Abstract
Background
Oesophageal adenocarcinoma is an aggressive neoplasm with poor prognosis as a result of early lymph node metastasis.
Aims
To measure lymphatic vessel density (LVD) in the neoplastic progression from Barrett's metaplasia to adenocarcinoma and determine whether LVD can predict the risk of cancer. In addition, to correlate LVD with lymph node metastasis and assess whether LVD could be used as a prognostic indicator for outcome or survival.
Methods
LVD and microvascular density (MVD) were assessed after immunohistochemical staining of vessels in Barrett's metaplasia, dysplasia, and adenocarcinoma tissues and were correlated with clinicopathological features.
Results
LVD was significantly reduced in adenocarcinoma, being half that seen in normal stomach/oesophagus or metaplasia/dysplasia. LVD did not correlate with tumour grade, stage, or clinical outcome; however, patients who had either lymph node metastasis or invasion of tumour cells into peritumorous lymphatic vessels had a significantly worse overall survival. MVD was also assessed as a prognostic marker; its increase appeared to be linked more with the development of Barrett's metaplasia than adenocarcinoma.
Conclusions
The reduction in lymphatic vessel numbers was not useful for determining disease outcome in the patient group studied. It is the entry of tumour cells into pre‐existing peritumorous lymphatic vessels that confers a significantly worse overall survival.
Keywords: lymphangiogenesis, lymphatic vessel density, Barrett's metaplasia, dysplasia, oesophageal adenocarcinoma
The incidence of adenocarcinoma of the distal oesophagus and gastro–oesophageal junction (gastric cardia) has increased considerably in the past three decades, particularly in the industrialised countries of North America, Europe, and Oceania.1,2 Gastro–oesophageal reflux disease is believed to be the major risk factor for the development of these junctional adenocarcinomas.3 Barrett's oesophagus is the condition in which columnar epithelium replaces the squamous epithelium that normally lines the distal oesophagus. The condition develops when gastro–oesophageal reflux damages the squamous oesophageal mucosa and the injury heals through a metaplastic process in which columnar cells replace squamous ones. The abnormal columnar epithelium that characterises Barrett's oesophagus is an incomplete form of intestinal metaplasia that predisposes patients to adenocarcinoma, which is estimated to develop in approximately 0.5% of patients with Barrett's oesophagus each year.4
“We measured lymphatic vessel density in non‐neoplastic, metaplastic, and dysplastic tissues to determine whether the neoplastic cascade was accompanied by a progressive increase in the density of lymphatic vessels”
Most patients with junctional adenocarcinoma present with advanced disease and have an overall five year survival of less than 15%.5 In an effort to detect early treatable lesions, endoscopic surveillance protocols have been developed to identify dysplasia, a histological feature suggesting that one or more clone of epithelial cells has acquired neoplastic genetic alterations. However, dysplasia is an imperfect predictor of malignancy, because of unsatisfactory interobserver agreement among pathologists and the inevitable sampling error during the endoscopic collection of biopsy specimens.6 In addition, the presence of dysplasia is a poor indicator of those patients who will go on to develop adenocarcinoma. Therefore, other markers of cancer risk (for example, the expression of p53 and flow cytometric results) have been evaluated, but there are insufficient data to justify their routine use in clinical practice.7
The best prognostic indicator for oesophageal adenocarcinoma is the presence of lymph node metastases, which occur early and follow fairly constant distribution patterns. In transgenic animal models of pancreatic β cell carcinogenesis, tumour lymphangiogenesis has been shown to correlate with the presence of lymph node metastasis.8 The recent characterisation of several lymphatic specific markers, such as lymphatic endothelium specific hyaluronan receptor (LYVE‐1),9 allows the accurate assessment of lymphatic vascular density (LVD) in tissue specimens by means of immunohistochemistry. Therefore, we designed a study to test the hypothesis that in oesophageal adenocarcinoma LVD correlates with the development of lymph node metastases and could serve as a prognostic indicator. Furthermore, we measured LVD in non‐neoplastic, metaplastic, and dysplastic tissues to determine whether the neoplastic cascade was accompanied by a progressive increase in the density of lymphatic vessels. If such a progression could be demonstrated, the measurement of LVD could be used as an adjunct for the diagnosis of dysplasia and perhaps to help predict the risk of neoplasia.
Patients and methods
Patients, biopsy samples, pathological review, and clinical follow up data
Samples and clinical and follow up data from 87 patients who underwent surgery between January 1979 and June 1998 for oesophageal or gastric adenocarcinoma were retrieved from the files of the departments of pathology and surgery of Geneva University Hospital and the Tumour Registry of Geneva, Switzerland. This retrospective study conformed to local ethical committee guidelines. Six patients were excluded either because no pathological material was available (five patients) or because the initial diagnosis was revised (one patient). All pathology reports and haematoxylin and eosin stained slides were reviewed by one pathologist (MAB). Tumours were staged according to the TNM classification.10 In addition, the presence of metaplastic columnar epithelium or dysplastic foci adjacent to the tumour was documented. Representative areas from the tumours and from metaplastic or dysplastic columnar mucosa were selected for subsequent immunohistochemical analysis.
Immunohistochemistry
Immunohistochemistry was performed on formalin fixed, paraffin wax embedded material. Slides were dewaxed and treated with 0.1% H2O2 to reduce non‐specific staining. After heat induced antigen retrieval by microwave, primary antibodies were applied for one hour at room temperature. Antibodies were rabbit polyclonal anti‐LYVE‐1 (a lymphatic specific marker11), a generous gift from Dr D Jackson (Oxford, UK), and monoclonal anti‐CD34 antibody (Q‐BEND/10; Dako, Glostrup, Denmark). Slides were then incubated with a biotinylated secondary antibody followed by 3,3‐diaminobenzadrine or 3‐amino‐9‐ethylcarbazole (Dako). For negative controls, primary antibodies were omitted; mucosal capillaries and lymphatic vessels served as internal positive controls for CD34 and LYVE‐1 staining.
Measurement of LVD
The extent of LVD was measured by a method comparable to the one used for microvascular density (MVD; see below). Anti‐LYVE‐1 was used to stain lymphatic vessels. Slides were scanned at low magnification (×40) and LVD was measured at a magnification of ×400 (field area, 0.237 mm2). LVD was measured in normal oesophagus, and in areas of columnar metaplasia, dysplasia, and adenocarcinoma by counting three random fields. In adenocarcinoma, five to 10 random fields were counted at the periphery and in the centre of each tumour. Maximum values were used for statistical analysis. In addition, slides containing peritumorous lymphatic vessels invaded by tumour cells were classified as tumour cell invasion positive.
Measurement of MVD
Microvessels were counted after IHC using anti‐CD34 (which does not stain lymphatics12), as described above. The extent of MVD was assessed by a modification of the method described by Weidner et al.13,14 Briefly, highly vascular areas, so called “hot spots”, were selected by low power scanning of the section (magnification, ×40) and MVD determined at ×200 magnification (field area, 0.949 mm2) in one to three microscopic fields for each slide. In the absence of apparent hot spots, three or more randomly selected areas were counted. In both instances, maximum values were retained for further analysis. MVD in metaplastic and dysplastic foci were counted randomly because we were unable to identify hot spots. Any red/brown stained cells or separate clusters of endothelial cells, with or without an identifiable lumen, were considered and counted as a single vessel.13,14,15,16,17 A pathologist and a technician (blinded to all clinical information) performed counts simultaneously at a double headed Nikon eclipse 6000 microscope. For the first 20 cases, fields were counted twice. Using this method, an intraobserver and interobserver variation of < 10% was achieved.
Statistical analysis
All statistical tests were carried out using SPSS for Windows, release 11.0.0. The association between LVD, MVD, and clinical, pathological, or morphological parameters was established using the t test. Survival curves were compared using the log rank test and multivariate survival analysis was performed using Cox's regression model.
Results
Patients, pathological review, and clinical follow up data
Clinical and follow up data were reviewed for 81 patients, 66 of whom were men (median age, 67 years; range, 37–88). Table 1 details the stage, type, and location of the tumours.
Table 1 Stage, type, and location of the 81 oesophageal or gastric adenocarcinomas.
| Tumour characteristics | N | % |
|---|---|---|
| T stage | ||
| T1a (intramucosal) | 6 | 7.4 |
| T1b (submucosal) | 8 | 9.9 |
| T2 | 10 | 12.3 |
| T3 | 53 | 65.4 |
| T4 | 2 | 2.5 |
| T0* | 2 | 2.5 |
| N stage | ||
| N0 | 28 | 35.4 |
| N+ | 51 | 64.6 |
| M stage | ||
| M0 | 71 | 89.9 |
| M+ (liver/distant lymph nodes) | 8 | 10.1 |
| Tumour location | ||
| Distal oesophagus | 26 | 32.9 |
| Cardia | 53 | 67.1 |
| Tumour differentiation | ||
| Well (G1) | 6 | 7.6 |
| Moderately (G2) | 39 | 49.4 |
| Poorly (G3) | 34 | 43 |
| Tumour type | ||
| Tubular | 56 | 70.9 |
| Tubulopapillary | 8 | 10.1 |
| Solid (cells grow in sheets) | 5 | 6.3 |
| Mucinous | 7 | 8.9 |
| Diffuse | 3 | 3.8 |
| Metaplasia | ||
| Specialised glandular mucosa | 26 | 96‡ |
| Intestinal metaplasia cardia† | 13 | 25§ |
| Dysplasia | ||
| Low grade | 11 | 34‡/4§ |
| High grade | 21 | 60‡/8§ |
*Patients with Barrett's oesophagus without associated carcinoma; †intestinal metaplasia confined to the gastric cardia, no other findings suggesting Barrett's oesophagus; ‡% of patients with Barrett's oesophagus; §% of patients with adenocarcinoma of cardia.
Forty nine deaths were reported, 32 of which were specifically disease related. The median clinical follow up (data available for 77 patients) was 18 months (range, 1–122 months).
LVD in the neoplastic progression from Barrett's metaplasia to oesophageal adenocarcinoma
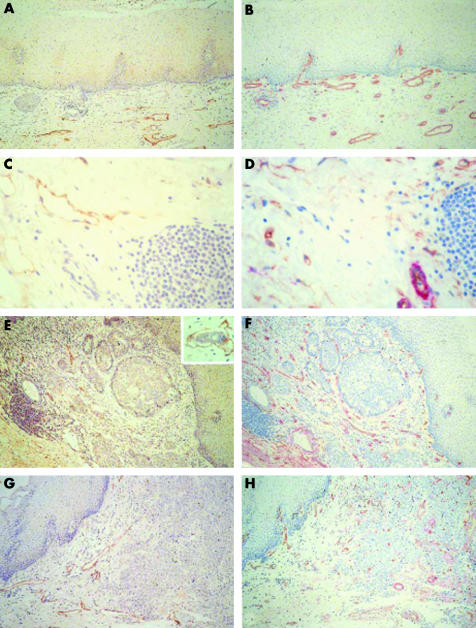
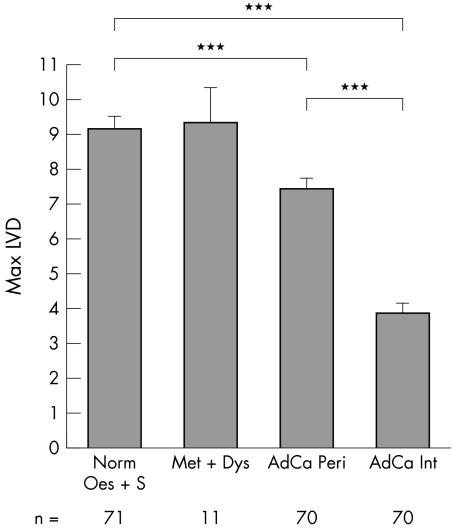
We assessed the extent of LVD by counting LYVE‐1 positive vessels (fig 1A, C, E, G). Lymphatic vessels were evenly distributed throughout the normal squamous lined oesophageal mucosa and were dispersed throughout the mid and lower lamina propria (fig 1A, C). They were also found around tumour tissue (fig 1E, G); however, lymphatic vessels were less frequent within the tumour mass in either differentiated or poorly differentiated tumours. When we measured the extent of LVD (fig 2), we saw no differences between LVD in normal oesophagus and stomach (LVD, 9.17) and LVD in metaplasia and dysplasia (LVD, 9.36). However we saw a significant decrease in LVD in adenocarcinoma (LVD, 5.65; p < 0.001). Interestingly, LVD was lower in the central tumour area (LVD, 3.98) than at the tumour periphery (LVD, 7.41; p < 0.001; figs 1E, G and 2).
Figure 1 Representative immunohistochemical staining of normal oesophagus ((A, B); (C, D) submucosa) and adenocarcinoma ((E, F) differentiated and (G, H) undifferentiated) stained for (A, C, E, G) LYVE‐1 or (B, D, F, H) CD34 (brown) and α smooth muscle actin (red). Original magnification, ×200 (A, B, E–H); ×400 (C, D). Insert in (E), tumour cell invasion into peritumorous lymphatic vessel.
Figure 2 Maximum lymphatic vessel density (LVD) in the neoplastic progression: normal oesophagus and stomach (Norm Oes + S), metaplasia and dysplasia (Met + Dys), and peripheral or internal adenocarcinoma (AdCa Peri/AdCa Int); unpaired samples. ***p < 0.001); n, number of patients/group. Representative immunohistochemical images stained with LYVE‐1 are shown in fig 1A and C (normal oesophagus) and fig 1E and G (adenocarcinoma).
Prognostic value of LVD in oesophageal adenocarcinoma
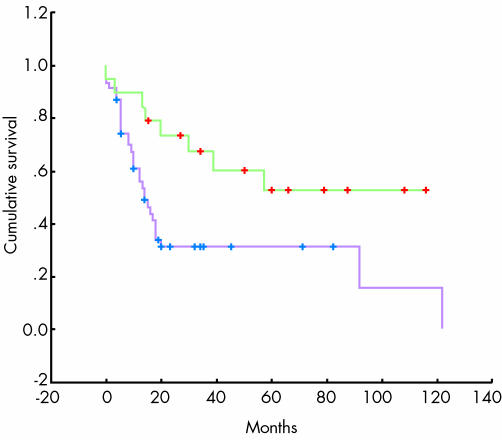
The decrease in LVD was independent of tumour stage, lymphatic metastatic spread, and overall survival (data not shown). Because LVD did not correlate with overall survival we looked for additional parameters, other than tumour stage, that could be used as independent prognostic factors. A combination of invasion of tumour cells into LYVE‐1‐positive peritumorous lymphatic vessels (fig 1E, insert) or lymph node metastasis (assessed by review of haematoxylin and eosin slides) proved to be the only other independent prognostic factor of outcome, and was associated with a significantly worse overall survival of 31.7% at two years compared with 73.3% (p = 0.0127; fig 3).
Figure 3 Overall survival of 69 individuals after surgical resection for oesophageal adenocarcinoma. Green: no lymph node metastasis or invasion of tumour cells into peritumorous lymphatics; purple: lymph node metastasis or invasion of tumour cells into peritumorous lymphatics (fig 1E; insert).
MVD in neoplastic progression from Barrett's metaplasia to oesophageal adenocarcinoma
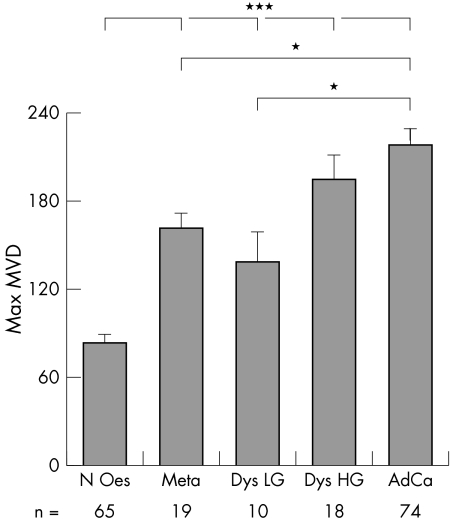
We measured MVD in normal oesophagus, metaplasia, dysplasia (low and high grade), and adenocarcinoma (fig 1). Microvessels were evenly distributed throughout the normal oesophageal mucosa (fig 1B, D) and were increasingly apparent around and within tumour tissue (fig 1F, H). MVD was significantly increased in all tissues (161.42, 138.1, 194.17, and 217.86 in metaplasia, low grade dysplasia, high grade dysplasia, and adenocarcinoma, respectively) compared with normal oesophagus (83.63; p < 0.001; fig 4). There was also a significant increase in MVD in adenocarcinoma (217.86) compared with both metaplasia (161.42) and low grade dysplasia (138.1; p < 0.01).
Figure 4 Maximum microvessel density (MVD) in the neoplastic sequence: normal oesophagus (N Oes), Barrett's metaplasia (Meta), low and high grade dysplasia (Dys LG/HG), and adenocarcinoma (AdCa). Correlation between MVD and histological stage. *p < 0.01; ***p < 0.001; n, number of patients/group; unpaired samples. Representative immunohistochemical images stained with CD34 (brown) and α smooth muscle actin (red) are shown in fig 1B and D (normal oesophagus) and fig 1F and H (adenocarcinoma).
Discussion
It is not known whether the early lymph node metastasis seen in oesophageal adenocarcinoma is dependent on lymphangiogenesis, invasion of tumour cells into pre‐existing lymphatic vessels, or other factors. We measured LVD and saw a decrease in lymphatic vessel numbers in adenocarcinoma compared with normal oesophagus. LVD was lower in the centre of tumours, with those lymphatics that were present occurring predominantly at the tumour periphery. This could be an underestimation of intratumorous lymphatics if lymphatic vessels are collapsed within tumours, and therefore more difficult to see, or it could be a real absence of lymphatic vessels. This decrease appears logical given the increased interstitial pressure in tumours and the physical properties of lymphatics18; however, lymphatic vessels that do not appear to be functional have been shown to be responsible for the dissemination of tumour cells.19 In this patient group, we saw no evidence of tumour associated lymphangiogenesis (viewed as an increase in lymphatics around or extending into a tumour), so that the frequent early lymph node metastasis seen in oesophageal adenocarcinoma is probably not primarily a result of increased LVD.
The only previous report that investigated lymphatics in oesophageal adenocarcinoma is from Auvinen et al,20 who reported tumour associated lymphangiogenesis using anti‐ vascular endothelial growth factor receptor 3 (VEGFR‐3) to stain lymphatics. VEGFR‐3 has since been shown not to be completely specific for lymphatic endothelium in the tumour vicinity because some blood vessels re‐express VEGFR‐3.21 We did not see an increase in intratumorous lymphatic vessels or an increase in total LVD. These apparent discrepancies may result from the fact that we used a more specific lymphatic endothelial marker, LYVE‐1.
LVD is not a suitable prognostic marker in oesophageal adenocarcinoma, because it does not correlate with disease progression or survival. LVD has been investigated as a prognostic marker in melanoma and squamous cell carcinoma of the head and neck with varying results.22,23,24,25,26 No clear consensus has been reached on the usefulness of LVD as a prognostic marker. We found that invasion of peritumorous lymphatic vessels by tumour cells and the presence of lymph node metastasis correlated significantly with worse overall survival. This confirms the use of lymph node status as the best prognostic indicator currently available.
Because MVD has previously been studied as a prognostic indicator, we concentrated on LVD. Nonetheless, to compare our patient group with those used in previous studies, we also measured MVD. The agreement between our MVD results and those of others20,27,28,29 shows that our patient group is representative. Although methodology and results varied in the different studies, the consensus is that MVD is not a good prognostic marker. We showed that MVD is increased in precancerous lesions compared with normal oesophagus, and increases progressively from metaplasia to adenocarcinoma. The formation of an increased vascular network is an early event associated with metaplasia formation and not with adenocarcinoma development. Increased MVD during metaplasia may be a direct result of the changes in the oesophageal epithelium from a squamous to a glandular phenotype expressing goblet cells. Goblet cells have previously been shown to express large quantities of vascular endothelial growth factor A.20,27 The local inappropriate expression of vascular endothelial growth factor A by goblet cells in the metaplastic glandular epithelium may cause the angiogenesis seen in metaplasia.
Take home messages
In patients with oesophageal adenocarcinoma, lymph vessel density (LVD) was significantly reduced in adenocarcinoma compared with normal stomach/oesophagus or metaplasia/dysplasia
Neither LVD nor microvessel density was associated with progression from a precancerous lesion to neoplasia, and neither is a suitable prognostic marker in oesophageal adenocarcinoma
Lymphangiogenesis was not detected, so that tumour associated lymphangiogenesis is probably not responsible for the frequent early occurrence of lymph node metastases seen in oesophageal adenocarcinoma
“We saw no evidence of tumour associated lymphangiogenesis (viewed as an increase in lymphatics around or extending into a tumour), so that the frequent early lymph node metastasis seen in oesophageal adenocarcinoma is probably not primarily a result of increased lymphatic vessel density”
In conclusion, we showed that in our patients with oesophageal adenocarcinoma neither LVD nor MVD was associated with progression from a precancerous lesion to neoplasia. We saw no evidence of lymphangiogenesis. Tumour associated lymphangiogenesis is therefore unlikely to be responsible for the frequent early occurrence of lymph node metastases seen in oesophageal adenocarcinoma. We hypothesise that some other factor is responsible for the early entry of tumour cells into peritumorous lymphatic vessels; the determination of such mechanisms may lead to the identification of strategies that can prevent tumour cell dissemination during the pathogenesis of oesophageal adenocarcinoma.
Acknowledgements
We are grateful to N Dupont and C Rumbelli for their technical assistance and to Professor R Genta for advice and critical reading of the manuscript. This work was funded by the Swiss National Science Foundation (number 3100‐064037.00 to MSP) and the Ligue Genevoise Contre le Cancer (to MAB and MSP).
Abbreviations
LVD - lymphatic vessel density
LYVE‐1 - lymphatic endothelium specific hyaluronan receptor
MVD - microvascular density
VEGFR‐3 - vascular endothelial growth factor receptor 3
References
- 1.Pera M. Trends in incidence and prevalence of specialized intestinal metaplasia, Barrett's esophagus, and adenocarcinoma of the gastroesophageal junction. World J Surg 200327999–1008. [DOI] [PubMed] [Google Scholar]
- 2.Sampliner R E. Adenocarcinoma of the esophagus and gastric cardia: is there progress in the face of increasing cancer incidence? Ann Intern Med 199913067–69. [DOI] [PubMed] [Google Scholar]
- 3.Spechler S J. Clinical practice. Barrett's esophagus. N Engl J Med 2002346836–842. [DOI] [PubMed] [Google Scholar]
- 4.Corey K E, Schmitz S M, Shaheen N J. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett's esophagus? A meta‐analysis. Am J Gastroenterol 2003982390–2394. [DOI] [PubMed] [Google Scholar]
- 5.Hawk E.Report of the stomach/esophageal cancers progress review group. National Cancer Institute, 2002 ( http://searchosp1.nci.nih.gov/stomach/stomach_esophageal.pdf )
- 6.Boyce H W. Barrett esophagus: endoscopic findings and what to biopsy. J Clin Gastroenterol 200336S6–18. [DOI] [PubMed] [Google Scholar]
- 7.Reid B J, Blount P L, Rabinovitch P S. Biomarkers in Barrett's esophagus. Gastrointest Endosc Clin N Am 200313369–397. [DOI] [PubMed] [Google Scholar]
- 8.Mandriota S J, Jussila L, Jeltsch M.et al Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumour metastasis. EMBO J 200120672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerji S, Ni J, Wang S X.et al LYVE‐1, a new homologue of the CD44 glycoprotein, is a lymph‐specific receptor for hyaluronan. J Cell Biol 1999144789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermanek P, Hutter R V P, Sobin L H.et al TNM atlas. Illustrated guide to the TNM/pTNM classification of malignant tumours. Berlin: Springer‐Verlag, 1997
- 11.Nisato R E, Harrison J A, Buser R.et al Generation and characterization of telomerase‐transfected human lymphatic endothelial cells with an extended life span. Am J Pathol 200416511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarijs R, Schalkwijk L, Hofmann U B.et al Induction of vascular endothelial growth factor receptor‐3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res 2002627059–7065. [PubMed] [Google Scholar]
- 13.Weidner N, Folkman J, Pozza F.et al Tumor angiogenesis: a new significant and independent prognostic indicator in early‐stage breast carcinoma. J Natl Cancer Inst 1992841875–1887. [DOI] [PubMed] [Google Scholar]
- 14.Weidner N, Semple J P, Welch W R.et al Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 19913241–8. [DOI] [PubMed] [Google Scholar]
- 15.Hall N R, Fish D E, Hunt N.et al Is the relationship between angiogenesis and metastasis in breast cancer real? Surg Oncol 19921223–229. [DOI] [PubMed] [Google Scholar]
- 16.Bosari S, Lee A K, DeLellis R A.et al Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 199223755–761. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen P B, Gasparini G, Fox S B.et al Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 199632A2474–2484. [DOI] [PubMed] [Google Scholar]
- 18.Leu A J, Berk D A, Lymboussaki A.et al Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 2000604324–4327. [PubMed] [Google Scholar]
- 19.Padera T P, Kadambi A, di Tomaso E.et al Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 20022961883–1886. [DOI] [PubMed] [Google Scholar]
- 20.Auvinen M I, Sihvo E I, Ruohtula T.et al Incipient angiogenesis in Barrett's epithelium and lymphangiogenesis in Barrett's adenocarcinoma. J Clin Oncol 2002202971–2979. [DOI] [PubMed] [Google Scholar]
- 21.Clarijs R, Schalkwijk L, Hofmann U B.et al Induction of vascular endothelial growth factor receptor‐3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res 2002627059–7065. [PubMed] [Google Scholar]
- 22.Straume O, Jackson D G, Akslen L A. Independent prognostic impact of lymphatic vessel density and presence of low‐grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 20039250–256. [PubMed] [Google Scholar]
- 23.Valencak J, Heere‐Ress E, Kopp T.et al Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer 200440358–364. [DOI] [PubMed] [Google Scholar]
- 24.Straume O, Akslen L A. Lymphatic vessel density and prognosis in cutaneous melanoma. Br J Cancer 2004911224–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maula S M, Luukkaa M, Grenman R.et al Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 2003631920–1926. [PubMed] [Google Scholar]
- 26.Padera T P, Boucher Y, Jain R K. Correspondence re: S. Maula, et al. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinoma of the head and neck. Cancer Res 2003638555–8556. [PubMed] [Google Scholar]
- 27.Couvelard A, Paraf F, Gratio V.et al Angiogenesis in the neoplastic sequence of Barrett's oesophagus. Correlation with VEGF expression. J Pathol 200019214–18. [DOI] [PubMed] [Google Scholar]
- 28.Torres C, Wang H, Turner J.et al Prognostic significance and effect of chemoradiotherapy on microvessel density (angiogenesis) in esophageal Barrett's esophagus‐associated adenocarcinoma and squamous cell carcinoma. Hum Pathol 199930753–758. [DOI] [PubMed] [Google Scholar]
- 29.Lord R V, Park J M, Wickramasinghe K.et al Vascular endothelial growth factor and basic fibroblast growth factor expression in esophageal adenocarcinoma and Barrett esophagus. J Thorac Cardiovasc Surg 2003125246–253. [DOI] [PubMed] [Google Scholar]