Abstract
Aims
To determine whether the G(−174)C interleukin 6 (IL‐6) polymorphism influences the development of peripheral arterial disease (PAD) in individuals with type 2 diabetes. This was investigated by comparing the distribution of G(−174)C genotypes between patients with type 2 diabetes and PAD (PAD+) and those with type 2 diabetes but without PAD (PAD−). Plasma concentrations of IL‐6, fibrinogen, C reactive protein (CRP), and vascular endothelial growth factor (VEGF) were also compared in PAD+ and PAD− patients.
Methods
Blood samples were collected from 146 PAD+ and 144 PAD− patients. SfaNI was used to determine the G(−174)C genotype. Plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF were measured by an enzyme linked immunosorbent assay.
Results
The GG genotype was more common in PAD+ patients than in PAD− patients. PAD+ patients also had increased mean plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF compared with PAD− patients. Mean plasma concentrations of IL‐6, fibrinogen, and CRP in both PAD+ and PAD− patients were higher in those with the GG genotype than in those with the GC or CC genotypes. In contrast, mean plasma concentrations of VEGF in PAD+ and PAD− patients were not significantly different between those with different G(−174)C genotypes.
Conclusions
These results support a model in which the GG genotype promotes PAD development among individuals with type 2 diabetes by inducing increased release of IL‐6. Higher concentrations of IL‐6 among those with the GG genotype is associated with increased plasma concentrations of fibrinogen and CRP.
Keywords: inflammation, interleukin‐6, peripheral arterial disease, type 2 diabetes mellitus
Type 2 diabetes is associated with several vascular pathologies. These include retinopathy, neuropathy, and peripheral arterial disease (PAD).1,2,3 Several mediators of inflammation have been associated with the pathogenesis of PAD.4 Interleukin 6 (IL‐6) is a multifunctional cytokine that has a major role in driving the acute inflammatory response. IL‐6 induces the expression of acute phase inflammatory proteins, including fibrinogen and C reactive protein (CRP).5,6 IL‐6 has also been shown to induce vascular endothelial growth factor (VEGF) expression.7 Increased serum concentrations of VEGF have been seen in patients with several vascular diseases, including PAD and proliferative retinopathy.8,9
“Interleukin 6 induces the expression of acute phase inflammatory proteins, including fibrinogen and C reactive protein”
Increased concentrations of IL‐6 are present in patients with carotid atherosclerosis and coronary artery disease.10 Raised IL‐6 values are also associated with an increased incidence of cerebral ischaemia and myocardial infarction.11,12 A common genetic polymorphism is located 174 nucleotides upstream of the major transcription initiation site of the IL‐6 gene.13 The presence of either guanine or cytosine at this position gives rise to two different IL‐6 alleles. These two different alleles give rise to three possible G(−174)C IL‐6 genotypes: GG, GC, and CC.
The G(−174)C polymorphism is one of several IL‐6 polymorphisms that have been suggested to affect IL‐6 expression.14 Fishman et al found that plasma IL‐6 concentrations were lower in patients with systemic onset juvenile chronic arthritis who had the CC genotype than in those with the GC or GG genotypes.13 However, Jones et al reported that plasma IL‐6 concentrations were higher in patients with abdominal aortic aneurysm who had the CC genotype than in those with the GG or GC genotypes.15 Other studies have indicated that the G(−174)C IL‐6 genotype does not significantly affect plasma IL‐6 values.16,17
The G(−174)C polymorphism has recently been suggested to influence the development of PAD.18 However, no previous studies have explored the relation between G(−174)C genotype and the presence of PAD among patients with type 2 diabetes. In our present study, the distribution of G(−174)C IL‐6 genotypes among patients with type 2 diabetes and PAD (PAD+ patients) was compared with that of patients with type 2 diabetes but without PAD (PAD− patients). Furthermore, plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF were analysed to determine whether they correlated with the G(−174)C genotype. These proteins were selected because each has been suggested to contribute to inflammation or vascular damage.19,20,21
Patients and methods
Patients
The patients enrolled in our present study comprised 290 consecutive patients with type 2 diabetes who were examined at the angiology unit of the University of Catania, Italy between 2001 and 2003. PAD was diagnosed in 146 of the 290 patients. An ankle/brachial index of less than 0.9 or the absence of one or more arteries of the lower legs, as determined by duplex ultrasonography, were the criteria used for the diagnosis of PAD. Duplex ultrasonography was performed with an Apogee CX 800 probe at 7–10 mHz (Philips Medical Systems, Bothell, Washington, USA). All patients adhered to a controlled diet and were treated with oral hypoglycaemic drugs, including glibenclamide, gliclazide, and repaglinide. None of the patients was treated with hydroxymethylglutaryl coenzyme A reductase inhibitors. Standard methods were used to measure fasting blood glucose concentrations, haemoglobin glycosylation, total cholesterol values, triglyceride concentrations, and high density lipoprotein values. Individuals with arterial hypertension were excluded because of its association with the development of PAD. Individuals with a history of ischaemic coronary artery disease, connective tissue disorders, or cancer were also excluded. Additional exclusion criteria included arterial hypertension, chronic renal failure, and current use of antibiotics or agents known to affect cytokine concentrations.
Venous blood samples were collected by the angiology unit of the University of Catania, Italy. Samples were divided into three portions for isolation of serum, plasma, and genomic DNA. Samples were centrifuged at 400 × g for 30 minutes in lithium heparin coated plastic tubes for the isolation of plasma. Samples were centrifuged under the same conditions in uncoated plastic tubes for the isolation of serum. Samples were kept at room temperature before centrifugation and were centrifuged within 60 minutes after collection. Plasma and serum were stored at −80°C until analysis. Genomic DNA was extracted from samples that had been deposited into lithium heparin coated plastic tubes. Genomic DNA was stored at −20°C until analysis. Informed consent was given by each patient and our study was approved by the University of Catania ethics committee. All procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki.
Measurement of protein concentrations
Plasma concentrations of IL‐6 and VEGF were measured in duplicate with the Quantikine enzyme linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota, USA). Detection limits for IL‐6 and VEGF were 0.1 pg/ml and 10 pg/ml, respectively. CRP was assayed with a BN II nephelometer (Dade Behring, Deerfield, Illinois, USA). This assay detects CRP concentrations as low as 0.15 mg/litre. The Clauss method was used to detect fibrinogen with Multifibren U and a BCS analyser (Dade Behring Diagnostics).
G(−174)C IL‐6 genotype analysis
Genotyping of the G(−174)C IL‐6 polymorphism was carried out by polymerase chain reaction (PCR) analysis, as described previously.18 A 198 bp fragment of the IL‐6 gene was amplified. Forward and reverse primer sequences were 5′‐TGACTTCAGCTTTACTCTTTGT‐3′ and 5′‐CTGATTGGAAACCTTATTAGG‐3′, respectively. DNA was denatured for nine minutes at 94°C then subjected to 35 amplification cycles. Each PCR cycle consisted of denaturation for 60 seconds at 94°C, annealing for 95 seconds at 55°C, and extension for 60 seconds at 72°C; followed by a final extension at 72°C for nine minutes. PCR products were incubated overnight with 0.1 Ut/μl SfaNI at 37°C then separated by electrophoresis in 3% agarose. DNA separated by electrophoresis was visualised by staining for 30 minutes in 5 μg/ml ethidium bromide. The presence of a single 198 bp band corresponds to the CC genotype; bands at 140 and 58 bp correspond to the GG genotype; and the presence of three bands corresponds to the GC genotype.
Statistical analysis
All values are expressed as mean (SD). The distribution of males and females in the PAD+ and PAD− groups was compared by means of the χ2 test.22 The G/C allele ratio in PAD+ and PAD− patients was compared by means of Fisher's exact test.22 The odds ratio and corresponding 95% confidence interval for each G(−174)C IL‐6 genotype for PAD+ and PAD− patients was calculated with SAS software (SAS Institute, Cary, North Carolina, USA). These calculations were performed with and without adjustment for sex and age (in five year blocks) by unconditional logistic regression analysis. All other comparisons between groups were made by the Wilcoxon rank test. Spearman coefficients were calculated to determine whether plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF correlated with one another. Two tailed tests were used for all statistical analyses. Significance was set at p < 0.01.
Results
Table 1 summarises the clinical characteristics of the PAD+ and PAD− patients. Table 2 details the mean plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF for PAD+ and PAD− patients. The mean plasma concentration of IL‐6 was higher in PAD+ patients than in PAD− patients (p < 0.01). Similar trends were also seen for fibrinogen, CRP, and VEGF. Table 3 shows the Spearman correlation coefficients for plasma concentration of IL‐6, fibrinogen, CRP, and VEGF among PAD+ and PAD− patients. Plasma concentrations of IL‐6 positively correlated with those of fibrinogen (p < 0.01) and CRP (p < 0.01) in PAD+ patients, but only with fibrinogen concentration (p < 0.01) in PAD− patients. A positive correlation between plasma concentrations of fibrinogen and CRP was also seen in PAD+ patients, but not in PAD− patients (p < 0.01). No other correlations between plasma protein concentrations were seen among the two groups of patients.
Table 1 Clinical characteristics of PAD+ and PAD− patients.
| Clinical feature | PAD+ patients (n = 146) | PAD− patients (n = 144) | p Value* |
|---|---|---|---|
| Sex (M/F) | 92/54 | 55/89 | <0.01 |
| Age (years) | 69.0 (7.9) | 61.0 (7.0) | <0.01 |
| Body mass index (kg/mq) | 24.5 (5.8) | 24.0 (5.3) | NS |
| Ankle/brachial index | 0.60 (0.25) | 1.14 (0.43) | <0.01 |
| Duration of diabetes (years) | 9.4 (1.1) | 8.7 (2.6) | NS |
| Fasting glucose (mmol/l) | 6.3 (1.1) | 6.5 (1.0) | NS |
| HbA1C (%) | 8.3 (2.7) | 8.5 (2.3) | NS |
| Total cholesterol (mg/l) | 1820 (160) | 1840 (140) | NS |
| Tryglicerides (mg/l) | 1500 (440) | 1460 (410) | NS |
| High density lipoprotein (mg/l) | 360 (82) | 351 (94) | NS |
Values are mean (SD).
*The Wilcoxon rank test was used to calculate p values for comparisons between PAD+ and PAD− patients for all categories except sex. The distribution of men and women among PAD+ patients was compared with that of PAD− patients by means of the χ2 test.
HbA1C, glycated haemoglobin; PAD, peripheral arterial disease.
Table 2 Plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF in PAD+ and PAD− patients.
| PAD+ patients (n = 146) | PAD− patients (n = 144) | p Value* | |
|---|---|---|---|
| IL‐6 (pg/ml) | 12.1 (10.7) | 4.0 (3.2) | <0.01 |
| Fibrinogen (mg/l) | 3570 (2030) | 2310 (1100) | <0.01 |
| CRP (mg/l) | 3.2 (2.5) | 2.1 (1.7) | <0.01 |
| VEGF (ng/ml) | 425 (174) | 163 (48) | <0.01 |
Values are mean (SD).
*The Wilcoxon rank test was used to calculate p values.
CRP, C reactive protein; IL‐6, interleukin 6; PAD, peripheral arterial disease; VEGF, vascular endothelial growth factor.
Table 3 Spearman rank correlation coefficients for comparison of plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF between PAD+ and PAD− patients.
| PAD+ patients | PAD− patients | |||||
|---|---|---|---|---|---|---|
| Fibrinogen | CRP | VEGF | Fibrinogen | CRP | VEGF | |
| IL‐6 | 0.86* | 0.75* | 0.17 | 0.69* | 0.15 | 0.02 |
| Fibrinogen | 0.71* | 0.19 | 0.30 | −0.05 | ||
| CRP | 0.11 | −0.05 | ||||
*p<0.01.
CRP, C reactive protein; IL‐6, interleukin 6; PAD, peripheral arterial disease; VEGF, vascular endothelial growth factor.
Table 4 lists the distribution of the G(−174)C IL‐6 genotypes for each group. The GG genotype was more common in PAD+ patients (51%) than PAD− patients (33%) (p < 0.01). The G/C allele ratio was 1.8 in PAD+ patients and 1.0 in PAD− patients. The difference in G/C allele ratio between PAD+ and PAD− patients was significant (p < 0.01). The odds ratio and corresponding 95% confidence interval for each G(−174)C IL‐6 genotype was calculated for PAD+ patients compared with PAD− patients (table 4). The ratio of individuals with the GG genotype to those with the CC genotype was 2.24 times higher in PAD+ patients than in PAD− patients (p < 0.01). After adjustment for sex and age (in five year blocks), the odds ratio was 2.84 times higher in PAD+ patients than in PAD− patients (p < 0.01).
Table 4 Distribution of G(−174)C genotypes in PAD+ and PAD− patients.
| Genotype | PAD+ patients (n = 146) | PAD− patients (n = 144) | Unadjusted model | Adjusted model* |
|---|---|---|---|---|
| N (%) | N (%) | OR (95% CI) | OR (95% CI) | |
| CC | 32 (22%) | 45 (31%) | 1† | 1† |
| GC | 39 (27%) | 52 (37%) | 1.06 (0.57 to 1.95) | 1.66 (0.81 to 3.38) |
| GG | 75 (51%) | 47 (33%) | 2.24 (1.25 to 4.02)‡ | 2.84 (1.45 to 5.57)‡ |
*Estimated from unconditional logistic regression adjusted for sex and age (in five year blocks); †reference value; ‡p<0.01.
CI, confidence interval; OR, odds ratio.
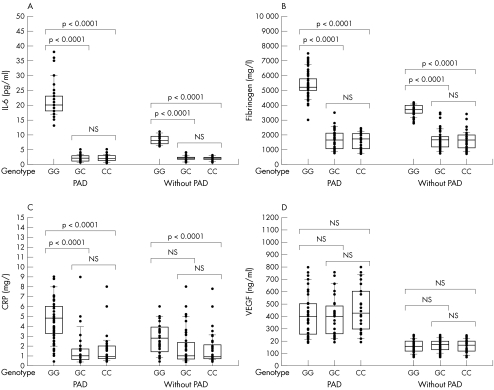
Table 5 shows the mean plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF in relation to each G(−174)C genotype for both groups of patients. Figure 1 shows the distribution of IL‐6, fibrinogen, CRP, and VEGF plasma concentrations in both groups of patients in relation to each G(−174)C genotype. PAD+ and PAD− patients with the GG genotype had higher mean plasma concentrations of IL‐6, fibrinogen, and CRP than those with the GC or CC genotypes. In contrast, mean plasma concentrations of VEGF did not differ significantly between the PAD+ and PAD− patients with different G(−174)C genotypes. Mean plasma concentrations of IL‐6, fibrinogen, and CRP were similar in PAD+ and PAD− patients with the GC or CC genotypes. In contrast, mean plasma concentrations of IL‐6, fibrinogen, and CRP in PAD+ patients with the GG genotype were higher than those of PAD− patients with the GG genotype. VEGF differs from IL‐6, fibrinogen, and CRP in that there were differences in mean plasma concentrations of VEGF between PAD+ and PAD− patients for all three G(−174)C genotypes.
Table 5 Mean protein concentrations of IL‐6, fibrinogen, CRP, and VEGF, in PAD+ and PAD− patients according to G(−174)C genotype.
| PAD+ patients (n = 146) | PAD− patients (n = 144) | p Value* | |
|---|---|---|---|
| IL‐6 (pg/ml) | |||
| GG | 21.67 (5.62) | 8.27 (1.51) | <0.01 |
| GC | 2.06 (1.13) | 1.98 (0.83) | NS |
| CC | 2.03 (1.08) | 1.95 (0.65) | NS |
| Fibrinogen (mg/l) | |||
| GG | 5410 (580) | 3680 (350) | <0.01 |
| GC | 1680 (690) | 1670 (630) | NS |
| CC | 1590 (560) | 1630 (580) | NS |
| CRP (mg/l) | |||
| GG | 4.8 (2.2) | 2.8 (1.5) | <0.01 |
| GC | 1.5 (1.7) | 1.8 (1.8) | NS |
| CC | 1.6 (1.6) | 1.6 (1.5) | NS |
| VEGF (ng/ml) | |||
| GG | 418 (174) | 160 (48) | <0.01 |
| GC | 406 (163) | 167 (47) | <0.01 |
| CC | 464 (185) | 160 (48) | <0.01 |
Values are mean (SD).
*The Wilcoxon rank test was used to calculate p values.
CRP, C reactive protein; IL‐6, interleukin 6; PAD, peripheral arterial disease; VEGF, vascular endothelial growth factor.
Figure 1 Plasma concentrations of (A) interleukin 6 (IL‐6), (B) fibrinogen, (C) C reactive protein (CRP), and (D) vascular endothelial growth factor (VEGF) in patients with and without peripheral arterial disease (PAD) for each G(−174)C IL‐6 genotype. Plasma concentrations within the second and third quartiles are enclosed in boxes. The Wilcoxon rank test was used to determine whether significant differences were present between patients with different G(−174)C IL‐6 genotypes. The results of this analysis are indicated by brackets.
Discussion
Several studies have shown that inflammatory processes contribute to the development of PAD.4 IL‐6 is a mediator of inflammation that induces the secretion of several acute phase proteins from hepatocytes, such as CRP.23,24,25 It has also been shown that increased serum concentrations of several markers of the acute response, including IL‐6, are raised in those with type 2 diabetes.26,27 Individuals with type 2 diabetes are twice as likely to have PAD than those without type 2 diabetes.28 The G(−174)C IL‐6 polymorphism has been suggested to influence IL‐6 release.13 The aim of our present study was to determine whether the G(−174)C IL‐6 polymorphism may influence the development of PAD in patients with type 2 diabetes. This possibility was investigated by comparing the distribution of G(−174)C genotypes in patients with type 2 diabetes with and without PAD. Plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF were also measured to determine whether they differed between PAD+ and PAD− patients.
The GG genotype was seen more frequently in PAD+ patients than in PAD− patients (table 4). This finding suggests that patients with type 2 diabetes and the GG genotype may develop PAD more often than those with the GC or CC genotypes. Previous studies correlating plasma IL‐6 concentrations with the G(−174)C genotype have yielded conflicting results. Some investigators found increased plasma IL‐6 concentrations in those with the GG genotype, whereas others found increased plasma IL‐6 concentrations in those with the CC genotype.13,15,29,30 Plasma IL‐6 concentrations have also been reported to be independent of the G(−174)C genotype.16,17 We found that both PAD+ and PAD− patients with the GG genotype had higher mean plasma IL‐6 concentrations than those with the GC or CC genotypes (fig 1).
It was of interest to determine whether the G(−174)C genotype may similarly affect plasma concentrations of proteins that are regulated by IL‐6. An analysis of plasma concentrations of IL‐6, fibrinogen, and CRP in patients with abdominal aortic aneurysm revealed that only IL‐6 plasma concentrations varied between patients with different G(−174)C genotypes.15 In contrast, we found that the GG genotype was associated with increased mean plasma concentrations of IL‐6, fibrinogen, and CRP in both PAD+ and PAD− patients (fig 1). These results support the possibility that the GG genotype facilitates increased IL‐6 release in patients with type 2 diabetes, which then causes increased release of fibrinogen and CRP. This possibility is supported by the observation that plasma concentrations of IL‐6, fibrinogen, and CRP in PAD+ patients correlated with one another (table 3). VEGF differed from the other proteins studied because its concentrations were independent of G(−174)C genotype in both PAD+ and PAD− patients. Furthermore, plasma VEGF concentrations did not correlate with IL‐6, fibrinogen, or CRP in PAD+ or PAD− patients. These results contradict previous reports indicating that IL‐6 induces VEGF expression.7
Plasma concentrations of IL‐6, fibrinogen, CRP, and VEGF were higher in PAD+ patients than in PAD− patients (table 2). VEGF differed from IL‐6, fibrinogen, and CRP in that we found differences in plasma VEGF concentrations between PAD+ and PAD− patients for each G(−174)C genotype. These results suggest that the increase in plasma VEGF concentrations in PAD+ patients is caused by factors that are independent of G(−174)C genotype. In contrast, mean plasma concentrations of IL‐6, fibinogen, and CRP were higher in PAD+ and PAD− patients with the GG genotype than in those with the GC or CC genotypes. The differences in plasma concentrations of IL‐6, fibrinogen, and CRP between PAD+ and PAD− patients arise in part from the fact that the GG genotype was more common in PAD+ patients. In addition, PAD+ patients with the GG genotype had higher plasma concentrations of these proteins than PAD− patients with the same genotype.
Take home messages
We investigated the different G(−174)C genotypes of the interleukin 6 (IL‐6) gene in patients with type 2 diabetes with and without peripheral arterial disease (PAD− and PAD+, respectively)
The GG genotype was more common in PAD+ patients than in PAD− patients, and PAD+ patients also had increased mean plasma concentrations of IL‐6, fibrinogen, C reactive protein (CRP), and vascular endothelial growth factor
Our results support a model in which the GG genotype promotes the development of PAD in individuals with type 2 diabetes by inducing increased release of IL‐6, which results in increased plasma concentrations of fibrinogen and CRP
“There may be a complex relation between G(−174)C genotype and the development of cardiovascular disease”
Our results support a model in which the GG genotype promotes the development of PAD in patients with type 2 diabetes by inducing increased release of IL‐6. Higher concentrations of IL‐6 in those with the GG genotype cause increased release of fibrinogen and CRP. These findings are consistent with previous studies that have documented a higher incidence of cardiovascular disease in individuals with the GG genotype than those with the CC genotype.18,29,30,31 However, other studies have associated cardiovascular disease with the CC genotype instead of the GG genotype.15,32,33,34 These conflicting results suggest that there may be a complex relation between G(−174)C genotype and the development of cardiovascular disease. Therefore, it is important to recognise that our present study was based entirely upon normotensive patients with type 2 diabetes. The relation between G(−174)C genotype and PAD development may differ in individuals with different clinical characteristics. Additional studies are needed to characterise the mechanisms by which the G(−174)C IL‐6 genotype and its influence upon IL‐6 release affects the development of cardiovascular disease in individuals with type 2 diabetes.
Acknowledgements
This work was supported by Fondo d'Ateneo, University of Catania, Italy.
Abbreviations
CRP - C reactive protein
IL‐6 - interleukin 6
PAD - peripheral arterial disease
PCR - polymerase chain reaction
VEGF - vascular endothelial growth factor
References
- 1.Al‐Delaimy W K, Merchant A T, Rimm E B.et al Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med 2004116236–240. [DOI] [PubMed] [Google Scholar]
- 2.Manaviat M R, Afkhami M, Shoja M R. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol 200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer C, Milat F, McGrath B P.et al Vascular dysfunction and autonomic neuropathy in type 2 diabetes. Diabet Med 200421746–751. [DOI] [PubMed] [Google Scholar]
- 4.Brevetti G, Silvestro A, Di Giacomo S.et al Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg 200338374–379. [DOI] [PubMed] [Google Scholar]
- 5.Dalmon J, Laurent M, Courtois G. The human β fibrinogen promoter contains a hepatocyte nuclear factor 1‐dependent interleukin‐6‐responsive element. Mol Cell Biol 1993131183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramji D P, Vitelli A, Tronche F.et al The two C/EBP isoforms, IL‐6DBP/NF‐IL6 and C/EBPδ/NF‐IL6β, are induced by IL‐6 to promote acute phase gene transcription via different mechanisms. Nucleic Acids Res 199321289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei L H, Kuo M L, Chen C A.et al Interleukin‐6 promotes cervical tumor growth by VEGF‐dependent angiogenesis via a STAT3 pathway. Oncogene 2003221517–1527. [DOI] [PubMed] [Google Scholar]
- 8.Matsui K, Yoshioka T, Murakami Y.et al Serum concentrations of vascular endothelial growth factor and monocyte‐colony stimulating factor in peripheral arterial disease. Circ J 200367660–662. [DOI] [PubMed] [Google Scholar]
- 9.Witmer A N, Vrensen G F, Van Noorden C J.et al Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003221–29. [DOI] [PubMed] [Google Scholar]
- 10.Biasucci L M, Vitelli A, Liuzzo G.et al Elevated levels of interleukin‐6 in unstable angina. Circulation 199694874–877. [DOI] [PubMed] [Google Scholar]
- 11.Grau A J, Aulmann M, Lichy C.et al Increased cytokine release by leucocytes in survivors of stroke at young age. Eur J Clin Invest 200131999–1006. [DOI] [PubMed] [Google Scholar]
- 12.Ridker P M, Rifai N, Stampfer M J.et al Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation 20001011767–1772. [DOI] [PubMed] [Google Scholar]
- 13.Fishman D, Faulds G, Jeffery R.et al The effect of novel polymorphisms in the interleukin‐6 (IL‐6) gene on IL‐6 transcription and plasma IL‐6 levels, and an association with systemic‐onset juvenile chronic arthritis. J Clin Invest 19981021369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terry C F, Loukaci V, Green F R. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem 200027518138–18144. [DOI] [PubMed] [Google Scholar]
- 15.Jones K G, Brull D J, Brown L C.et al Interleukin‐6 (IL‐6) and the prognosis of abdominal aortic aneurysms. Circulation 20011032260–2265. [DOI] [PubMed] [Google Scholar]
- 16.Rauramaa R, Vaisanen S B, Luong L A.et al Stromelysin‐1 and interleukin‐6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 2000202657–2662. [DOI] [PubMed] [Google Scholar]
- 17.Nauck M, Winkelmann B R, Hoffmann M M.et al The interleukin‐6 G(−174)C promoter polymorphism in the LURIC cohort: no association with plasma interleukin‐6, coronary artery disease, and myocardial infarction. J Mol Med 200280507–513. [DOI] [PubMed] [Google Scholar]
- 18.Flex A, Gaetani E, Pola R.et al The −174 G/C polymorphism of the interleukin‐6 gene promoter is associated with peripheral artery occlusive disease. Eur J Vasc Endovasc Surg 200224264–268. [DOI] [PubMed] [Google Scholar]
- 19.Andreotti F, Burzotta F, Maseri A. Fibrinogen as a marker of inflammation: a clinical view. Blood Coagul Fibrinolysis 199910(suppl 1)S3–S4. [PubMed] [Google Scholar]
- 20.Signorelli S S, Mazzarino M C, Di Pino L.et al High circulating levels of cytokines (IL‐6 and TNFalpha), adhesion molecules (VCAM‐1 and ICAM‐1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med 2003815–19. [DOI] [PubMed] [Google Scholar]
- 21.Maksimowicz‐McKinnon K, Bhatt D L, Calabrese L H. Recent advances in vascular inflammation: C‐reactive protein and other inflammatory biomarkers. Curr Opin Rheumatol 20041618–24. [DOI] [PubMed] [Google Scholar]
- 22.Armitage P, Berry G.Statistical methods in medical research. Oxford: Blackwell Scientific Publications, 1987
- 23.Ng S B, Tan Y H, Guy G R. Differential induction of the interleukin‐6 gene by tumor necrosis factor and interleukin‐1. J Biol Chem 199426919021–19027. [PubMed] [Google Scholar]
- 24.Heinrich P C, Castell J V, Andus T. Interleukin‐6 and the acute phase response. Biochem J 1990265621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann H, Gauldie J. The acute phase response. Immunol Today 19941574–80. [DOI] [PubMed] [Google Scholar]
- 26.Kado S, Nagase T, Nagata N. Circulating levels of interleukin‐6, its soluble receptor and interleukin‐6/interleukin‐6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol 19993667–72. [DOI] [PubMed] [Google Scholar]
- 27.Yu H I, Sheu W H, Song Y M.et al C‐reactive protein and risk factors for peripheral vascular disease in subjects with type 2 diabetes mellitus. Diabet Med 200421336–341. [DOI] [PubMed] [Google Scholar]
- 28.Walters D P, Gatling W, Mullee M A.et al The prevalence, detection, and epidemiological correlates of peripheral vascular disease: a comparison of diabetic and non‐diabetic subjects in an English community. Diabet Med 19929710–715. [DOI] [PubMed] [Google Scholar]
- 29.Burzotta F, Iacoviello L, Di Casterlnuovo A.et al Relation of the ‐174 G/C polymorphism of interleukin‐6 to interleukin‐6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am J Cardiol 2001881125–1128. [DOI] [PubMed] [Google Scholar]
- 30.Giacconi R, Cipriano C, Albanese F.et al The −174G/C polymorphism of IL‐6 is useful to screen old subjects at risk for atherosclerosis or to reach successful ageing. Exp Gerontol 200439621–628. [DOI] [PubMed] [Google Scholar]
- 31.Greisenegger S, Endler G, Haering D.et al The (−174) G/C polymorphism in the interleukin‐6 gene is associated with the severity of acute cerebrovascular events. Thromb Res 2003110181–186. [DOI] [PubMed] [Google Scholar]
- 32.Chapman C M L, Beilby J P, Humphries S E.et al Association of an allelic variant of interleukin‐6 with subclinical carotid atherosclerosis in an Australian community population. Eur Heart J 2003241494–1499. [DOI] [PubMed] [Google Scholar]
- 33.Exner M, Schillinger M, Minar E.et al Interleukin‐6 promoter genotype and restenosis after femoropopliteal balloon angioplasty: initial observations. Radiology 2004231839–844. [DOI] [PubMed] [Google Scholar]
- 34.Jenny N S, Tracy R P, Ogg M S.et al In the elderly, interleukin‐6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol 2002222066–2071. [DOI] [PubMed] [Google Scholar]