, careful light microscopy examination in conjunction with a panel of appropriate immunohistochemical stains will aid in the detection of Langerhans cells and hence the diagnosis.
References
- 1.Cualing H, Schroder L, Perme C. Allergic granulomatosis secondary to a limited form of Churg‐Strauss syndrome. A case report with histologic and immunophenotypic analysis. Arch Pathol Lab Med 2001125954–957. [DOI] [PubMed] [Google Scholar]
- 2.Hosaka N, Minato T, Yoshida S.et al Kimura's disease with unusual eosinophilic epithelioid granulomatous reaction : a finding possibly related to eosinophil apoptosis. Hum Pathol 200233561–564. [DOI] [PubMed] [Google Scholar]
- 3.Weiss S, Goldblum J R. Benign tumors and tumor‐like lesions of blood vessels. In: Weiss SW, Goldblum JR, editors. Enzinger and Weiss's soft tissue tumors, 4th edition. St Louis, Missouri: CV Mosby, 2001863–864.
- 4.Zweig A, Karasik A, Hiss J. Dirofilaria in a cervical lymph node in Israel. Hum Pathol 198112939–940. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein A L A. Diagnostic features and differential diagnosis of Churg‐Strauss syndrome in the lung. Am J Clin Pathol 2000114767–772.11068552 [Google Scholar]
- 6.Weiss L M, Grogan T M, Muller‐Hermelink H K.et al Langerhans cell histiocytosis. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organisation classification of tumours. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001280–282.
- 7.Lieberman P H, Jones C R, Steinman R M.et al Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol 199620519–552. [DOI] [PubMed] [Google Scholar]
- 8.Howarth D M, Gilchrist G S, Mullan B P.et al Langerhans cell histiocytosis. Diagnosis, natural history, management and outcome. Cancer 1999852278–2290. [DOI] [PubMed] [Google Scholar]